Synthesis and Anti-Breast Cancer Evaluation of Novel N-(Guanidinyl)benzenesulfonamides
Abstract
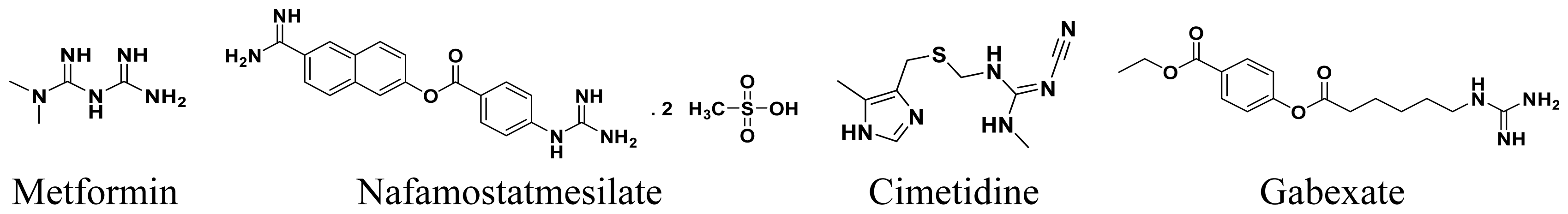
:1. Introduction
2. Results and Discussion
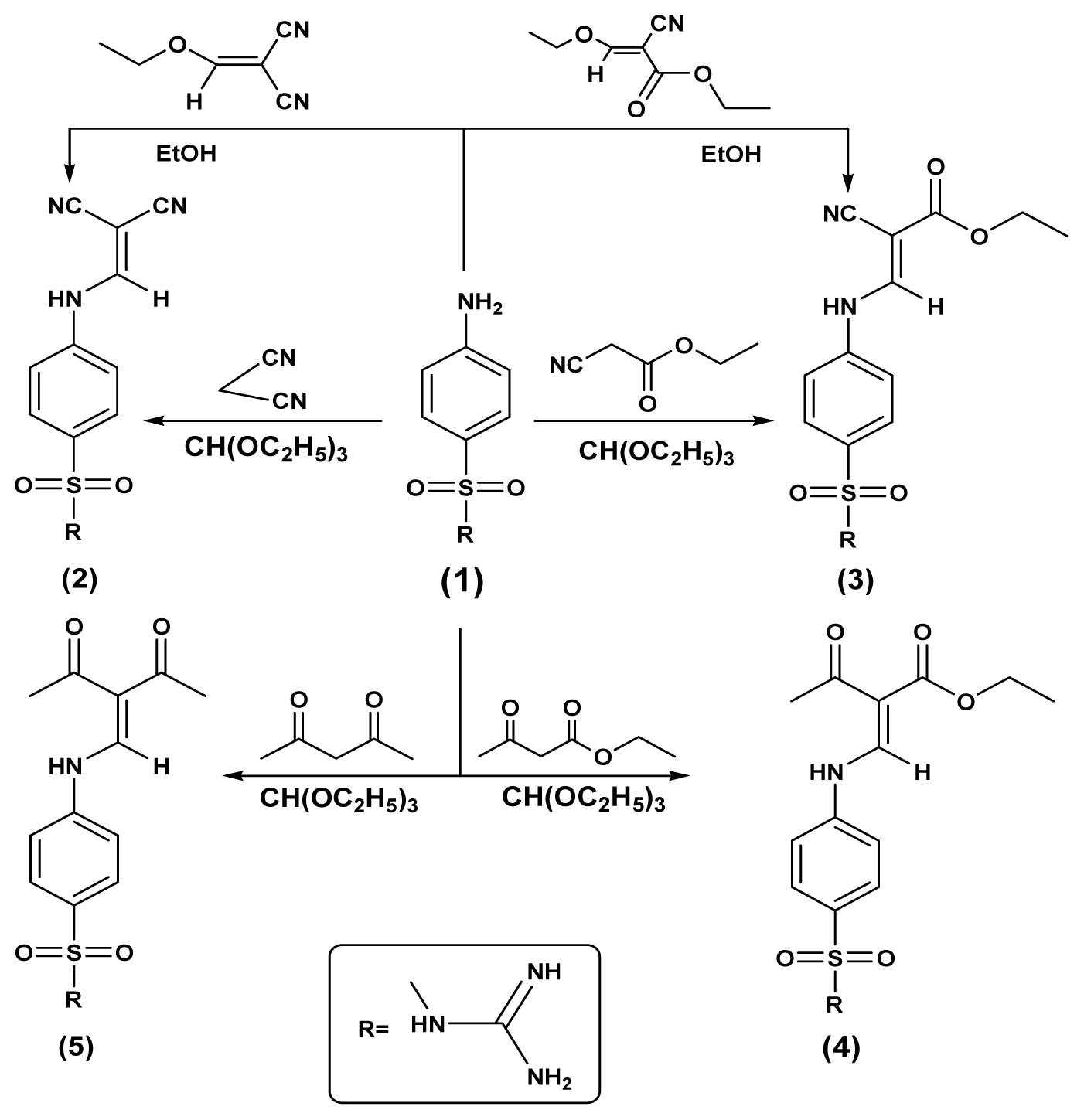
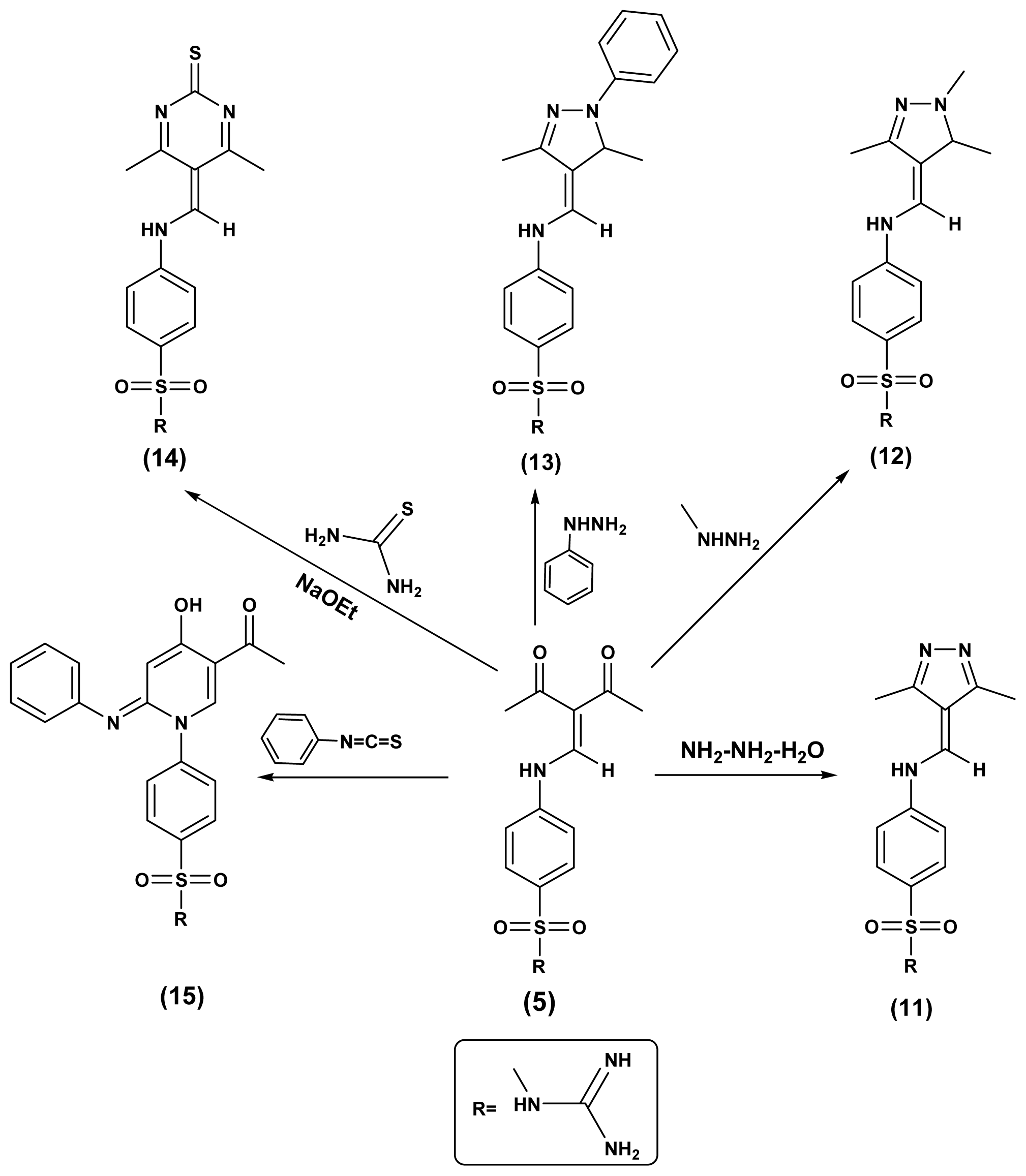
2.1. Chemistry
2.2. In-Vitro Anticancer Screening
3. Experimental Section
3.1. Chemistry
3.1.1. N-Carbamimidoyl-4-(2,2-cyanovinylamino)benzenesulfonamide (2)
3.1.2. (E)-Ethyl-3-(4-(N-carbamimidoylsulfamoyl)phenylamino)-2-cyanoacrylate (3)
3.1.3. (E)-Ethyl-2-((4-(N-carbamimidoylsulfamoyl)phenylamino)methylene)-3-oxobutanoate (4)
3.1.4. 4-(2-Acetyl-3-oxobut-1-enylamino)-N-carbamimidoylbenzenesulfonamide (5)
3.1.5. N-Carbamimidoyl-4-((3,5-diamino-4H-pyrazol-4-ylidene)methylamino)benzensulfonamide (6)
3.1.6. 4-((3-Amino-5-imino-1-methyl-1H-pyrazol-4-(5H)-ylidene)methylamino)-N-carbamimidoyl-benzenesulfonamide (7)
3.1.7. N-Carbamimidoyl-4-(5-cyano-4-oxo-3-phenyl-2-thioxo-3,4-dihydropyrimidin-1(2H)-yl)-benzenesulfonamide (8)
3.1.8. 4-(4-Amino-3-chloro-5-cyano-2-oxopyridin-1(2H)-yl)-N-carbamimidoylbenzenesulfonamide (9)
3.1.9. N-Carbamimidoyl-4-((4,6-diamino-2-thioxopyrimidin-5(2H)-ylidene)methylamino)-benzenesul-fonamide (10)
3.1.10. N-Carbamimidoyl-4-((3,5-dimethyl-4H-pyrazol-4-ylidene)methylamino)benzensulfonamide (11)
3.1.11. N-Carbamimidoyl-4-((1,3,5-trimethyl-1H-pyrazol-4-yl)methyleneamino)benzenesulfonamide (12)
3.1.12. N-Carbamimidoyl-4-((3,5-dimethyl-1-phenyl-1H-pyrazol-4-yl)methyleneamino)-benzenesulf-onamide (13)
3.1.13. N-Carbamimidoyl-4-((4,6-dimethyl-2-thioxopyrimidin-5(2H)-ylidene)methylamino) benzene-sulfonamide (14)
3.1.14. (E)-4-(5-Acetyl-4-hydroxy-2-(phenylimino)pyridine-1(2H)-yl)-N-cabamimidoylbenzenesul-fonamide (15)
3.2. In-Vitro Anticancer Screening
4. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsM.M.G. suggested the research idea and contributed in the experimental work. M.G.E.-G. contributed in the experimental work and in writing the paper. M.S.A. contributed in the experimental work and the biological activity.
References
- Hooley, R.J.; Scoutt, L.M.; Philpotts, L.E. Breast ultrasonography: State of the art. Radiology 2013, 268, 642–659. [Google Scholar]
- Gizzo, S.; Saccardi, C.; Patrelli, T.S.; Berretta, R.; Capobianco, G.; di Gangi, S.; Vacilotto, A.; Bertocco, A.; Noventa, M.; Ancona, E.; et al. Update on raloxifene: Mechanism of action, clinical efficacy, adverse effects, and contraindications. Obstet. Gynecol. Surv 2013, 68, 467–481. [Google Scholar]
- Piva, R.; Spandidos, D.A.; Gambari, R. From microRNA functions to microRNA therapeutics: Novel targets and novel drugs in breast cancer research and treatment. Int. J. Oncol 2013, 43, 985–994. [Google Scholar]
- Jackson, C.; Browell, D.; Gautrey, H.; Tyson-Capper, A. Clinical significance of HER-2 splice variants in breast cancer progression and drug resistance. Int. J. Cell Biol 2013, 2013, 973584:1–973584:8. [Google Scholar]
- Reimers, L.; Crew, K.D. Tamoxifenvs raloxifenevs exemestane for chemoprevention. Curr. Breast Cancer Rep 2012, 4, 207–215. [Google Scholar]
- Silber, J.H.; Rosenbaum, P.R.; Clark, A.S.; Giantonio, B.J.; Ross, R.N.; Teng, Y.; Wang, M.; Niknam, B.A.; Ludwig, J.M.; Wang, W.; et al. Characteristics associated with differences in survival among black and white women with breast cancer. J. Am. Med. Assoc 2013, 310, 389–397. [Google Scholar]
- Chakrabarti, S.; Karmakar, R.; Barui, G.; Maity, P.K.; Bandyopadhyay, A.; Roy, A. Prevalence of known prognostic factors in female breast carcinoma including oestrogen receptor, progesterone receptor and Her-2/neu status—A study in a tertiary care centre. J. Indian Med. Assoc 2012, 110, 876–879. [Google Scholar]
- Thumar, N.J.; Patel, M.P. Synthesis, characterization, and antimicrobial evaluation of carbostyril derivatives of 1H-pyrazole. Saudi Pharm. J 2011, 19, 75–83. [Google Scholar]
- Kumar, V.; Kaur, K.; Gupta, G.K.; Gupta, A.K.; Kumar, S. Developments in synthesis of the anti-inflammatory drug, celecoxib: A review. Recent Pat. Inflamm. Allergy Drug Discov 2013, 7, 124–134. [Google Scholar]
- Zaninetti, R.; Cortese, S.V.; Aprile, S.; Massarotti, A.; Canonico, P.L.; Sorba, G.; Grosa, G.; Genazzani, A.A.; Pirali, T. A concise synthesis of pyrazole analogues of combretastatin A1 as potent anti-tubulin agents. Chem. Med. Chem 2013, 8, 633–643. [Google Scholar]
- Ibrahim, H.M.; Behbehani, H.; Elnagdi, M.H. Approaches towards the synthesis of a novel class of 2-amino-5-arylazonicotinate, pyridazinone and pyrido[2,3-d]pyrimidine derivatives as potent antimicrobial agents. Chem. Cent. J 2013, 7, 123:1–123:16. [Google Scholar]
- Ivanov, M.A.; Aleksandrova, L.A. Bicyclic furano[2,3-d] derivatives of pyrimidine nucleosides—Synthesis and antiviral properties. Bioorganicheskaia Khimiia 2013, 39, 26–45. [Google Scholar]
- Singh, K.; Kaur, H.; Chibale, K.; Balzarini, J. Synthesis of 4-aminoquinoline-pyrimidine hybrids as potent antimalarials and their mode of action studies. Eur. J. Med. Chem 2013, 66, 314–323. [Google Scholar]
- Shaquiquzzaman, M.; Khan, S.A.; Amir, M.; Alam, M.M. Synthesis, anticonvulsant and neurotoxicity evaluation of some new pyrimidine-5-carbonitrile derivatives. Saudi Pharm. J 2012, 20, 149–154. [Google Scholar]
- Lauria, A.; Patella, C.; Abbate, I.; Martorana, A.; Almerico, A.M. An unexpected Dimroth rearrangement leading to annelatedthieno[3,2-d][1,2,3]triazolo[1,5-a]pyrimidines with potent antitumor activity. Eur. J. Med. Chem 2013, 65, 381–388. [Google Scholar]
- Rezende, V.M.; Rivellis, A.J.; Gomes, M.M.; Dörr, F.A.; Novaes, M.M.; Nardinelli, L.; Costa, A.L.; Chamone, D.A.; Bendit, I. Determination of serum levels of imatinibmesylate in patients with chronic myeloid leukemia: Validation and application of a new analytical method to monitor treatment compliance. Rev. Bras. Hematol. Hemoter 2013, 35, 103–108. [Google Scholar]
- Wei, X.; Zhou, D.; Wang, H.; Ding, N.; Cui, X.X.; Wang, H.; Verano, M.; Zhang, K.; Conney, A.H.; Zheng, X.; et al. Effects of pyridine analogs of curcumin on growth, apoptosis and NF-κB activity in prostate cancer PC-3 cells. Anticancer Res 2013, 33, 1343–1350. [Google Scholar]
- Wang, X.M.; Xu, J.; Li, Y.P.; Li, H.; Jiang, C.S.; Yang, G.D.; Lu, S.M.; Zhang, S.Q. Synthesis and anticancer activity evaluation of a series of [1,2,4]triazolo[1,5-a]pyridinylpyridines in vitro and in vivo. Eur. J. Med. Chem. 2013, 67C, 243–251. [Google Scholar]
- Li, L.; Du, K.; Wang, Y.; Jia, H.; Hou, X.; Chao, H.; Ji, L. Self-activating nuclease and anticancer activities of copper(ii) complexes with aryl-modified 2,6-di(thiazol-2-yl)pyridine. Dalton Trans 2013, 42, 11576–11588. [Google Scholar]
- AlSaid, M.S.; El-Gazzar, M.G.; Ghorab, M.M. Anticancer activity of novel thiophenes containing a biological active diphenylsulfone, diazepin, piperidine, oxazepine, acryladehyde and sulfonamide moieties. Drug Res. (Stuttg) 2013, 63, 263–269. [Google Scholar]
- Al-Dosari, M.S.; Ghorab, M.M.; AlSaid, M.S.; Nissan, Y.M. Discovering some novel 7-chloroquinolines carrying a biologically active benzenesulfonamide moiety as a new class of anticancer agents. Chem. Pharm. Bull 2013, 61, 50–58. [Google Scholar]
- AlSaid, M.S.; El-Gazzar, M.G.; Ghorab, M.M. In-vitro cytotoxic and radiosensitizing evaluation of novel 2-pyridone, isoquinoline, chromene and chromenopyridone derivatives. Eur. J. Chem 2012, 3, 228–234. [Google Scholar]
- El-Said, M.S.; El-Gazzar, M.G.; Al-Dosari, M.S.; Ghorab, M.M. Synthesis, anticancer activity and radiosensitizing: Evaluation of some new 2-pyridone derivatives. Arzneimittelforschung 2012, 62, 149–156. [Google Scholar]
- Ghorab, M.M.; Ragab, F.A.; Heiba, H.I.; Youssef, H.A.; El-Gazzar, M.G. Synthesis of novel pyrazole and pyrimidine derivatives bearing sulfonamide moiety as antitumor and radiosensitizing agents. Med. Chem. Res 2012, 21, 1376–1383. [Google Scholar]
- Ghorab, M.M.; Ragab, F.A.; Heiba, H.I.; El-Gazzar, M.G.; El-Gazzar, M.G. Synthesis, in vitro anticancer screening and radiosensitizing evaluation of some new 4-[3-(substituted)thioureido]-N-(quinoxalin-2-yl)-benzenesulfonamide derivatives. Acta Pharm 2011, 61, 415–425. [Google Scholar]
- Brożewicz, K.; Sławiński, J. 1-(2-Mercaptobenzenesulfonyl)-3-hydroxyguanidines—Novel potent antiproliferatives, synthesis and in vitro biological activity. Eur. J. Med. Chem 2012, 55, 384–394. [Google Scholar]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Orta, M.L.; Pastor, N.; Perez-Guerrero, C.; Austin, C.A.; Mateos, S.; López-Lázaro, M. Guanidine-reactive agent phenylglyoxal induces DNA damage and cancer cell death. Pharmacol. Rep 2012, 64, 1515–1525. [Google Scholar]
- Duo, J.; Ma, Y.; Wang, G.; Han, X.; Zhang, C. Metformin synergistically enhances antitumor activity of histone deacetylase inhibitor trichostatina against osteosarcoma cell line. DNA Cell Biol 2013, 32, 156–164. [Google Scholar]
- Würth, R.; Pattarozzi, A.; Gatti, M.; Bajetto, A.; Corsaro, A.; Parodi, A.; Sirito, R.; Massollo, M.; Marini, C.; Zona, G.; et al. Metformin selectively affects human glioblastoma tumor-initiating cell viability: A role for metformin-induced inhibition of Akt. Cell Cycle 2013, 12, 145–156. [Google Scholar]
- Sinnett-Smith, J.; Kisfalvi, K.; Kui, R.; Rozengurt, E. Metformin inhibition of mTORC1 activation, DNA synthesis and proliferation in pancreatic cancer cells: Dependence on glucose concentration and role of AMPK. Biochem. Biophys. Res. Commun 2013, 430, 352–357. [Google Scholar]
- Furukawa, K.; Uwagawa, T.; Iwase, R.; Haruki, K.; Fujiwara, Y.; Gocho, T.; Shiba, H.; Misawa, T.; Yanaga, K. Prognostic factors of unresectable pancreatic cancer treated with nafamostatmesilate combined with gemcitabine chemotherapy. Anticancer Res 2012, 32, 5121–5126. [Google Scholar]
- Georgopoulos, S.; Mastorakos, D.; Kondi-Pafiti, A.; Katsenis, K.; Arkadopoulos, N.; Kannas, D.; Archontaki, M.; Vestarchis, N.; Kokkalis, G. Hydroxyzine, cimetidine and vitamin C in reducing skin flap necrosis in ischemia-reperfusion injury in rats: A comparative study. J. BUON 2012, 17, 377–382. [Google Scholar]
- Ozeki, T.; Natori, T. The specific inhibition of HepG2 cells proliferation by apoptosis induced by gabexatemesilate. Int. J. Clin. Exp. Pathol 2010, 3, 710–717. [Google Scholar]
- Fornari, F.A.; Randolph, J.K.; Yalowich, J.C.; Ritke, M.K.; Gewirtz, D.A. Interference by Doxorubicin with DNA unwinding in MCF-7 breast tumor cells. Mol. Pharmacol 1994, 45, 649–656. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst 1990, 82, 1107–1112. [Google Scholar]
- Rubinstein, L.V.; Shoemaker, R.H.; Paull, K.D.; Simon, R.M.; Tosini, S.; Skehan, P.; Scudiero, D.A.; Monks, A.; Boyd, M.R. Comparison of in vitro anticancer-drug-screening data generated with a tetrazolium assay vs. a protein assay against a diverse panel of human tumor cell lines. J. Natl. Cancer Inst 1990, 82, 1113–1118. [Google Scholar]

| Compound No. | IC50 (μg/mL) | IC50 (μM) |
|---|---|---|
| 3 | 16.7 | 49.5 |
| 4 | 19.4 | 54.8 |
| 5 | 24.4 | 75.3 |
| 6 | 19.0 | 59.0 |
| 7 | 23.6 | 70.2 |
| 8 | 29.0 | 68.1 |
| 9 | 21.7 | 59.2 |
| 10 | 23.3 | 76.1 |
| 11 | 24.2 | 75.6 |
| 12 | 34.4 | 102.9 |
| 13 | 22.9 | 57.8 |
| 14 | NA | NA |
| Doxorubicin | 39.0 | 71.8 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ghorab, M.M.; El-Gazzar, M.G.; Alsaid, M.S. Synthesis and Anti-Breast Cancer Evaluation of Novel N-(Guanidinyl)benzenesulfonamides. Int. J. Mol. Sci. 2014, 15, 5582-5595. https://doi.org/10.3390/ijms15045582
Ghorab MM, El-Gazzar MG, Alsaid MS. Synthesis and Anti-Breast Cancer Evaluation of Novel N-(Guanidinyl)benzenesulfonamides. International Journal of Molecular Sciences. 2014; 15(4):5582-5595. https://doi.org/10.3390/ijms15045582
Chicago/Turabian StyleGhorab, Mostafa M., Marwa G. El-Gazzar, and Mansour S. Alsaid. 2014. "Synthesis and Anti-Breast Cancer Evaluation of Novel N-(Guanidinyl)benzenesulfonamides" International Journal of Molecular Sciences 15, no. 4: 5582-5595. https://doi.org/10.3390/ijms15045582




