Effect of Fiber Length on Carbon Nanotube-Induced Fibrogenesis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization of Single-Walled Carbon Nanotubes (SWCNT)
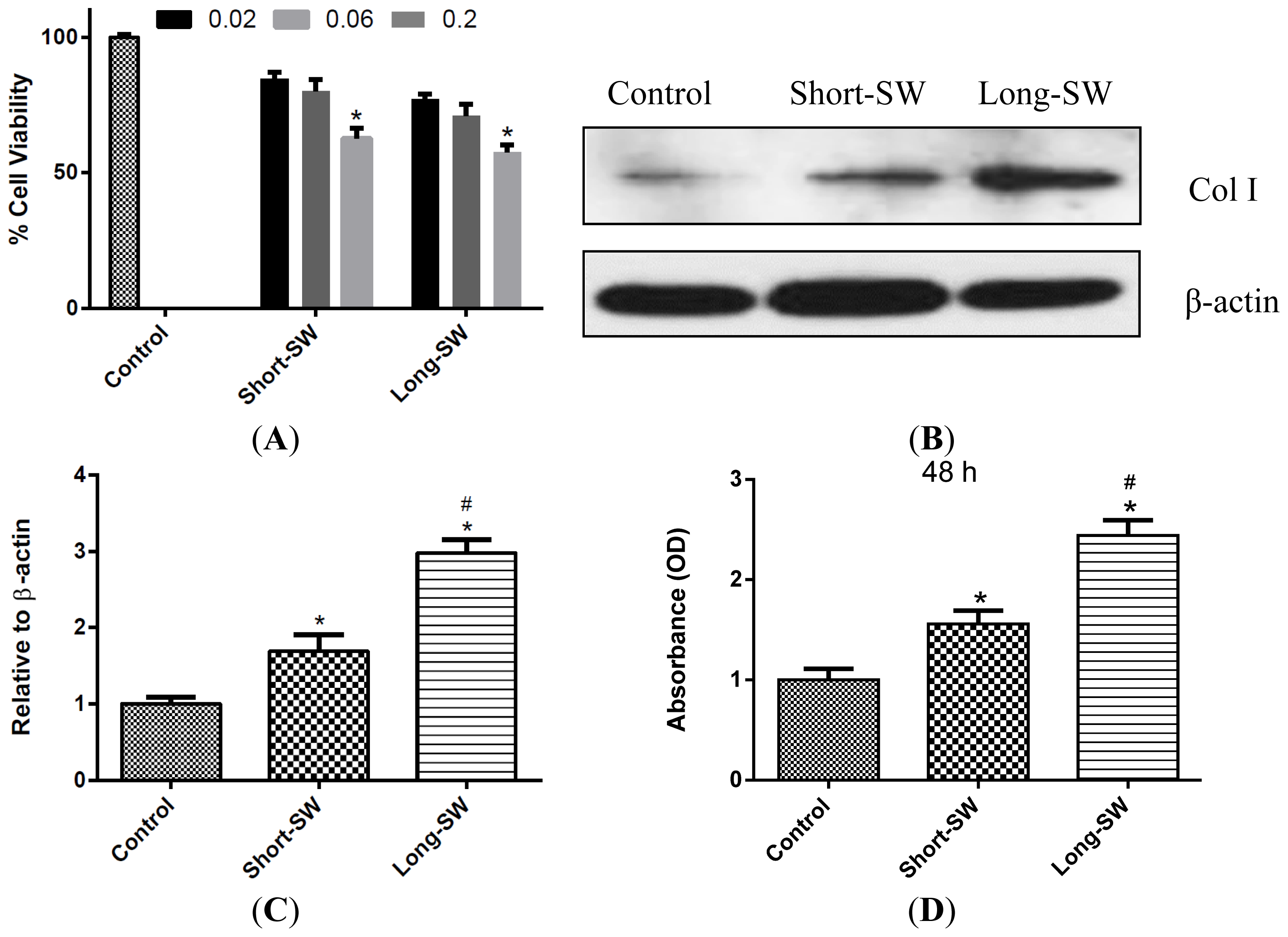
2.2. Dose- and Length-Dependent Effects of SWCNTs on Cell Viability and Collagen Expression
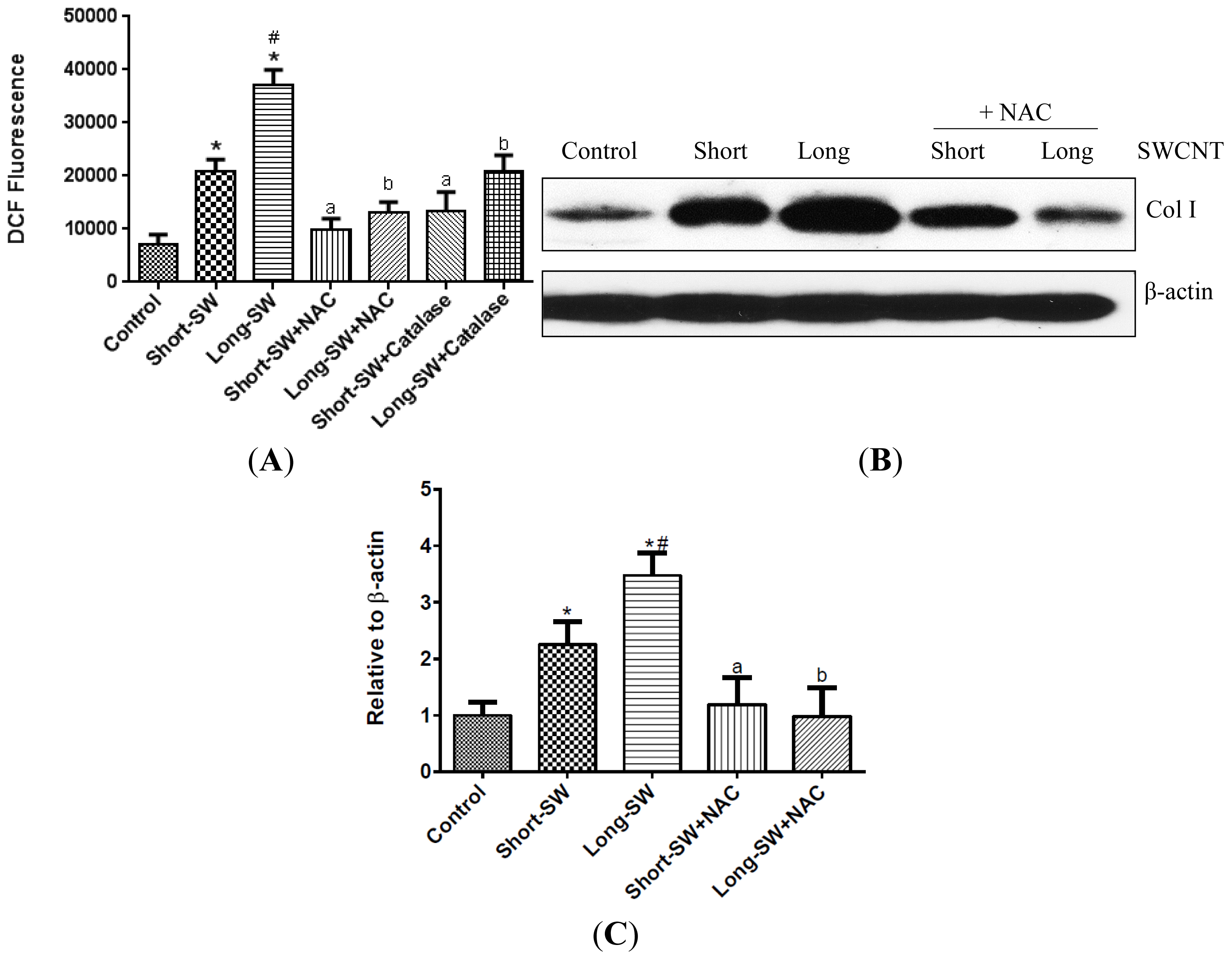
2.3. SWCNTs Induced Cellular Oxidative Stress and Fibrogenic Response
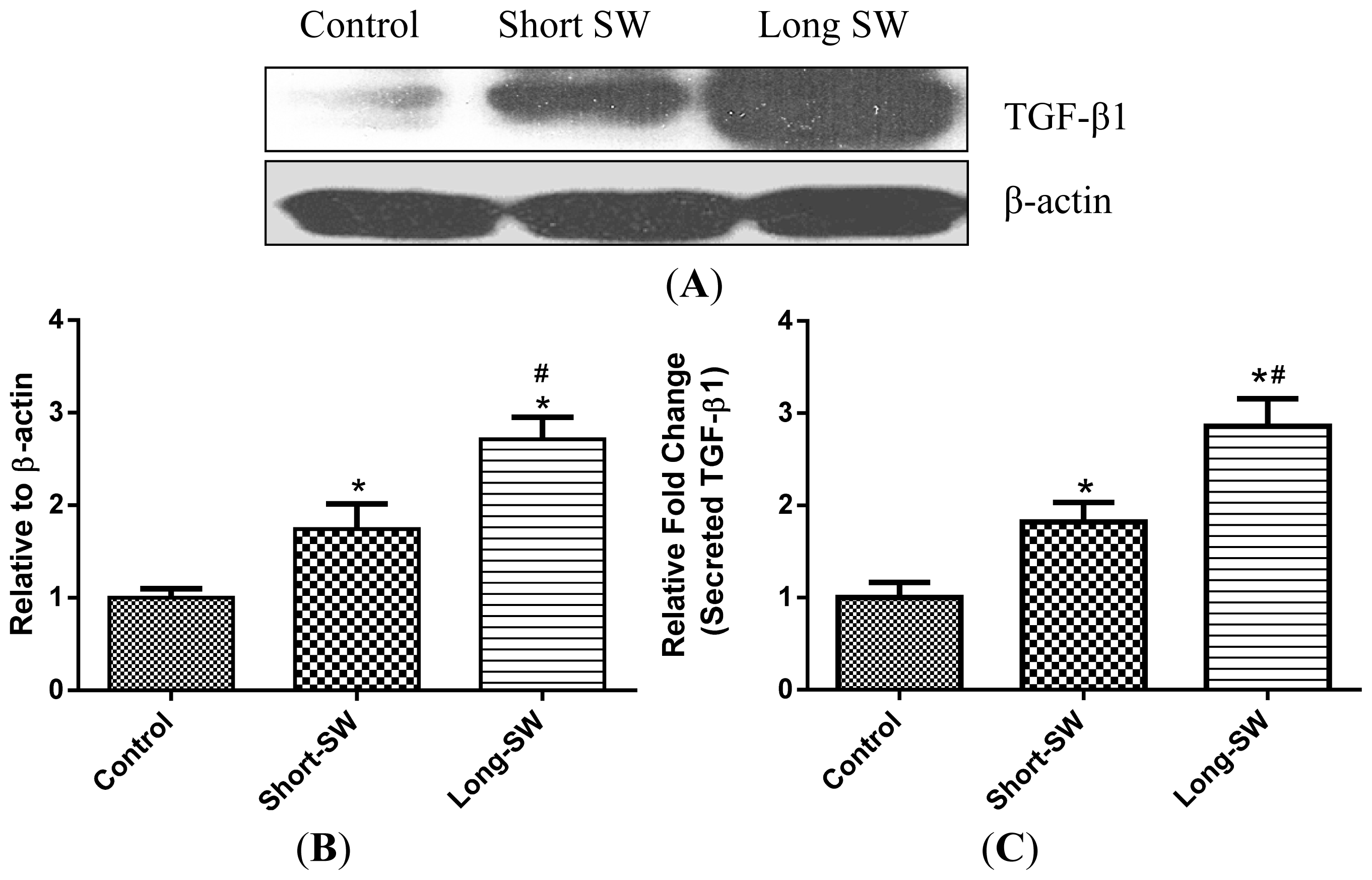
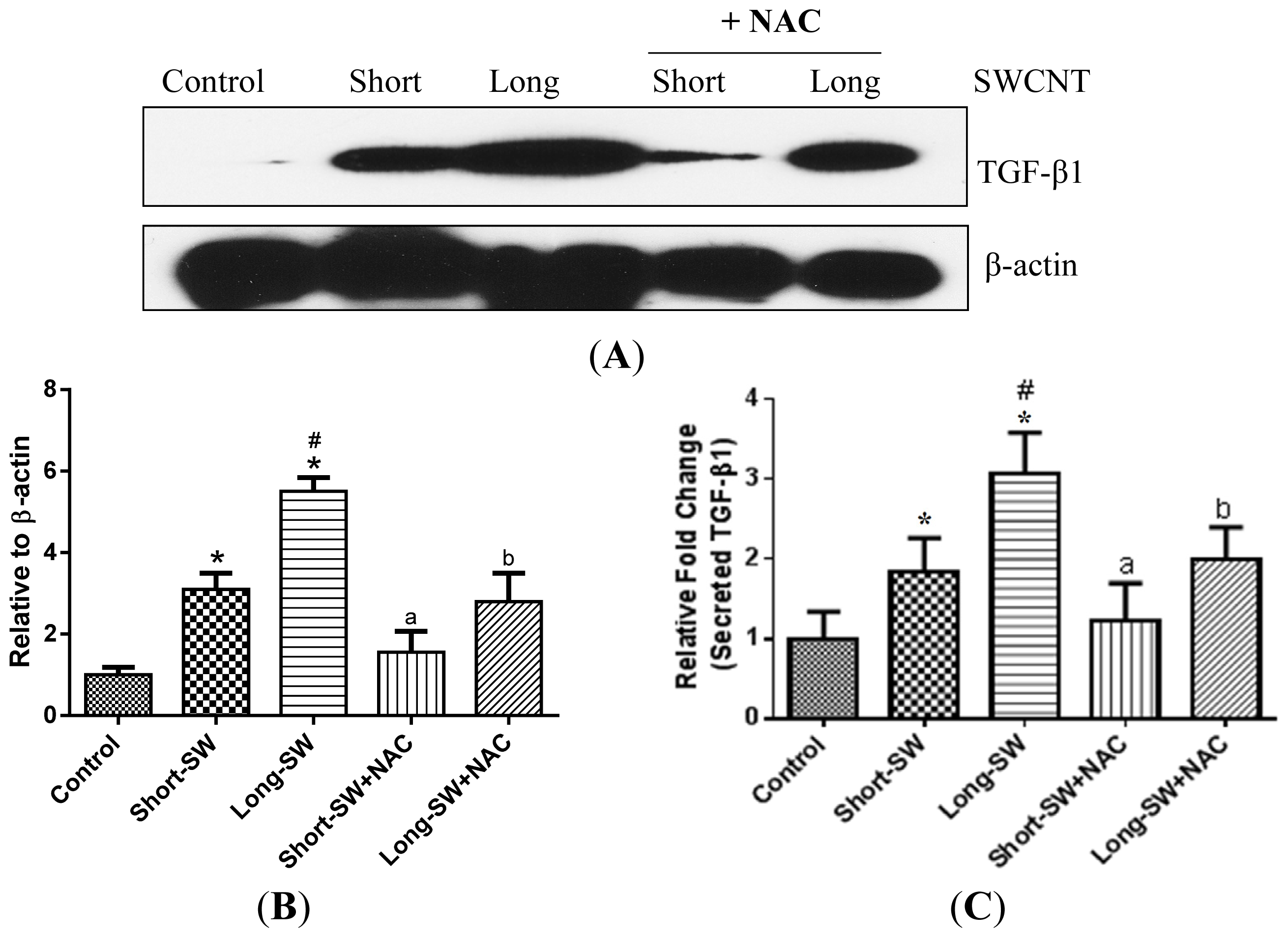
2.4. Effect of SWCNT Length on TGF-β Expression and Secretion
2.5. Role of Reactive Oxygen Species (ROS) in SWCNT-Induced TGF-β Expression
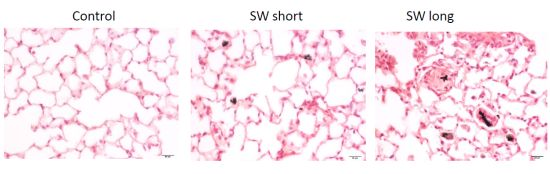
2.6. In Vivo Validation of the Pulmonary Fibrogenic Effect of SWCNTs
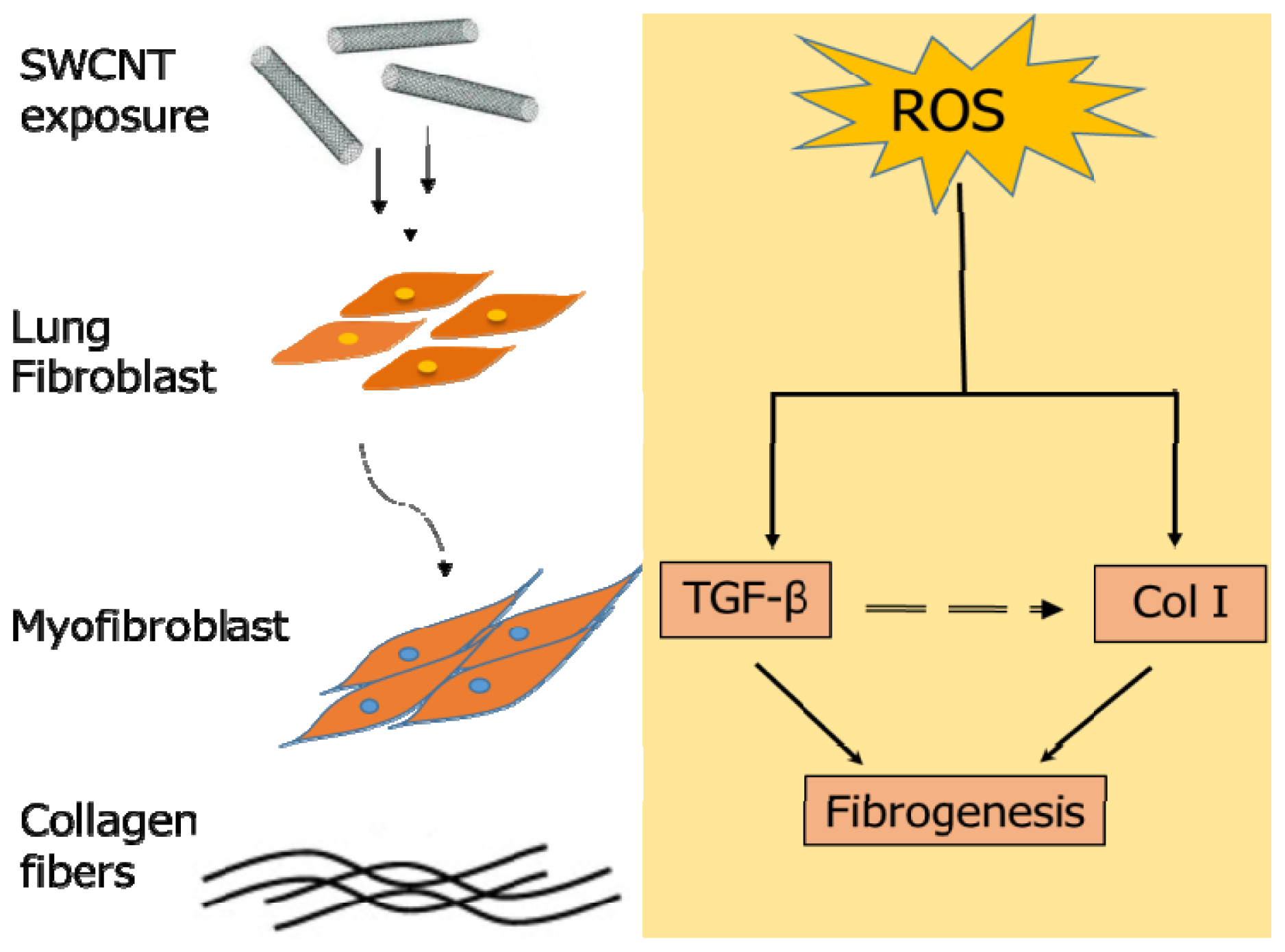
2.7. Discussion
3. Experimental Section
3.1. SWCNT Preparation
3.2. Chemicals and Reagents
3.3. Energy Dispersive X-ray Spectroscopy (EDX-S)
3.4. Atomic Force Microscopy (AFM)
3.5. Cell Culture
3.6. Cytotoxicity Assay
3.7. Sircol® Collagen Assay
3.8. Western Blot Analysis
3.9. DCF Fluorometric Assay for ROS Detection
3.10. TGF-β Enzyme-Linked Immunosorbent Assay (ELISA)
3.11. SWCNT Animal Model
3.12. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsA.M. conceptualized the research design, carried out the in vitro and in vivo experiments, as well as drafted the manuscript. S.L. was involved in the conceptualizing the research design. C.D., H.G., N.W. and C.Z.D. carried out the CNT characterization and elemental analysis. X.H. performed the Sirius red staining. L.W., D.P., To.S. and R.M. participated in the in vivo study design and the evaluation of results. L.B. conducted lung preparation for histopathology. R.D. assisted with preparation of CNT dispersions for in vivo exposure. Y.R. conceived the study and coordinated the project.
- DisclaimersResearch findings and conclusions are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- Donaldson, K.; Aitken, R.; Tran, L.; Stone, V.; Duffin, R.; Forrest, G.; Alexander, A. Carbon nanotubes: A review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol. Sci 2006, 92, 5–22. [Google Scholar]
- Oberdörster, G.; Maynard, A.; Donaldson, K.; Castranova, V.; Fitzpatrick, J.; Ausman, K.; Carter, J.; Karn, B.; Kreyling, W.; Lai, D.; et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Part. Fibre Toxicol 2005, 2, 8. [Google Scholar]
- Lam, C.; James, J.T.; McCluskey, R.; Hunter, R.L. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol. Sci 2004, 77, 126–134. [Google Scholar]
- Kolosnjaj, J.; Szwarc, H.; Moussa, F. Toxicity studies of carbon nanotubes. Adv. Exp. Med. Biol 2007, 620, 181–204. [Google Scholar]
- Jaurand, M.F.; Renier, A.; Daubriac, J. Mesothelioma: Do asbestos and carbon nanotubes pose the same health risk? Part. Fibre Toxicol 2009, 6, 16. [Google Scholar] [Green Version]
- Stella, G.M. Carbon nanotubes and pleural damage: Perspectives of nanosafety in the light of asbestos experience. Biointerphases 2011, 6, P1–P17. [Google Scholar]
- Shvedova, A.A.; Kisin, E.R.; Mercer, R.; Murray, A.R.; Johnson, V.J.; Potapovich, A.I.; Tyurina, Y.Y.; Gorelik, O.; Arepalli, S.; Schwegler-Berry, D.; et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am. J. Physiol. Lung Cell. Mol. Physiol 2005, 289, L698–L708. [Google Scholar]
- Shvedova, A.A.; Kisin, E.; Murray, A.R.; Johnson, V.J.; Gorelik, O.; Arepalli, S.; Hubbs, A.F.; Mercer, R.R.; Keohavong, P.; Sussman, N.; et al. Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: Inflammation, fibrosis, oxidative stress, and mutagenesis. Am. J. Physiol. Lung Cell. Mol. Physiol 2008, 295, L552–L565. [Google Scholar]
- Mercer, R.R.; Scabilloni, J.; Wang, L.; Kisin, E.; Murray, A.R.; Schwegler-Berry, D.; Shvedova, A.A.; Castranova, V. Alteration of deposition pattern and pulmonary response as a result of improved dispersion of aspirated single-walled carbon nanotubes in a mouse model. Am. J. Physiol. Lung Cell. Mol. Physiol 2008, 294, L87–L97. [Google Scholar]
- Park, E.; Roh, J.; Kim, S.; Kang, M.; Han, Y.; Kim, Y.; Hong, J.T.; Choi, K. A single intratracheal instillation of single-walled carbon nanotubes induced early lung fibrosis and subchronic tissue damage in mice. Arch. Toxicol 2011, 85, 1121–1131. [Google Scholar]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.H.; Seaton, A.; Stone, V.; Brown, S.; Macnee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol 2008, 3, 423–428. [Google Scholar]
- Murphy, F.A.; Poland, C.A.; Duffin, R.; Al-Jamal, K.; Ali-Boucetta, H.; Nunes, A.; Byrne, F.; Prina-Mello, A.; Volkov, Y.; Li, S.; et al. Length-dependent retention of carbon nanotubes in the pleural space of mice initiates sustained inflammation and progressive fibrosis on the parietal pleura. Am. J. Pathol 2011, 178, 2587–2600. [Google Scholar]
- Liu, J.; Legros, S.; Ma, G.; Veinot, J.G.C.; von, D.K.; Hofmann, T. Influence of surface functionalization and particle size on the aggregation kinetics of engineered nanoparticles. Chemosphere 2012, 87, 918–924. [Google Scholar]
- Palomäki, J.; Välimäki, E.; Sund, J.; Vippola, M.; Clausen, P.A.; Jensen, K.A.; Savolainen, K.; Matikainen, S.; Alenius, H. Long, needle-like carbon nanotubes and asbestos activate the nlrp3 inflammasome through a similar mechanism. ACS Nano 2011, 5, 6861–6870. [Google Scholar]
- Vietti, G.; Ibouraadaten, S.; Palmai-Pallag, M.; Yakoub, Y.; Bailly, C.; Fenoglio, I.; Marbaix, E.; Lison, D.; van den Brule, S. Towards predicting the lung fibrogenic activity of nanomaterials: Experimental validation of an in vitro fibroblast proliferation assay. Part. Fibre Toxicol 2013, 10, 52. [Google Scholar]
- Wang, L.; Castranova, V.; Mishra, A.; Chen, B.; Mercer, R.R.; Schwegler-Berry, D.; Rojanasakul, Y. Dispersion of single-walled carbon nanotubes by a natural lung surfactant for pulmonary in vitro and in vivo toxicity studies. Part. Fibre Toxicol 2010, 7, 31. [Google Scholar]
- Wang, L.; Mercer, R.R.; Rojanasakul, Y.; Qiu, A.; Lu, Y.; Scabilloni, J.F.; Wu, N.; Castranova, V. Direct fibrogenic effects of dispersed single-walled carbon nanotubes on human lung fibroblasts. J. Toxicol. Environ. Health A 2010, 73, 410–422. [Google Scholar]
- Murray, A.R.; Kisin, E.R.; Tkach, A.V.; Yanamala, N.; Mercer, R.; Young, S.; Fadeel, B.; Kagan, V.E.; Shvedova, A.A. Factoring-in agglomeration of carbon nanotubes and nanofibers for better prediction of their toxicity versus asbestos. Part. Fibre Toxicol 2012, 9, 10. [Google Scholar]
- Leask, A.; Abraham, D.J. TGF-β signaling and the fibrotic response. FASEB J 2004, 18, 816–827. [Google Scholar]
- Rhyu, D.Y.; Yang, Y.; Ha, H.; Lee, G.T.; Song, J.S.; Uh, S.; Lee, H.B. Role of reactive oxygen species in TGF-β1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J. Am. Soc. Nephrol 2005, 16, 667–675. [Google Scholar]
- Porter, D.W.; Hubbs, A.F.; Mercer, R.R.; Wu, N.; Wolfarth, M.G.; Sriram, K.; Leonard, S.; Battelli, L.; Schwegler-Berry, D.; Friend, S.; et al. Mouse pulmonary dose- and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology 2010, 269, 136–147. [Google Scholar]
- Mercer, R.R.; Hubbs, A.F.; Scabilloni, J.F.; Wang, L.; Battelli, L.A.; Friend, S.; Castranova, V.; Porter, D.W. Pulmonary fibrotic response to aspiration of multi-walled carbon nanotubes. Part. Fibre Toxicol 2011, 8, 21. [Google Scholar]
- Shvedova, A.A.; Kapralov, A.A.; Feng, W.H.; Kisin, E.R.; Murray, A.R.; Mercer, R.R.; St Croix, C.M.; Lang, M.A.; Watkins, S.C.; Konduru, N.V.; et al. Impaired clearance and enhanced pulmonary inflammatory/fibrotic response to carbon nanotubes in myeloperoxidase-deficient mice. PLoS One 2012, 7, e30923. [Google Scholar]
- Johnston, H.J.; Hutchison, G.R.; Christensen, F.M.; Peters, S.; Hankin, S.; Aschberger, K.; Stone, V. A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: The contribution of physico-chemical characteristics. Nanotoxicology 2010, 4, 207–246. [Google Scholar]
- Chang, C.; Tsai, M.; Huang, H.; Chen, C.; Dai, S. Epithelial-mesenchymal transition contributes to SWCNT-induced pulmonary fibrosis. Nanotoxicology 2012, 6, 600–610. [Google Scholar]
- Mangum, J.B.; Turpin, E.A.; Antao-Menezes, A.; Cesta, M.F.; Bermudez, E.; Bonner, J.C. Single-walled carbon nanotube (SWCNT)-induced interstitial fibrosis in the lungs of rats is associated with increased levels of PDGF mRNA and the formation of unique intercellular carbon structures that bridge alveolar macrophages in situ. Part. Fibre Toxicol. 2006, 3, 15. [Google Scholar]
- He, X.; Young, S.H.; Fernback, J.E.; Ma, Q. Single-walled carbon nanotubes induce fibrogenic effect by disturbing mitochondrial oxidative stress and activating NF-κb signaling. J. Clin. Toxicol 2012, 2012. [Google Scholar] [CrossRef]
- Sharma, C.S.; Sarkar, S.; Periyakaruppan, A.; Barr, J.; Wise, K.; Thomas, R.; Wilson, B.L.; Ramesh, G.T. Single-walled carbon nanotubes induces oxidative stress in rat lung epithelial cells. J. Nanosci. Nanotechnol 2007, 7, 2466–2472. [Google Scholar]
- Kagan, V.E.; Tyurina, Y.Y.; Tyurin, V.A.; Konduru, N.V.; Potapovich, A.I.; Osipov, A.N.; Kisin, E.R.; Schwegler-Berry, D.; Mercer, R.; Castranova, V.; et al. Direct and indirect effects of single walled carbon nanotubes on RAW 264.7 macrophages: Role of iron. Toxicol. Lett 2006, 165, 88–100. [Google Scholar]
- Pacurari, M.; Yin, X.J.; Zhao, J.; Ding, M.; Leonard, S.S.; Schwegler-Berry, D.; Ducatman, B.S.; Sbarra, D.; Hoover, M.D.; Castranova, V.; et al. Raw single-wall carbon nanotubes induce oxidative stress and activate MAPKs, AP-1, NF-κB, and Akt in normal and malignant human mesothelial cells. Environ. Health Perspect 2008, 116, 1211–1217. [Google Scholar]
- Azad, N.; Iyer, A.K.V.; Wang, L.; Liu, Y.; Lu, Y.; Rojanasakul, Y. Reactive oxygen species-mediated p38 MAPK regulates carbon nanotube-induced fibrogenic and angiogenic responses. Nanotoxicology 2013, 7, 157–168. [Google Scholar]
- Bonner, J.C. Lung fibrotic responses to particle exposure. Toxicol. Pathol 2007, 35, 148–153. [Google Scholar]
- Shvedova, A.A.; Castranova, V.; Kisin, E.R.; Schwegler-Berry, D.; Murray, A.R.; Gandelsman, V.Z.; Maynard, A.; Baron, P. Exposure to carbon nanotube material: Sssessment of nanotube cytotoxicity using human keratinocyte cells. J. Toxicol. Environ. Health A 2003, 66, 1909–1926. [Google Scholar]
- Liu, D.; Wang, L.; Wang, Z.; Cuschieri, A. Different cellular response mechanisms contribute to the length-dependent cytotoxicity of multi-walled carbon nanotubes. Nanoscale Res. Lett 2012, 7, 361–361. [Google Scholar]
- Tabet, L.; Bussy, C.; Amara, N.; Setyan, A.; Grodet, A.; Rossi, M.J.; Pairon, J.C.; Boczkowski, J.; Lanone, S. Adverse effects of industrial multiwalled carbon nanotubes on human pulmonary cells. J. Toxicol. Environ. Health A 2009, 72, 60–73. [Google Scholar]
- Ding, L.; Stilwell, J.; Zhang, T.; Elboudwarej, O.; Jiang, H.; Selegue, J.P.; Cooke, P.A.; Gray, J.W.; Chen, F.F. Molecular characterization of the cytotoxic mechanism of multiwall carbon nanotubes and nano-onions on human skin fibroblast. Nano Lett 2005, 5, 2448–2464. [Google Scholar]
- Thannickal, V.J.; Fanburg, B.L. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J. Biol. Chem 1995, 270, 30334–30338. [Google Scholar]
- Fernandez, I.E.; Eickelberg, O. The impact of TGF-β on lung fibrosis: From targeting to biomarkers. Proc. Am. Thorac. Soc 2012, 9, 111–116. [Google Scholar]
- Wang, P.; Nie, X.; Wang, Y.; Li, Y.; Ge, C.; Zhang, L.; Wang, L.; Bai, R.; Chen, Z.; Zhao, Y.; et al. Multiwall carbon nanotubes mediate macrophage activation and promote pulmonary fibrosis through TGF-β/Smad signaling pathway. Small 2013, 9, 3799–3811. [Google Scholar]
- Yu, Q.; Stamenkovic, I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev 2000, 14, 163–176. [Google Scholar]
- Muller, J.; Huaux, F.; Moreau, N.; Misson, P.; Heilier, J.-F.; Delos, M.; Arras, M.; Fonseca, A.; Nagy, J.B.; Lison, D. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol. Appl. Pharmacol 2005, 207, 221–231. [Google Scholar]
- Mercer, R.R.; Scabilloni, J.F.; Hubbs, A.F.; Battelli, L.A.; McKinney, W.; Friend, S.; Wolfarth, M.G.; Andrew, M.; Castranova, V.; Porter, D.W. Distribution and fibrotic response following inhalation exposure to multi-walled carbon nanotubes. Part. Fibre Toxicol 2013, 10, 33. [Google Scholar]

| SWCNT type | Purity | Length (μm) | Diameter (nm) | |
|---|---|---|---|---|
| Solution form | Dry form | |||
| Long | >90% | 12.31 ± 0.53 | 13.4 ± 0.62 | 11.3 ± 6.20 |
| Short | >90% | 1.13 ± 0.39 | 0.89 ± 0.21 | 10.8 ± 5.41 |
| Element | SWCNT type | |
|---|---|---|
| Short (wt %) | Long (wt %) | |
| C | 92.82 | 90.9 |
| O | 5.77 | 8 |
| Al | 0.06 | 0.01 |
| Si | 0.06 | 0.08 |
| S | 0.11 | 0.1 |
| Cl | 0.3 | 0.2 |
| Ca | 0.1 | 0.12 |
| Cr | 0.16 | 0.31 |
| Fe | 0.13 | 0.12 |
| Co | 0.48 | 0.1 |
| Mg | – | 0.04 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Manke, A.; Luanpitpong, S.; Dong, C.; Wang, L.; He, X.; Battelli, L.; Derk, R.; Stueckle, T.A.; Porter, D.W.; Sager, T.; et al. Effect of Fiber Length on Carbon Nanotube-Induced Fibrogenesis. Int. J. Mol. Sci. 2014, 15, 7444-7461. https://doi.org/10.3390/ijms15057444
Manke A, Luanpitpong S, Dong C, Wang L, He X, Battelli L, Derk R, Stueckle TA, Porter DW, Sager T, et al. Effect of Fiber Length on Carbon Nanotube-Induced Fibrogenesis. International Journal of Molecular Sciences. 2014; 15(5):7444-7461. https://doi.org/10.3390/ijms15057444
Chicago/Turabian StyleManke, Amruta, Sudjit Luanpitpong, Chenbo Dong, Liying Wang, Xiaoqing He, Lori Battelli, Raymond Derk, Todd A. Stueckle, Dale W. Porter, Tina Sager, and et al. 2014. "Effect of Fiber Length on Carbon Nanotube-Induced Fibrogenesis" International Journal of Molecular Sciences 15, no. 5: 7444-7461. https://doi.org/10.3390/ijms15057444