Human Bone Marrow Mesenchymal Stem Cell-Derived Hepatocytes Improve the Mouse Liver after Acute Acetaminophen Intoxication by Preventing Progress of Injury
Abstract
:1. Introduction
2. Results
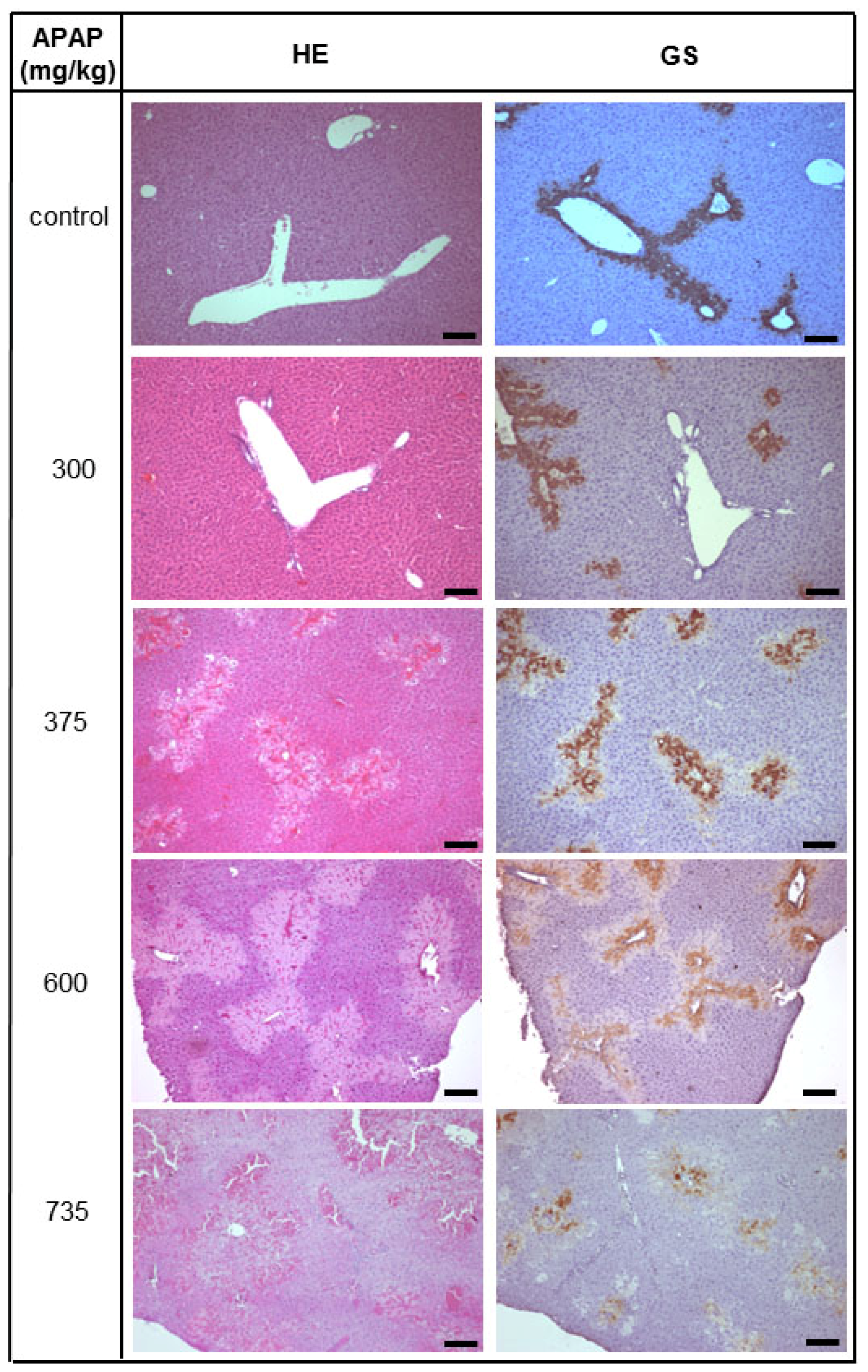
2.1. Acute Liver Injury Induced by APAP in Immunodeficient Pfp/Rag2−/− Mice
2.2. Periportal Localisation of Transplanted hMSC-HC without APAP Treatment
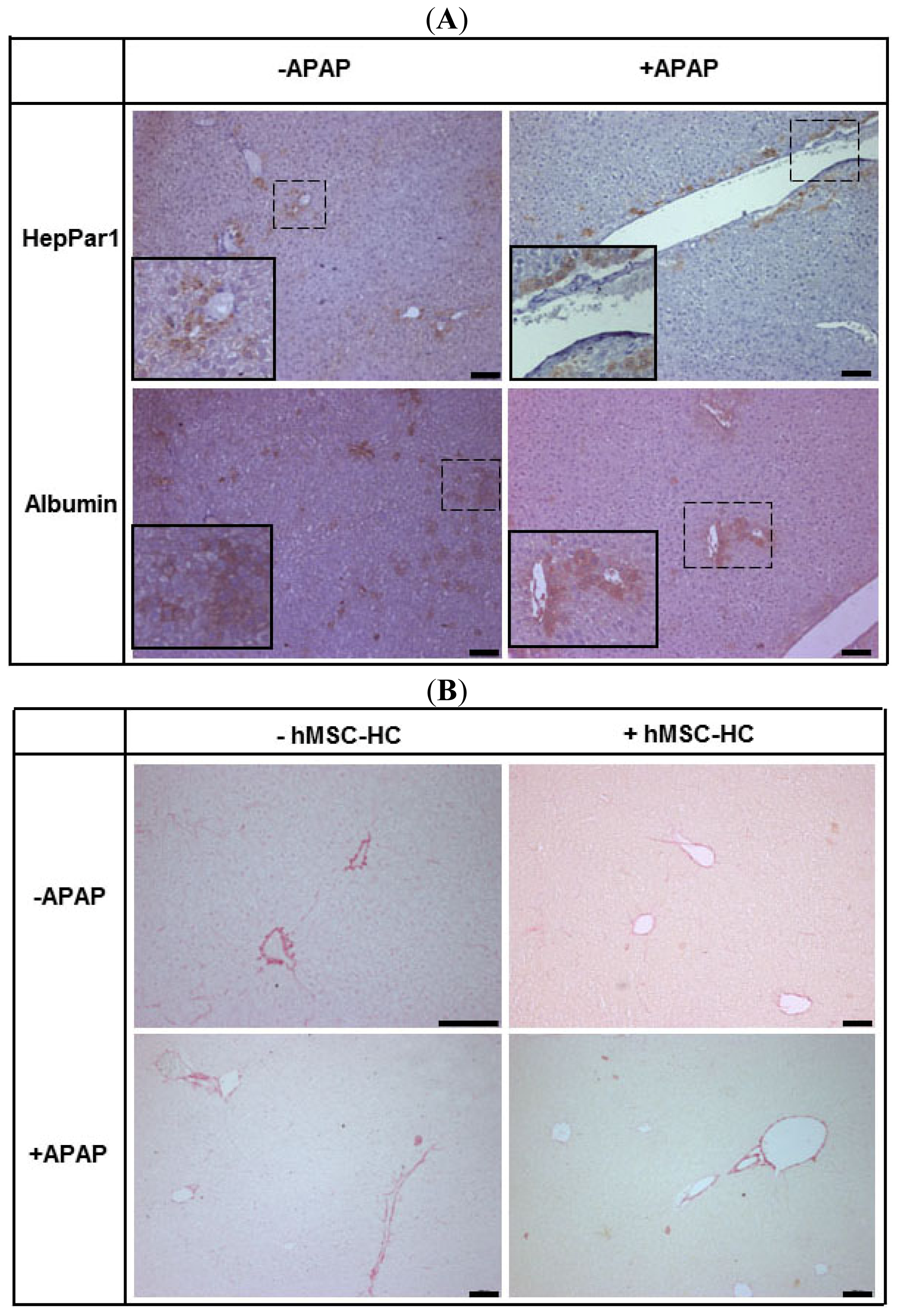
2.3. Tissue Distribution of Transplanted hMSC-HC after APAP Treatment
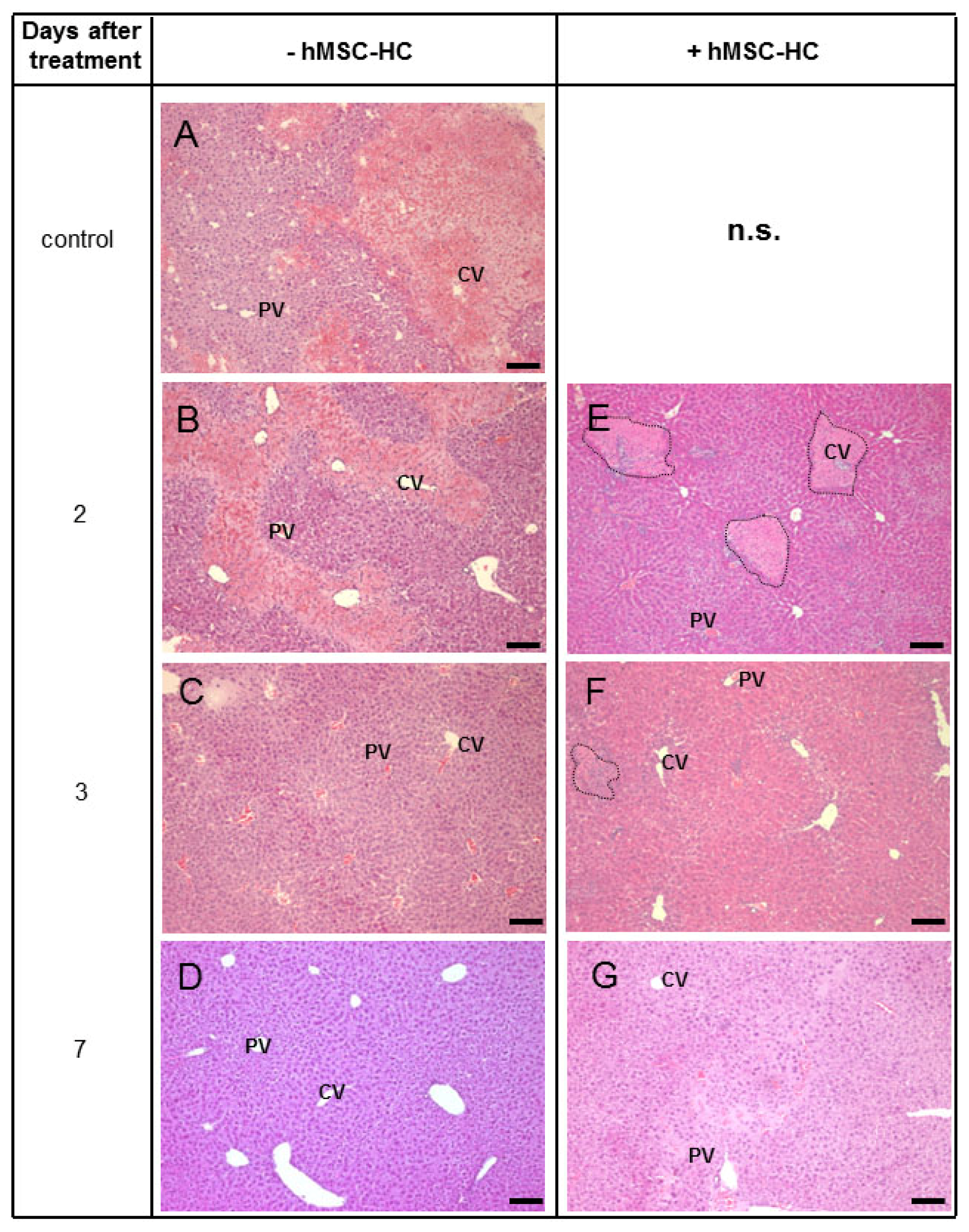
2.4. Perivenous Localisation of Transplanted hMSC-HC after APAP Treatment
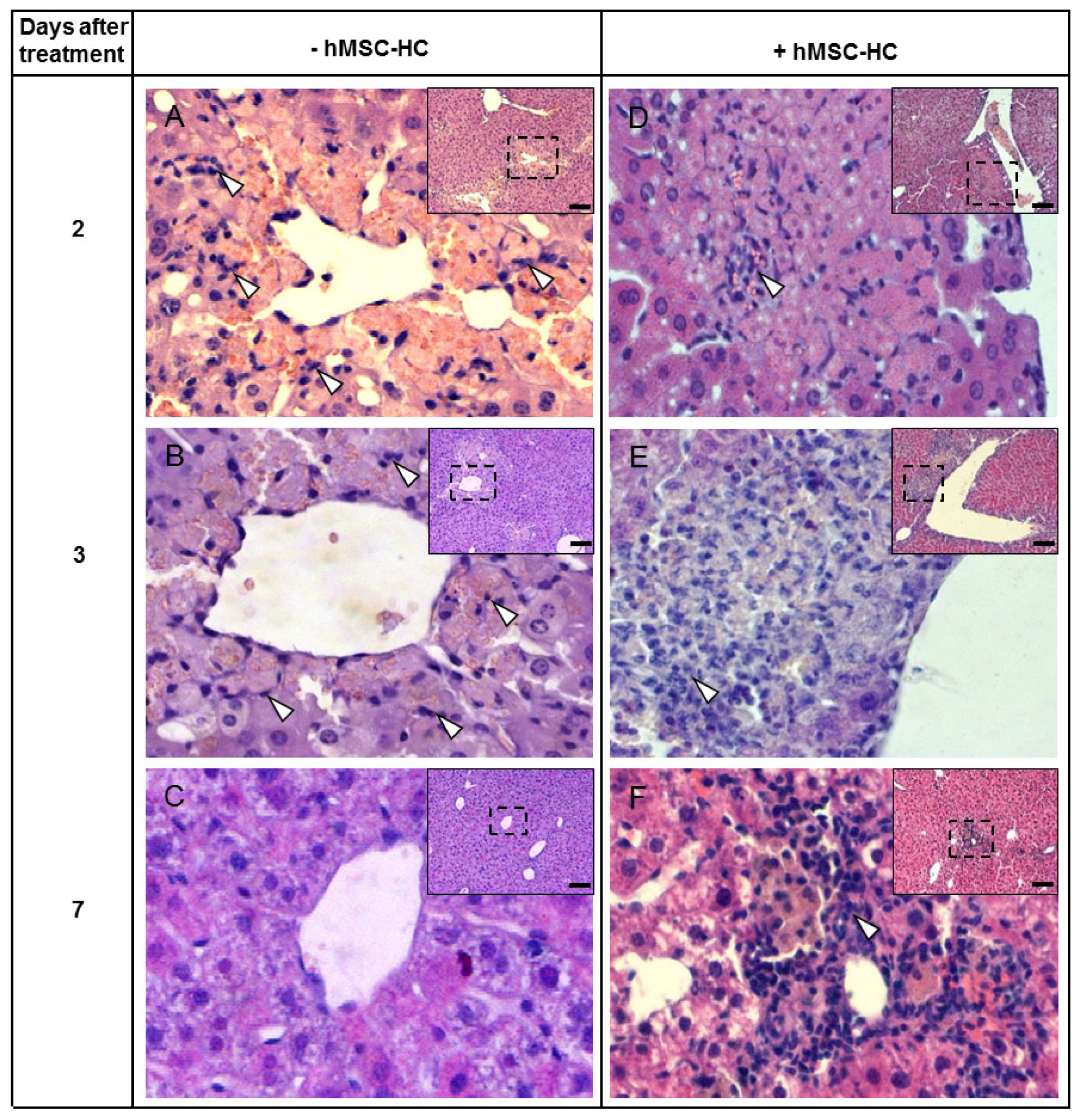
2.5. Attenuation of Hepatocyte Damage and Stimulation of Proliferation by Transplanted hMSC-HC
2.6. Maintenance of Functional Capacity by hMSC-HC after APAP-Induced Liver Injury
2.7. Long-Term Functional Engraftment of hMSC-HC into Mouse Livers after APAP-Induced Injury
2.8. Tissue Re-Arrangement after hMSC-HC Transplantation
3. Discussion
3.1. Histopathologic Impact of hMSC-HC on Liver Regeneration after APAP Injury
3.2. How Do hMSC-HC Ameliorate Liver Damage Caused by APAP?
4. Materials and Methods
4.1. Isolation, Propagation and Hepatocyte Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells (hBM-MSC)
4.2. Acetaminophen Treatment of Immunodeficient Mice and Transplantation of hBM-MSC
4.3. Histological Procedures
4.3.1. HE Staining
4.3.2. TUNEL-Assay
4.3.3. Immunohistochemistry
4.3.4. Sirius Red Staining
4.4. Tools and Assays
4.4.1. Determination of Serum Transaminase Activity
4.4.2. Western-Blotting
4.4.3. Statistics
5. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsPeggy Stock and Sandra Brückner performed animal experiments and biochemical analyses, Sandra Winkler did the artwork, Matthias Dollinger wrote parts of the paper, Bruno Christ was the supervisor of the project and wrote the manuscript.
References
- Alison, M.R.; Vig, P.; Russo, F.; Bigger, B.W.; Amofah, E.; Themis, M.; Forbes, S. Hepatic stem cells: From inside and outside the liver? Cell Prolif 2004, 37, 1–21. [Google Scholar]
- Fausto, N. Liver regeneration and repair: Hepatocytes, progenitor cells, and stem cells. Hepatology 2004, 39, 1477–1487. [Google Scholar]
- Russo, F.P.; Kallis, Y.N.; Alison, M.R.; Forbes, S.J. Bone marrow cells in the liver: Diverse cells, diverse effects. Hepatology 2007, 46, 604–605. [Google Scholar]
- Grompe, M. The role of bone marrow stem cells in liver regeneration. Semin. Liver Dis 2003, 23, 363–372. [Google Scholar]
- Kallis, Y.N.; Alison, M.R.; Forbes, S.J. Bone marrow stem cells and liver disease. Gut 2007, 56, 716–724. [Google Scholar]
- Oertel, M.; Shafritz, D.A. Stem cells, cell transplantation and liver repopulation. Biochim. Biophys. Acta 2008, 1782, 61–74. [Google Scholar]
- Fox, I.J.; Strom, S.C. To be or not to be: Generation of hepatocytes from cells outside the liver. Gastroenterology 2008, 134, 878–881. [Google Scholar]
- Willenbring, H.; Bailey, A.S.; Foster, M.; Akkari, Y.; Dorrell, C.; Olson, S.; Finegold, M.; Fleming, W.H.; Grompe, M. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nat. Med 2004, 10, 744–748. [Google Scholar]
- Wang, X.; Willenbring, H.; Akkari, Y.; Torimaru, Y.; Foster, M.; Al-Dhalimy, M.; Lagasse, E.; Finegold, M.; Olson, S.; Grompe, M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 2003, 422, 897–901. [Google Scholar]
- Bunnell, B.A.; Deng, W.; Robinson, C.M.; Waldron, P.R.; Bivalacqua, T.J.; Baber, S.R.; Hyman, A.L.; Kadowitz, P.J. Potential application for mesenchymal stem cells in the treatment of cardiovascular diseases. Can. J. Physiol. Pharmacol 2005, 83, 529–539. [Google Scholar]
- Christopeit, M.; Schendel, M.; Foll, J.; Muller, L.P.; Keysser, G.; Behre, G. Marked improvement of severe progressive systemic sclerosis after transplantation of mesenchymal stem cells from an allogeneic haploidentical-related donor mediated by ligation of CD137L. Leukemia 2008, 22, 1062–1064. [Google Scholar]
- Ohnishi, S.; Nagaya, N. Prepare cells to repair the heart: Mesenchymal stem cells for the treatment of heart failure. Am. J. Nephrol 2007, 27, 301–307. [Google Scholar]
- Ringdén, O.; Uzunel, M.; Rasmusson, I.; Remberger, M.; Sundberg, B.; Lönnies, H.; Marschall, H.-U.; Dlugosz, A.; Szakos, A.; Hassan, Z.; et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation 2006, 81, 1390–1397. [Google Scholar]
- Christ, B.; Dollinger, M.M. The generation of hepatocytes from mesenchymal stem cells and engraftment into the liver. Curr. Opin. Org. Transplant 2011, 16, 69–75. [Google Scholar]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar]
- Krampera, M.; Pasini, A.; Pizzolo, G.; Cosmi, L.; Romagnani, S.; Annunziato, F. Regenerative and immunomodulatory potential of mesenchymal stem cells. Curr. Opin. Pharmacol 2006, 6, 435–441. [Google Scholar]
- Le Blanc, K.; Ringden, O. Immunomodulation by mesenchymal stem cells and clinical experience. J. Intern. Med 2007, 262, 509–525. [Google Scholar]
- Lysy, P.A.; Campard, D.; Smets, F.; Malaise, J.; Mourad, M.; Najimi, M.; Sokal, E.M. Persistence of a chimerical phenotype after hepatocyte differentiation of human bone marrow mesenchymal stem cells. Cell Prolif 2008, 41, 36–58. [Google Scholar]
- Lee, K.D.; Kuo, T.K.; Whang-Peng, J.; Chung, Y.F.; Lin, C.T.; Chou, S.H.; Chen, J.R.; Chen, Y.P.; Lee, O.K. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology 2004, 40, 1275–1284. [Google Scholar]
- Aurich, I.; Mueller, L.P.; Aurich, H.; Luetzkendorf, J.; Tisljar, K.; Dollinger, M.M.; Schormann, W.; Walldorf, J.; Hengstler, J.G.; Fleig, W.E.; et al. Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers. Gut 2007, 56, 405–415. [Google Scholar]
- Oyagi, S.; Hirose, M.; Kojima, M.; Okuyama, M.; Kawase, M.; Nakamura, T.; Ohgushi, H.; Yagi, K. Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J. Hepatol 2006, 44, 742–748. [Google Scholar]
- Banas, A.; Teratani, T.; Yamamoto, Y.; Tokuhara, M.; Takeshita, F.; Quinn, G.; Okochi, H.; Ochiya, T. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology 2007, 46, 219–228. [Google Scholar]
- Kuo, T.K.; Hung, S.-P.; Chuang, C.-H.; Chen, C.-T.; Shih, Y.-R.V.; Fang, S.-C.Y.; Yang, V.W.; Lee, O.K. Stem cell therapy for liver disease: Parameters Governing the success of using bone marrow mesenchymal stem cells. Gastroenterology 2008, 134, 2111–2121. [Google Scholar]
- Banas, A.; Teratani, T.; Yamamoto, Y.; Tokuhara, M.; Takeshita, F.; Osaki, M.; Kawamata, M.; Kato, T.; Okochi, H.; Ochiya, T. IFATS series: In vivo therapeutic potential of human adipose tissue mesenchymal stem cells (at-mscs) after transplantation into mice with liver injury. Stem Cells 2008, 26, 2705–2712. [Google Scholar]
- Parekkadan, B.; van Poll, D.; Suganuma, K.; Carter, E.A.; Berthiaume, F.; Tilles, A.W.; Yarmush, M.L. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One 2007, 2, e941. [Google Scholar]
- Van Poll, D.; Parekkadan, B.; Cho, C.H.; Berthiaume, F.; Nahmias, Y.; Tilles, A.W.; Yarmush, M.L. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology 2008, 47, 1634–1643. [Google Scholar]
- Sgodda, M.; Aurich, H.; Kleist, S.; Aurich, I.; Konig, S.; Dollinger, M.M.; Fleig, W.E.; Christ, B. Hepatocyte differentiation of mesenchymal stem cells from rat peritoneal adipose tissue in vitro and in vivo. Exp. Cell Res. 2007, 313, 2875–2886. [Google Scholar]
- Stock, P.; Staege, M.S.; Müller, L.P.; Sgodda, M.; Völker, A.; Volkmer, I.; Lützkendorf, J.; Christ, B. Hepatocytes derived from adult stem cells. Transplant. Proc 2008, 40, 620–623. [Google Scholar]
- Christ, B.; Pelz, S. Implication of hepatic stem cells in functional liver repopulation. Cytometry A 2013, 8, 90–102. [Google Scholar]
- Hughes, R.D.; Mitry, R.R.; Dhawan, A. Current status of hepatocyte transplantation. Transplantation 2012, 93, 342–347. [Google Scholar]
- O’Grady, J.G. Acute liver failure. Postgrad. Med. J 2005, 81, 148–154. [Google Scholar]
- Lee, W.M. Acetaminophen and the U.S. acute liver failure study group: Lowering the risks of hepatic failure. Hepatology 2004, 40, 6–9. [Google Scholar]
- James, L.P.; Mayeux, P.R.; Hinson, J.A. Acetaminphen-induced hepatotoxicity. Drug Metab. Dispos 2003, 31, 1499–1506. [Google Scholar]
- Jaeschke, H.; Bajt, M.L. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol. Sci 2006, 89, 31–41. [Google Scholar]
- Mehendale, H.M. Tissue repair: An important determinant of final outcome of toxicant-induced injury. Toxicol. Pathol 2005, 33, 41–51. [Google Scholar]
- Lee, V.M.; Cameron, R.G.; Archer, M.C. Zonal location of compensatory hepatocyte proliferation following chemically induced hepatotoxicity in rats and humans. Toxicol. Pathol 1998, 26, 621–627. [Google Scholar]
- Kofman, A.V.; Morgan, G.; Kirschenbaum, A.; Osbeck, J.; Hussain, M.; Swenson, S.; Theise, N.D. Dose- and time-dependent oval cell reaction in acetaminophen-induced murine liver injury. Hepatology 2005, 41, 1252–1261. [Google Scholar]
- Fisher, R.A.; Strom, S.C. Human hepatocyte transplantation. Transplantation 2006, 82, 441–449. [Google Scholar]
- Smets, F.; Najimi, M.; Sokal, E.M. Cell transplantation in the treatment of liver diseases. Pediatr. Transplant 2008, 12, 6–13. [Google Scholar]
- Francois, S.; Mouiseddine, M.; Allenet-Lepage, B.; Voswinkel, J.; Douay, L.; Benderitter, M.; Chapel, A. Human mesenchymal stem cells provide protection against radiation-induced liver injury by antioxidative process, vasculature protection, hepatocyte differentiation, and trophic effects. BioMed Res. Int 2013, 2013, 151679. [Google Scholar]
- Stock, P.; Brueckner, S.; Ebensing, S.; Hempel, M.; Dollinger, M.M.; Christ, B. The generation of hepatocytes from mesenchymal stem cells and engraftment into murine liver. Nat. Protoc 2010, 5, 617–627. [Google Scholar]
- Kallis, Y.N.; Forbes, S.J. The bone marrow and liver fibrosis: Friend or foe? Gastroenterology 2009, 137, 1218–1221. [Google Scholar]
- Mitchell, J.R.; Jollow, D.J.; Potter, W.Z.; Gillette, J.R.; Brodie, B.B. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J. Pharmacol. Exp. Therap 1973, 187, 211–217. [Google Scholar]
- Jollow, D.J.; Thorgeirsson, S.S.; Potter, W.Z.; Hashimoto, M.; Mitchell, J.R. Acetaminophen-induced hepatic necrosis. VI. Metabolic disposition of toxic and nontoxic doses of acetaminophen. Pharmacology 1974, 12, 251–271. [Google Scholar]
- Jaeschke, H.; Gores, G.J.; Cederbaum, A.I.; Hinson, J.A.; Pessayre, D.; Lemasters, J.J. Mechanisms of hepatotoxicity. Toxicol. Sci 2002, 65, 166–176. [Google Scholar]
- Doi, K.; Ishida, K. Diabetes and hypertriglyceridemia modify the mode of acetaminophen-induced hepatotoxicity and nephrotoxicity in rats and mice. J. Toxicol. Sci 2009, 34, 1–11. [Google Scholar]
- Christ, B.; Brückner, S. Rodent animal models for surrogate analysis of cell therapy in acute liver failure. Front. Physiol 2012, 3, 78. [Google Scholar]
- Ni, H.-M.; Williams, J.A.; Jaeschke, H.; Ding, W.-X. Zonated induction of autophagy and mitochondrial spheroids limits acetaminophen-induced necrosis in the liver. Redox Biol 2013, 1, 427–432. [Google Scholar]
- Blazka, M.E.; Wilmer, J.L.; Holladay, S.D.; Wilson, R.E.; Luster, M.I. Role of proinflammatory cytokines in acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol 1995, 133, 43–52. [Google Scholar]
- Bourdi, M.; Masubuchi, Y.; Reilly, T.P.; Amouzadeh, H.R.; Martin, J.L.; George, J.W.; Shah, A.G.; Pohl, L.R. Protection against acetaminophen-induced liver injury and lethality by interleukin 10: Role of inducible nitric oxide synthase. Hepatology 2002, 35, 289–298. [Google Scholar]
- Gardner, C.R.; Laskin, J.D.; Dambach, D.M.; Chiu, H.; Durham, S.K.; Zhou, P.; Bruno, M.; Gerecke, D.R.; Gordon, M.K.; Laskin, D.L. Exaggerated hepatotoxicity of acetaminophen in mice lacking tumor necrosis factor receptor-1: Potential role of inflammatory mediators. Toxicol. Appl. Pharmacol 2003, 192, 119–130. [Google Scholar]
- Götherström, C. Immunomodulation by multipotent mesenchymal stromal cells. Transplantation 2007, 84, S35–S37. [Google Scholar]
- Aurich, H.; Sgodda, M.; Kaltwasser, P.; Vetter, M.; Weise, A.; Liehr, T.; Brulport, M.; Hengstler, J.G.; Dollinger, M.M.; Fleig, W.E.; et al. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut 2009, 58, 570–581. [Google Scholar]
- Xie, J.; Wang, W.; Si, J.-W.; Miao, X.-Y.; Li, J.-C.; Wang, Y.-C.; Wang, Z.-R.; Ma, J.; Zhao, X.-C.; Li, Z.; et al. Notch signaling regulates CXCR4 expression and the migration of mesenchymal stem cells. Cell. Immunol 2013, 281, 68–75. [Google Scholar]
- Huang, C.-K.; Lee, S.O.; Lai, K.-P.; Ma, W.-L.; Lin, T.-H.; Tsai, M.-Y.; Luo, J.; Chang, C. Targeting androgen receptor in bone marrow mesenchymal stem cells leads to better transplantation therapy efficacy in liver cirrhosis. Hepatology 2013, 57, 1550–1563. [Google Scholar]
- Zagoura, D.S.; Roubelakis, M.G.; Bitsika, V.; Trohatou, O.; Pappa, K.I.; Kapelouzou, A.; Antsaklis, A.; Anagnou, N.P. Therapeutic potential of a distinct population of human amniotic fluid mesenchymal stem cells and their secreted molecules in mice with acute hepatic failure. Gut 2012, 61, 894–906. [Google Scholar]
- Salomone, F.; Barbagallo, I.; Puzzo, L.; Piazza, C.; Li Volti, G. Efficacy of adipose tissue-mesenchymal stem cell transplantation in rats with acetaminophen liver injury. Stem Cell Res 2013, 11, 1037–1044. [Google Scholar]
- Koenig, S.; Aurich, H.; Schneider, C.; Krause, P.; Haftendorn, R.; Becker, H.; Christ, B. Zonal expression of hepatocytic marker enzymes during liver repopulation. Histochem. Cell Biol 2007, 128, 105–114. [Google Scholar]
- Aurich, H.; Koenig, S.; Schneider, C.; Walldorf, J.; Krause, P.; Fleig, W.E.; Christ, B. Functional characterization of serum-free cultured rat hepatocytes for downstream transplantation applications. Cell Transplant 2005, 14, 497–506. [Google Scholar]
- Butler, S.L.; Dong, H.; Cardona, D.; Jia, M.; Zheng, R.; Zhu, H.; Crawford, J.M.; Liu, C. The antigen for Hep Par 1 antibody is the urea cycle enzyme carbamoyl phosphate synthetase 1. Lab. Investig 2007, 88, 78–88. [Google Scholar]
- Sato, Y.; Araki, H.; Kato, J.; Nakamura, K.; Kawano, Y.; Kobune, M.; Sato, T.; Miyanishi, K.; Takayama, T.; Takahashi, M.; et al. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood 2005, 106, 756–763. [Google Scholar]
- Seo, M.J.; Suh, S.Y.; Bae, Y.C.; Jung, J.S. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem. Biophys. Res. Commun. 2005, 328, 258–264. [Google Scholar]
- Yukawa, H.; Noguchi, H.; Oishi, K.; Takagi, S.; Hamaguchi, M.; Hamajima, N.; Hayashi, S. Cell transplantation of adipose tissue-derived stem cells in combination with heparin attenuated acute liver failure in mice. Cell Transplant 2009, 18, 611–618. [Google Scholar]
- Yagi, H.; Soto-Gutierrez, A.; Kitagawa, Y.; Tilles, A.W.; Tompkins, R.G.; Yarmush, M.L. Bone marrow mesenchymal stromal cells attenuate organ injury induced by lps and burn. Cell Transplant 2010, 19, 823–830. [Google Scholar]
- Chen, Y.; Xiang, L.-X.; Shao, J.-Z.; Pan, R.-L.; Wang, Y.-X.; Dong, X.-J.; Zhang, G.-R. Recruitment of endogenous bone marrow mesenchymal stem cells towards injured liver. J. Cell. Mol. Med 2010, 14, 1494–1508. [Google Scholar]
- Jin, S.Z.; Meng, X.W.; Han, M.Z.; Sun, X.; Sun, L.Y.; Liu, B.R. Stromal cell derived factor-1 enhances bone marrow mononuclear cell migration in mice with acute liver failule. World J. Gastroenterol 2009, 15, 2657–2664. [Google Scholar]
- Collino, F.; Deregibus, M.C.; Bruno, S.; Sterpone, L.; Aghemo, G.; Viltono, L.; Tetta, C.; Camussi, G. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of mirnas. PLoS One 2010, 5, e11803. [Google Scholar]
- Deregibus, M.C.; Tetta, C.; Camussi, G. The dynamic stem cell microenvironment is orchestrated by microvesicle-mediated transfer of genetic information. Histol. Histopathol 2010, 25, 397–404. [Google Scholar]
- Williams, C.D.; Bajt, M.L.; Sharpe, M.R.; McGill, M.R.; Farhood, A.; Jaeschke, H. Neutrophil activation during acetaminophen hepatotoxicity and repair in mice and humans. Toxicol. Appl. Pharmacol 2014, 275, 122–133. [Google Scholar]
- Liu, Z.-X.; Kaplowitz, N. Role of innate immunity in acetaminophen-induced hepatotoxicity. Expert Opin. Drug Metab. Toxicol 2006, 2, 493–503. [Google Scholar]
- Jaeschke, H.; Williams, C.D.; Ramachandran, A.; Bajt, M.L. Acetaminophen hepatotoxicity and repair: The role of sterile inflammation and innate immunity. Liver Int 2012, 32, 8–20. [Google Scholar]
- Walldorf, J.; Hillebrand, C.; Aurich, H.; Stock, P.; Hempel, M.; Ebensing, S.; Fleig, W.E.; Seufferlein, T.; Dollinger, M.M.; Christ, B. Propranolol impairs liver regeneration after partial hepatectomy in C57Bl/6-mice by transient attenuation of hepatic lipid accumulation and increased apoptosis. Scand. J. Gastroenterol 2010, 45, 468–476. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 1976, 72, 248–254. [Google Scholar]







| Days after treatment | AST activity [μkat/L] | |||
|---|---|---|---|---|
| PBS | PBS + hMSC-HC | APAP | APAP + hMSC-HC | |
| 1 | 1.07 | n.d. | 141.92 ± 19.46 | n.d. |
| 2 | 8.96 ± 4.09 | 11.12 ± 4.64 | 18.46 ± 2.07 | 80.80 ± 13.49 |
| 3 | 1.63 | 4.32 ± 0.29 | 2.42 | 8.09 ± 2.95 |
| 7 | 1.54 | 1.05 ± 0.12 | 0.90 | 1.34 ± 0.07 |
| Antigen | Primary antibody | Secondary antibody | ||||
|---|---|---|---|---|---|---|
| Company | Species | Dilution | Company | Species | Dilution | |
| PCNA | Genetex (Aachen, Germany) | mouse-anti mouse | 1:250 | Vector Laboratories (Burlingame, CA, USA) | Biotin-conjugated horse anti-mouse IgG | 1:200 |
| HepPar1 | Dako (Hamburg, Germany) | Monoclonal mouse anti-human hepatocyte clone OCH1E5 | 1:50 | BD (Heidelberg, Germany) | HRP-conjugated goat anti-mouse IgG | 1:200 |
| Albumin | Abcam (Cambridge, UK) | polyclonal rabbit anti-human | 1:1000 | Dianova (Hamburg, Germany) | biotin-conjugated donkey anti-rabbit | 1:200 |
| Glutamine synthetase | BD (Heidelberg, Germany) | monoclonal mouse anti-mouse, human | 1:1000 | Dianova (Hamburg, Germany) | HRP-conjugated donkey anti-mouse | 1:200 |
| Antigen | Primary antibody | Secondary antibody | ||||
|---|---|---|---|---|---|---|
| Company | Species | Dilution | Company | Species | Dilution | |
| Glutamine synthetase | BD (Heidelberg, Germany) | mouse anti-human, rat, mouse | 1:6000 | BD (Heidelberg, Germany) | HRP-conjugated anti-mouse | 1:8000 |
| CYP1A1 | Natutec (Frankfurt, Germany) | goat anti-rat, human | 1:2000 | Dianova (Hamburg, Germany) | HRP-conjugated anti-goat | 1:9000 |
| CYP2B | Natutec (Frankfurt, Germany) | goat anti-rat | 1:2000 | Dianova (Hamburg, Germany) | HRP-conjugated anti-goat | 1:9000 |
| PCK | Aviva Systems Biology (San Diego, CA, USA) | rabbit anti-human, mouse, rat, dog | 1:700 | BD (Heidelberg, Germany) | HRP-conjugated anti-rabbit | 1:7500 |
| Ferritin | Self-made | rabbit anti-human | 1:3000 | BD (Heidelberg, Germany) | HRP-conjugated anti-rabbit | 1:7500 |
| Albumin | Dunn Labortechnik (Asbach, Germany) | goat anti-human | 1:450 | Dianova (Hamburg, Germany) | HRP-conjugated anti-goat | 1:9000 |
| β-Actin | Merck Millipore (Darmstadt, Germany) | mouse anti-human, rat, mouse | 1:5000 | BD (Heidelberg, Germany) | HRP-conjugated anti-mouse | 1:10,000 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Stock, P.; Brückner, S.; Winkler, S.; Dollinger, M.M.; Christ, B. Human Bone Marrow Mesenchymal Stem Cell-Derived Hepatocytes Improve the Mouse Liver after Acute Acetaminophen Intoxication by Preventing Progress of Injury. Int. J. Mol. Sci. 2014, 15, 7004-7028. https://doi.org/10.3390/ijms15047004
Stock P, Brückner S, Winkler S, Dollinger MM, Christ B. Human Bone Marrow Mesenchymal Stem Cell-Derived Hepatocytes Improve the Mouse Liver after Acute Acetaminophen Intoxication by Preventing Progress of Injury. International Journal of Molecular Sciences. 2014; 15(4):7004-7028. https://doi.org/10.3390/ijms15047004
Chicago/Turabian StyleStock, Peggy, Sandra Brückner, Sandra Winkler, Matthias M. Dollinger, and Bruno Christ. 2014. "Human Bone Marrow Mesenchymal Stem Cell-Derived Hepatocytes Improve the Mouse Liver after Acute Acetaminophen Intoxication by Preventing Progress of Injury" International Journal of Molecular Sciences 15, no. 4: 7004-7028. https://doi.org/10.3390/ijms15047004
APA StyleStock, P., Brückner, S., Winkler, S., Dollinger, M. M., & Christ, B. (2014). Human Bone Marrow Mesenchymal Stem Cell-Derived Hepatocytes Improve the Mouse Liver after Acute Acetaminophen Intoxication by Preventing Progress of Injury. International Journal of Molecular Sciences, 15(4), 7004-7028. https://doi.org/10.3390/ijms15047004







