Microwave Assistant Synthesis, Antifungal Activity and DFT Theoretical Study of Some Novel 1,2,4-Triazole Derivatives Containing Pyridine Moiety
Abstract
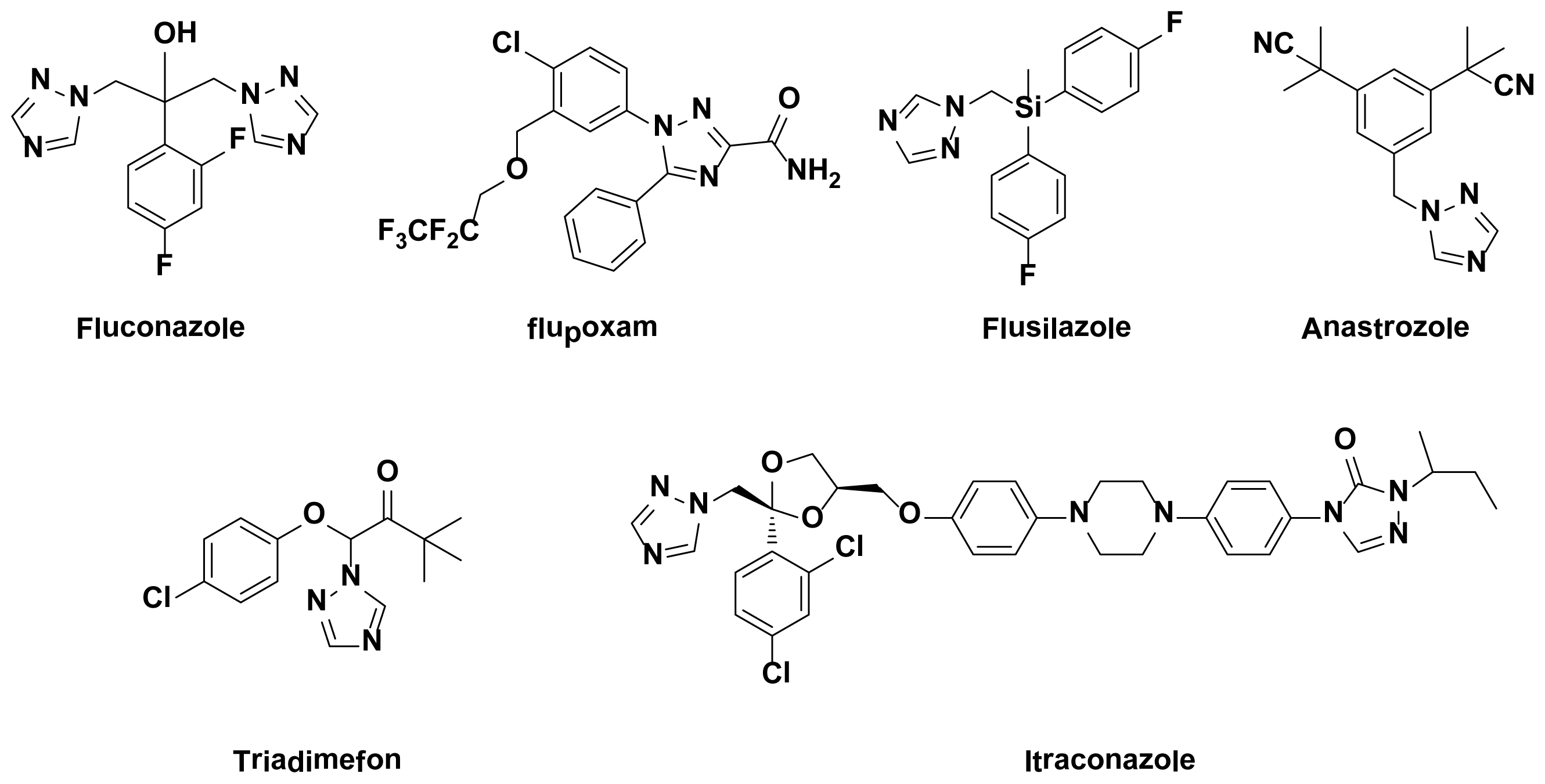
:1. Introduction
2. Results and Discussion
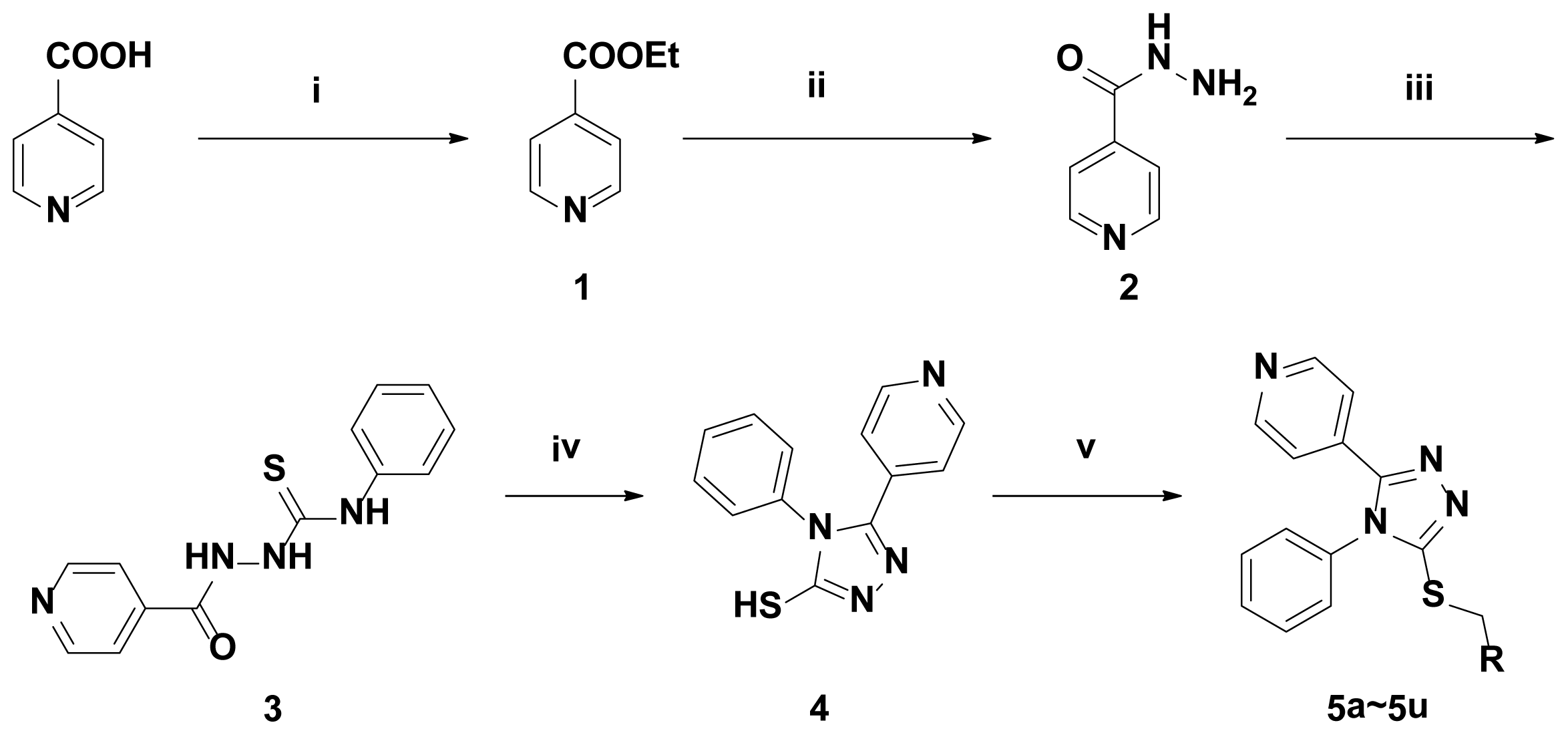
2.1. Chemistry
2.2. Fungicidal Activities and Structure–Activity Relationship (SAR)
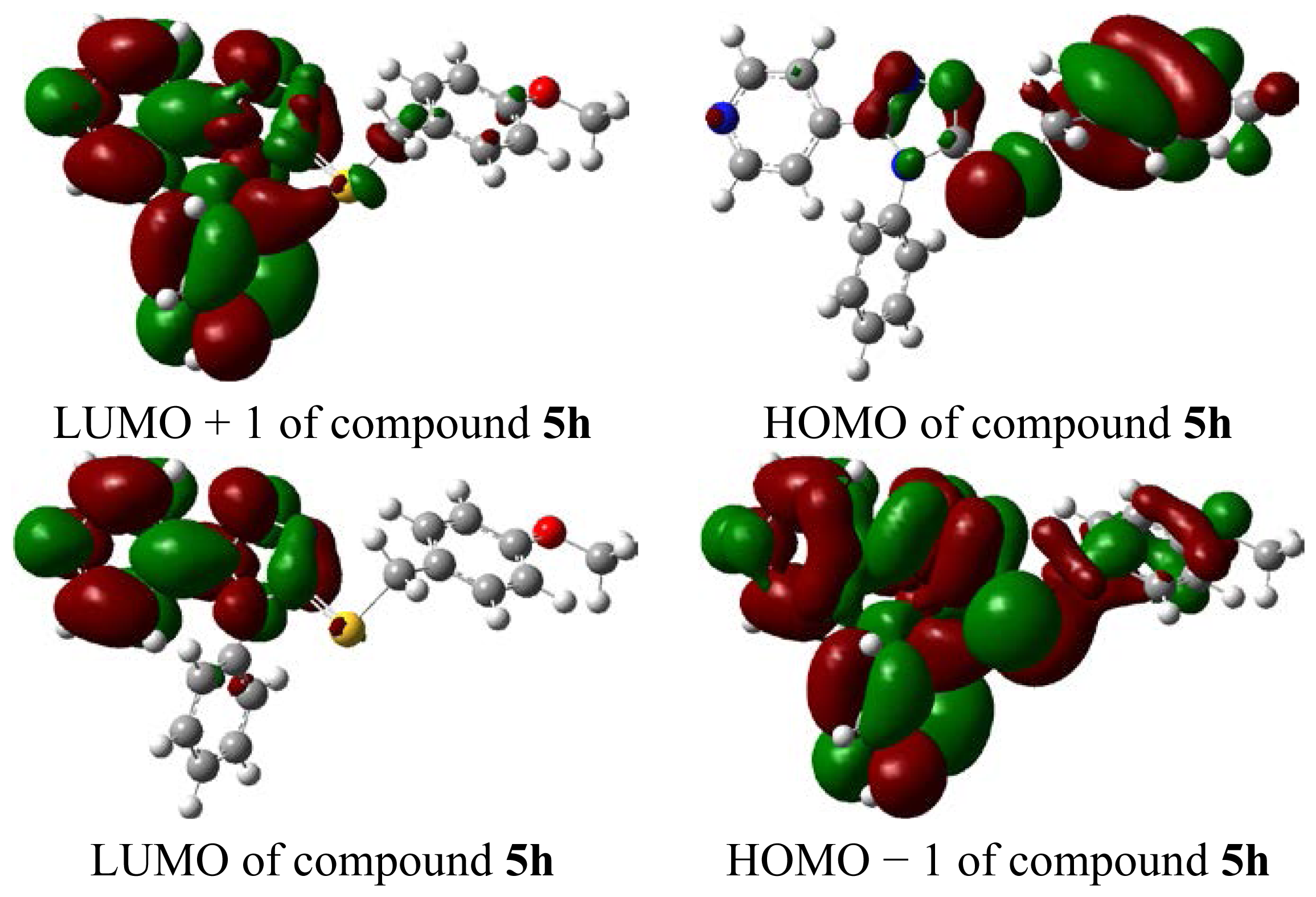
2.3. Molecular Total Energies and Frontier Orbital Energy Analysis
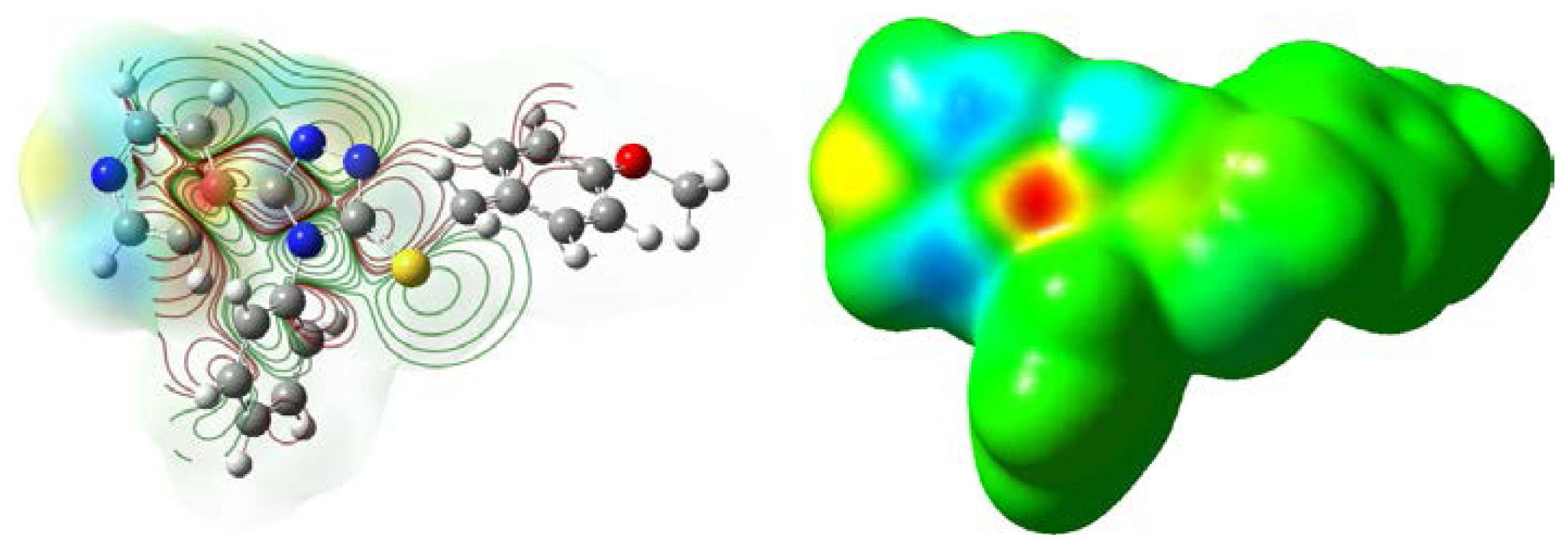
2.4. Mulliken Atomic Charges and Electrostatic Potential Analyses
3. Experimental Section
3.1. Materials and Reagents
3.2. Theoretical Calculations
3.3. Chemical Synthesis
Synthesis of Intermediates
Ethyl Isonicotinate (1)
Isonicotinyi Hydrazine (2)
2-(4-Methyl-1, 2, 3-thiadiazole-5-carbonyl)-N-phenylhydrazinecarbothioamide (3)
5-Pyridyl-4-phenyl-4H-1,2,4-triazole-3-thiol (4)
General Procedure for Thioether (5)
4-((5-(2,4-Dichlorobenzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyridine (5a)
3-(((4-Phenyl-5-(pyridin-4-yl)-4H-1,2,4-triazol-3-yl)thio)methyl)benzonitrile (5b)
4-((5-(2-Fluorobenzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyridine (5c)
4-(((4-Phenyl-5-(pyridin-4-yl)-4H-1,2,4-triazol-3-yl)thio)methyl)benzonitrile (5d)
2-Chloro-5-(((4-phenyl-5-(pyridin-4-yl)-4H-1,2,4-triazol-3-yl)thio)methyl)pyridine (5e)
4-(5-((3-Chlorobenzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyridine (5f)
4-(5-((4-(Tert-butyl)benzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyridine (5g)
4-(5-((4-Methoxybenzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyridine (5h)
4-(5-((3,4-Dichlorobenzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyridine (5i)
4-(5-((2-Chlorobenzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyridine (5j)
4-(5-((4-Bromobenzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyridine (5k)
4-(5-((4-Chlorobenzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyridine (5l)
4-(5-(Butylthio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyridine (5m)
2-Chloro-5-(((4-phenyl-5-(pyridin-4-yl)-4H-1,2,4-triazol-3-yl)thio)methyl)thiazole (5n)
2-((4-Phenyl-5-(pyridin-4-yl)-4H-1,2,4-triazol-3-yl)thio)acetonitrile (5o)
(E)-Methyl-2-(methoxyimino)-2-(2-(((4-phenyl-5-(pyridin-4-yl)-4H-1,2,4-triazol-3-yl)thio)methyl) phenyl)acetate (5p)
4-(5-((3-Fluorobenzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyridine (5q)
4-(5-((2-Methylbenzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyridine (5r)
4-(4-Phenyl-5-(undecylthio)-4H-1,2,4-triazol-3-yl)pyridine (5s)
4-(5-(Allylthio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyridine (5t)
Ethyl-2-((4-phenyl-5-(pyridin-4-yl)-4H-1,2,4-triazol-3-yl)thio)acetate (5u)
3.4. Fungicidal Activities
4. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsG.X., M.Y., Z.H., X.H. designed and synthesis the compound. B.J. and Y.X do the antifungal experiments. Y.G. and X.H. do the theoretical calculations. H.K. give some discuss and chemical characterization. X.H. wrote the manuscript.
References
- Malik, M.A.; Al-Thabaiti, S.A.; Malik, M.A. Synthesis, structure optimization and antifungal screening of novel tetrazole ring bearing acyl-hydrazones. Int. J. Mol. Sci 2012, 13, 10880–10898. [Google Scholar]
- Xu, N.; Yang, C.; Gan, X.; Wei, S.; Ji, Z. Synthesis of 1-isopropyl-3-acyl-5-methyl-benzimidazolone derivatives and their antimicrobial activity. Int. J. Mol. Sci 2013, 14, 6790–6804. [Google Scholar]
- Liu, X.H.; Pan, L.; Tan, C.X.; Weng, J.Q.; Wang, B.L.; Li, Z.M. Synthesis, crystal structure, bioactivity and DFT calculation of new oxime ester derivatives containing cyclopropane moiety. Pestic. Biochem. Physiol 2011, 101, 143–147. [Google Scholar]
- Mohamed, N.A.; Fahmy, M.M. Synthesis and antimicrobial activity of some novel cross-linked chitosan hydrogels. Int. J. Mol. Sci 2012, 13, 11194–11209. [Google Scholar]
- Kheder, N.A.; Mabkhot, Y.N. Synthesis and antimicrobial studies of some novel bis-[1,3,4]thiadiazole and bis-thiazole pendant to thieno[2,3-b]thiophene moiety. Int. J. Mol. Sci 2012, 13, 3661–3670. [Google Scholar]
- Liu, X.H.; Weng, J.Q.; Tan, C.X. Synthesis, crystal structure, and fungicidal activity of 5-(4-cyclopropyl-5-((3-fluorobenzyl)thio)-4H-1,2,4-triazol-3-yl)-4-methyl-1,2,3-thiadiazole. J. Chem 2013, 2013, 306361. [Google Scholar]
- Nitulescu, G.M.; Draghici, C.; Olaru, O.T. New potential antitumor pyrazole derivatives: Synthesis and cytotoxic evaluation. Int. J. Mol. Sci 2013, 14, 21805–21818. [Google Scholar]
- Weng, J.Q.; Wang, L.; Liu, X.H. Synthesis, crystal structure and herbicidal activity of a 1,2,4-triazol-5(4H)-one derivative. J. Chem. Soc. Pak 2012, 34, 1248–1252. [Google Scholar]
- Liu, X.H.; Zhao, W.G.; Wang, B.L.; Li, Z.M. Synthesis, bioactivity and DFT structure-activity relationship study of novel 1,2,3-thiadiazole derivatives. Chem. Res. Intermed 2012, 38, 1999–2008. [Google Scholar]
- Sun, N.B.; Tong, J.Y.; Wu, H.K. Synthesis and fungicidal activity of 1,3,4-oxadiazole derivatives containing pyrazole moiety. Chin. J. Org. Chem 2013, 33, 101–105. [Google Scholar]
- Tan, C.X.; Shi, Y.X.; Weng, J.Q.; Liu, X.H.; Li, B.J.; Zhao, W.G. Synthesis and antifungal activity of 1, 2,4-triazole derivatives containing cyclopropane moiety. Lett. Drug Des. Discov 2012, 9, 431–435. [Google Scholar]
- Kumar, D.; Narayanam, M.K.; Chang, K.H.; Shah, K. Synthesis of novel indolyl-1,2,4-triazoles as potent and selective anticancer agents. Chem. Biol. Drug Des 2011, 77, 182–188. [Google Scholar]
- Franklim, T.N.; Freire-de-Lima, L.; Diniz, J.D.S.; Previato, J.O.; Castro, R.N.; Mendonca-Previato, L.; de Lima, M.E.F. Design, synthesis and trypanocidal evaluation of novel 1,2,4-triazoles-3-thiones derived from natural piperine. Molecules 2013, 18, 6366–6382. [Google Scholar]
- Zhang, J.-W.; Hu, Z.; Gao, P.; Wang, J.-R.; Hu, Z.-N.; Wu, W.-J. Synthesis and larvicidal activity against Culex pipiens pallens of New Triazole Derivatives of Phrymarolin from Phryma leptostachya L. Int. J. Mol. Sci 2013, 14, 24064–24073. [Google Scholar]
- Kocyigit-Kaymakcioglu, B.; Celen, A.O.; Tabanca, N.; Ali, A.; Khan, S.I.; Khan, I.A.; Wedge, D.E. Synthesis and biological activity of substituted urea and thiourea derivatives containing 1,2,4-triazole moieties. Molecules 2013, 18, 3562–3576. [Google Scholar]
- Ke, W.; Sun, N.B.; Wu, H.K. Microwave assistant synthesis, crystal structure and biological activity of a 1,2,4-triazole compound. J. Chem. Soc. Pak 2013, 35, 1239–1244. [Google Scholar]
- Wang, B.L.; Liu, X.H.; Zhang, X.L.; Zhang, J.F.; Song, H.B.; Li, Z.M. Synthesis, structure and biological activity of novel 1,2,4-triazole mannich bases containing a substituted benzylpiperazine moiety. Chem. Biol. Drug Des 2011, 78, 42–49. [Google Scholar]
- Maddila, S.; Kumar, A.S.; Gorle, S.; Singh, M.; Lavanya, P.; Jonnalagadda, S.B. Synthesis and antioxidant activity of 1,2,4-triazole linked thieno[2,3-d]pyrimidine derivatives. Lett. Drug Des. Discov 2013, 10, 186–193. [Google Scholar]
- Benci, K.; Mandic, L.; Suhina, T.; Sedic, M.; Klobucar, M.; Pavelic, S.K.; Pavelic, K.; Wittine, K.; Mintas, M. Novel coumarin derivatives containing 1,2,4-triazole, 4,5-dicyanoimidazole and purine moieties: Synthesis and evaluation of their cytostatic activity. Molecules 2012, 17, 11010–11025. [Google Scholar]
- Yamada, K.; Yoshizawa, Y.; Oh, K. Synthesis of 2RS,4RS-1-[2-phenyl-4-[2-(2-trifluromethoxy-phenoxy)-ethyl]-1,3-dioxolan-2-yl-methyl]-1H-1,2,4-triazole derivatives as potent inhibitors of brassinosteroid biosynthesis. Molecules 2012, 17, 4460–4473. [Google Scholar]
- Yunus, U.; Bhatti, M.H.; Rahman, N.; Mussarat, N.; Asghar, S.; Masood, B. Synthesis, characterization, and biological activity of novel Schiff and Mannich bases of 4-amino-3-(N-phthalimidomethyl)-1,2,4-triazole-5-thione. J. Chem 2013, 2013, 638520. [Google Scholar]
- Dilmaghani, K.A.; Pur, F.N.; Jazani, N.H.; Alavi, A.; Niknam, Z.; Mirfakhraee, F. Synthesis of new 1,2,4-triazole-5-thiones and their thioglycoside derivatives as potential antibacterial agent. Phosphorus Sulfur Silicon Relat. Elem 2014, 189, 81–87. [Google Scholar]
- Plech, T.; Wujec, M.; Kosikowska, U.; Malm, A. Synthesis and antibacterial activity of 4,5-disubstituted-1,2,4-triazole-3-thiones. Lett. Drug Des. Discov 2013, 10, 917–922. [Google Scholar]
- Kaldrikyan, M.A.; Melik-Oganjanyan, R.G.; Aresnyan, F.H. Synthesis and antitumor activity of 5-methylbenzofuryl-substituted 1,2,4-triazoles and triazoline-5-thiones. Pharm. Chem. J 2013, 47, 191–194. [Google Scholar]
- Zia, M.; Akhtar, T.; Hameed, S.; Al-Masoudi, N.A. New aryl-1,3-thiazole-4-carbohydrazides, their 1,3,4-oxadiazole-2-thione, 1,2,4-triazole, isatin-3-ylidene and carboxamide derivatives. Synthesis and anti-HIV activity. Z. Naturforschung B 2012, 67, 747–758. [Google Scholar]
- Xiong, Q.Z.; Lin, X.F.; Liu, J.H.; Bi, L.; Bao, X.P. Synthesis and bioactivities of novel 1,2,4-triazolo[1,5-a]pyrimidine derivatives containing 1,2,4-triazole-5-thione Schiff base unit. Chin. J. Org. Chem 2012, 32, 1255–1260. [Google Scholar]
- Chaban, T.I.; Ogurtsov, V.V.; Chaban, I.G.; Klenina, O.V.; Komarytsia, J.D. Synthesis and antioxidant activity evaluation of novel 5,7-dimethyl-3h-thiazolo[4,5-b]pyridine. Phosphorus Sulfur Silicon Relat. Elem 2013, 188, 1611–1620. [Google Scholar]
- Janardhan, B.; Vijayalaxmi, S.; Rajitha, B. Sulfamic acid-catalyzed multicomponent one-pot synthesis of poly-substituted pyridines and their antimicrobial activity. J. Heterocycl. Chem 2013, 50, 1230–1235. [Google Scholar]
- Szymański, P.; Lázničková, A.; Lázniček, M.; Bajda, M.; Malawska, B.; Markowicz, M.; Mikiciuk-Olasik, E. 2,3-Dihydro-1H-cyclopenta[b]quinoline derivatives as acetylcholinesterase inhibitors—Synthesis, radiolabeling and biodistribution. Int. J. Mol. Sci 2012, 13, 10067–10090. [Google Scholar]
- El-Emary, T.I.; El-Mohsen, S.S.A. Multi-component one-pot synthesis and antimicrobial activities of 3-methyl-1,4-diphenyl-7-thioxo-4,6,8,9-tetrahydropyrazolo[5,4-b]pyrimidino[5,4-e]pyridine-5-one and related derivatives. Molecules 2012, 17, 14464–14483. [Google Scholar]
- Altalbawy, F.M.A. Synthesis and antimicrobial evaluation of some novel bis-α, β-unsaturated ketones, nicotinonitrile, 1,2-dihydropyridine-3-carbonitrile, fused thieno[2,3-b]pyridine and pyrazolo[3,4-b]pyridine derivatives. Int. J. Mol. Sci 2013, 14, 2967–2979. [Google Scholar]
- Al-Abdullah, E.S. Synthesis and anticancer activity of some novel tetralin-6-yl-pyrazoline, 2-thioxopyrimidine, 2-oxopyridine, 2-thioxo-pyridine and 2-iminopyridine derivatives. Molecules 2011, 16, 3410–3419. [Google Scholar]
- Mohareb, R.M.; Fleita, D.H.; Sakka, O.K. Novel synthesis of hydrazide-hydrazone derivatives and their utilization in the synthesis of coumarin, pyridine, thiazole and thiophene derivatives with antitumor activity. Molecules 2011, 16, 16–27. [Google Scholar]
- Chen, D.; Liu, Y.J.; Zhang, S.L.; Guo, D.X.; Liu, C.H.; Li, S.; Gong, P. Synthesis and in vitro anti-hepatitis B virus activity of ethyl 6-bromo-8-hydroxyimidazo[1,2-a]pyridine-3-carboxylates. Arch. Der. Pharm 2011, 344, 158–164. [Google Scholar]
- Wu, J.; Kang, S.H.; Song, B.A.; Hu, D.Y.; He, M.; Jin, L.H.; Yang, S. Synthesis and antibacterial activity against ralstonia solanacearum for novel hydrazone derivatives containing a pyridine moiety. Chem. Cent. J 2012, 6, 28. [Google Scholar]
- Li, D.J.; Long, D.Q.; Fu, H.Q. The synthesis and fungicidal activities of 2,6-bis[(3-aryl)-S-triazolo[3,4-b]-[1,3,4]thiadiazole-6-yl]pyridines. Phosphorus Sulfur Silicon Relat. Elem 2006, 181, 2079–2087. [Google Scholar]
- Liu, X.H.; Pan, L.; Ma, Y.; Weng, J.Q.; Tan, C.X.; Li, Y.H.; Shi, Y.X.; Li, B.J.; Li, Z.M.; Zhang, Y.G. Design, synthesis, biological activities, and 3D-QSAR of new N,N′-diacylhydrazines containing 2-(2,4-dichlorophenoxy)propane moiety. Chem. Biol. Drug Des 2011, 78, 689–694. [Google Scholar]
- Sun, G.X.; Sun, Z.H.; Yang, M.Y.; Liu, X.H.; Ma, Y.; Wei, Y.Y. Design, synthesis, biological activities and 3D-QSAR of new N,N′-diacylhydrazines containing 2,4-dichlorophenoxy moieties. Molecules 2013, 18, 14876–14891. [Google Scholar]
- Sun, N.-B.; Fu, J.-Q.; Weng, J.-Q.; Jin, J.-Z.; Tan, C.-X.; Liu, X.-H. Microwave assisted synthesis, antifungal activity and DFT theoretical study of some novel 1,2,4-triazole derivatives containing the 1,2,3-thiadiazole moiety. Molecules 2013, 18, 12725–12739. [Google Scholar]
- Sun, N.-B.; Shi, Y.-X.; Liu, X.-H.; Ma, Y.; Tan, C.-X.; Weng, J.-Q.; Jin, J.-Z.; Li, B.-J. Design, synthesis, antifungal activities and 3D-QSAR of new N,N′-diacylhydrazines containing 2,4-dichlorophenoxy moiety. Int. J. Mol. Sci 2013, 14, 21741–21756. [Google Scholar]
- Liu, X.H.; Chen, P.Q.; Wang, B.L.; Li, Y.H.; Wang, S.H.; Li, Z.M. Synthesis, bioactivity, theoretical and molecular docking study of 1-cyano-N-substituted-cyclopropanecarboxamide as ketol-acid reductoisomerase inhibitor. Bioorg. Med. Chem. Lett 2007, 17, 3784–3788. [Google Scholar]
- Frisch, M.-J.; Trucks, G.-W.; Schlegel, H.-B.; Scuseria, G.-E.; Robb, M.-A.; Cheeseman, J.-R.; Montgomery, J.-A., Jr.; Vreven, T.; Kudin, K.-N.; Burant, J.-C.; et al. Gaussian 03, Revision C. 01; Gaussian, Inc: Wallingford, CT, USA, 2004. [Google Scholar]

| No. | Method | Time | Temperature/°C | Yield/% |
|---|---|---|---|---|
| 5a | No-MW | 24 h | r.t. | 80 |
| 5a | No-MW | 10 min | 90 | 38 |
| 5a | MW | 10 min | 90 | 81 |
| 5a | MW | 15 min | 90 | 83 |
| 5a | MW | 20 min | 90 | 83 |
| No. | Stemphylium lycopersici (Enjoji) Yamamoto | Fusarium oxysporum. sp. cucumebrium | Botrytis cinerea |
|---|---|---|---|
| 5a | 53.57 | 66.67 | 24.44 |
| 5b | 5.21 | 64.86 | 20.42 |
| 5c | 50.30 | 65.56 | 28.89 |
| 5d | 33.04 | 66.67 | 23.33 |
| 5e | 43.15 | 64.44 | 24.44 |
| 5f | 46.23 | 66.94 | 14.44 |
| 5g | 71.43 | 63.93 | 20.00 |
| 5h | 85.12 | 64.44 | 20.00 |
| 5i | 73.51 | 63.33 | 16.67 |
| 5j | 62.20 | 66.67 | 15.56 |
| 5k | 42.86 | 64.44 | 6.67 |
| 5l | 66.98 | 64.44 | 17.78 |
| 5m | 54.17 | 64.81 | 26.67 |
| 5n | 46.58 | 65.56 | 10.00 |
| 5o | 65.67 | 48.89 | 13.33 |
| 5p | 66.07 | 54.44 | 15.56 |
| 5q | 67.86 | 53.33 | 22.22 |
| 5r | 75.00 | 53.33 | 15.56 |
| 5s | 51.79 | 53.33 | 12.22 |
| 5t | 79.76 | 55.56 | 12.22 |
| 5u | 75.89 | 48.89 | 20.00 |
| Zhongshengmycin | 59.58 | ||
| Thiophanate-Methyl | 81.69 | ||
| Cyprodinil | 45.56 | ||
| E | DFT |
|---|---|
| Etotal/Hartree b | −1503.11499092 |
| EHOMO/Hartree | −0.21450 |
| ELUMO/Hartree | −0.05529 |
| ΔE a/Hartree | 0.15921 |
 | |
|---|---|
| Atom | Charge(DFT) |
| S18 | 0.326749 |
| N14 | −0.359494 |
| N4 | −0.757709 |
| N2 | −0.319626 |
| N1 | −0.308887 |
| C16 | 0.035720 |
| C15 | 0.125270 |
| C13 | 0.135365 |
| C12 | 0.056497 |
| C17 | 0.127367 |
| C3 | 0.361752 |
| C5 | 0.175208 |
| C6 | 0.170471 |
| C7 | 0.092394 |
| C8 | −0.002097 |
| C9 | 0.040403 |
| C10 | −0.001456 |
| C11 | 0.093994 |
| C19 | −0.146321 |
| C20 | 0.128847 |
| C21 | −0.032559 |
| C22 | −0.012577 |
| C23 | 0.287098 |
| C24 | 0.008964 |
| C25 | 0.034697 |
| O26 | −0.557033 |
| C27 | 0.296964 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sun, G.-X.; Yang, M.-Y.; Shi, Y.-X.; Sun, Z.-H.; Liu, X.-H.; Wu, H.-K.; Li, B.-J.; Zhang, Y.-G. Microwave Assistant Synthesis, Antifungal Activity and DFT Theoretical Study of Some Novel 1,2,4-Triazole Derivatives Containing Pyridine Moiety. Int. J. Mol. Sci. 2014, 15, 8075-8090. https://doi.org/10.3390/ijms15058075
Sun G-X, Yang M-Y, Shi Y-X, Sun Z-H, Liu X-H, Wu H-K, Li B-J, Zhang Y-G. Microwave Assistant Synthesis, Antifungal Activity and DFT Theoretical Study of Some Novel 1,2,4-Triazole Derivatives Containing Pyridine Moiety. International Journal of Molecular Sciences. 2014; 15(5):8075-8090. https://doi.org/10.3390/ijms15058075
Chicago/Turabian StyleSun, Guo-Xiang, Ming-Yan Yang, Yan-Xia Shi, Zhao-Hui Sun, Xing-Hai Liu, Hong-Ke Wu, Bao-Ju Li, and Yong-Gang Zhang. 2014. "Microwave Assistant Synthesis, Antifungal Activity and DFT Theoretical Study of Some Novel 1,2,4-Triazole Derivatives Containing Pyridine Moiety" International Journal of Molecular Sciences 15, no. 5: 8075-8090. https://doi.org/10.3390/ijms15058075
APA StyleSun, G.-X., Yang, M.-Y., Shi, Y.-X., Sun, Z.-H., Liu, X.-H., Wu, H.-K., Li, B.-J., & Zhang, Y.-G. (2014). Microwave Assistant Synthesis, Antifungal Activity and DFT Theoretical Study of Some Novel 1,2,4-Triazole Derivatives Containing Pyridine Moiety. International Journal of Molecular Sciences, 15(5), 8075-8090. https://doi.org/10.3390/ijms15058075




