Potential Roles of Bone Morphogenetic Protein (BMP)-9 in Human Liver Diseases
Abstract
:1. BMPs and Liver
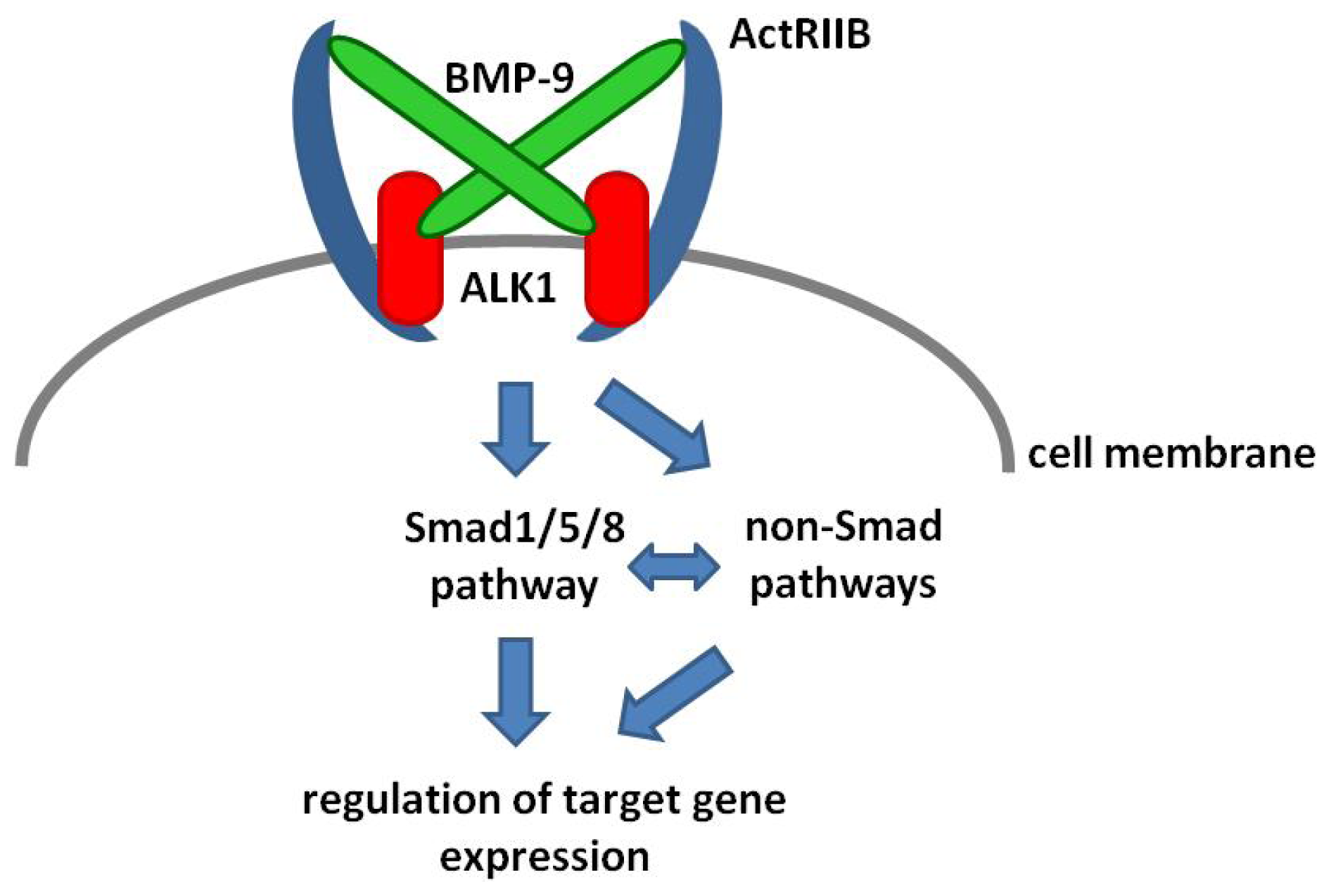
2. BMP-9 Signaling
3. Co-Receptors and Extracellular Regulators
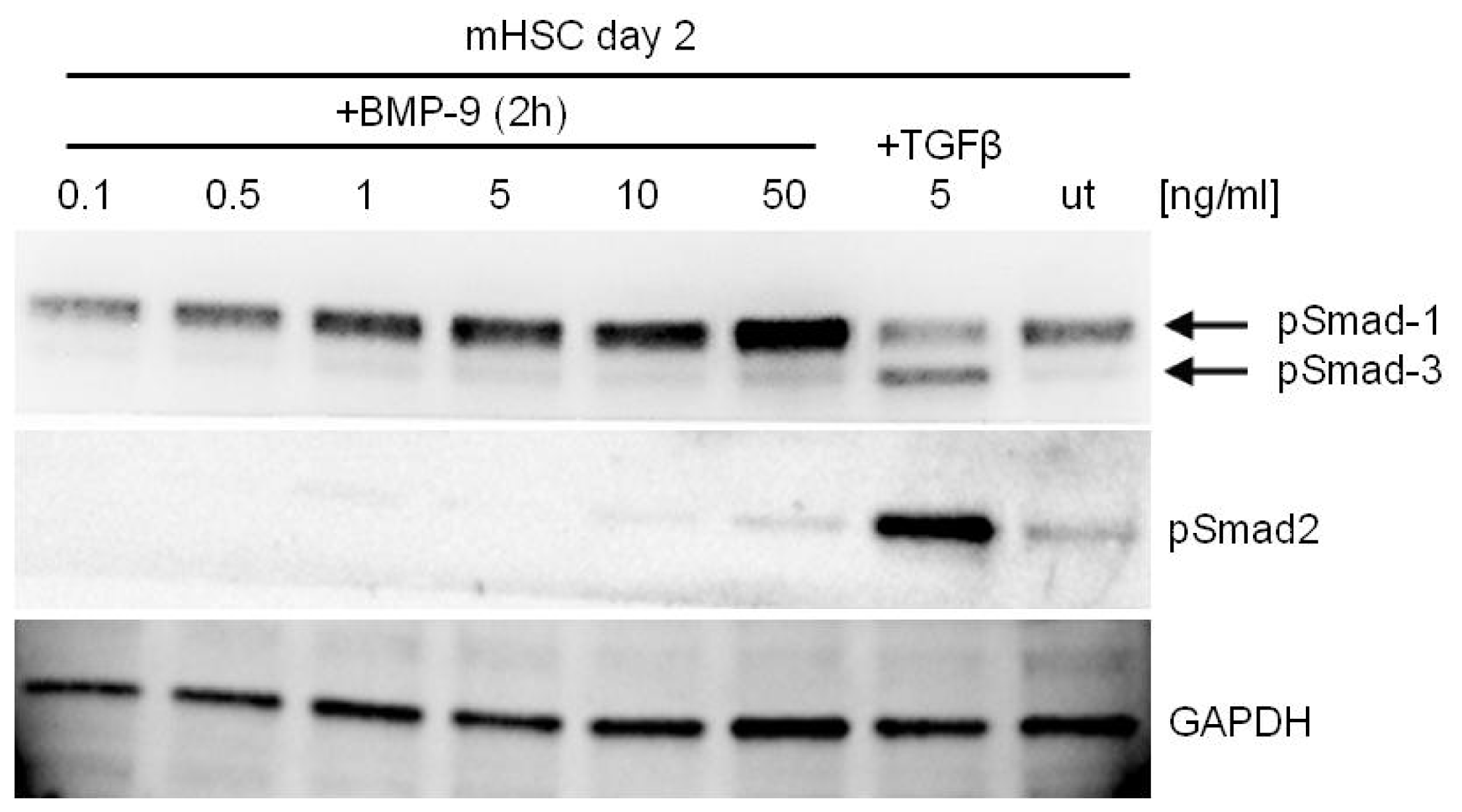
4. BMP-9—Smad Dependent Signaling
5. BMP-9—Non-Smad Dependent Signaling
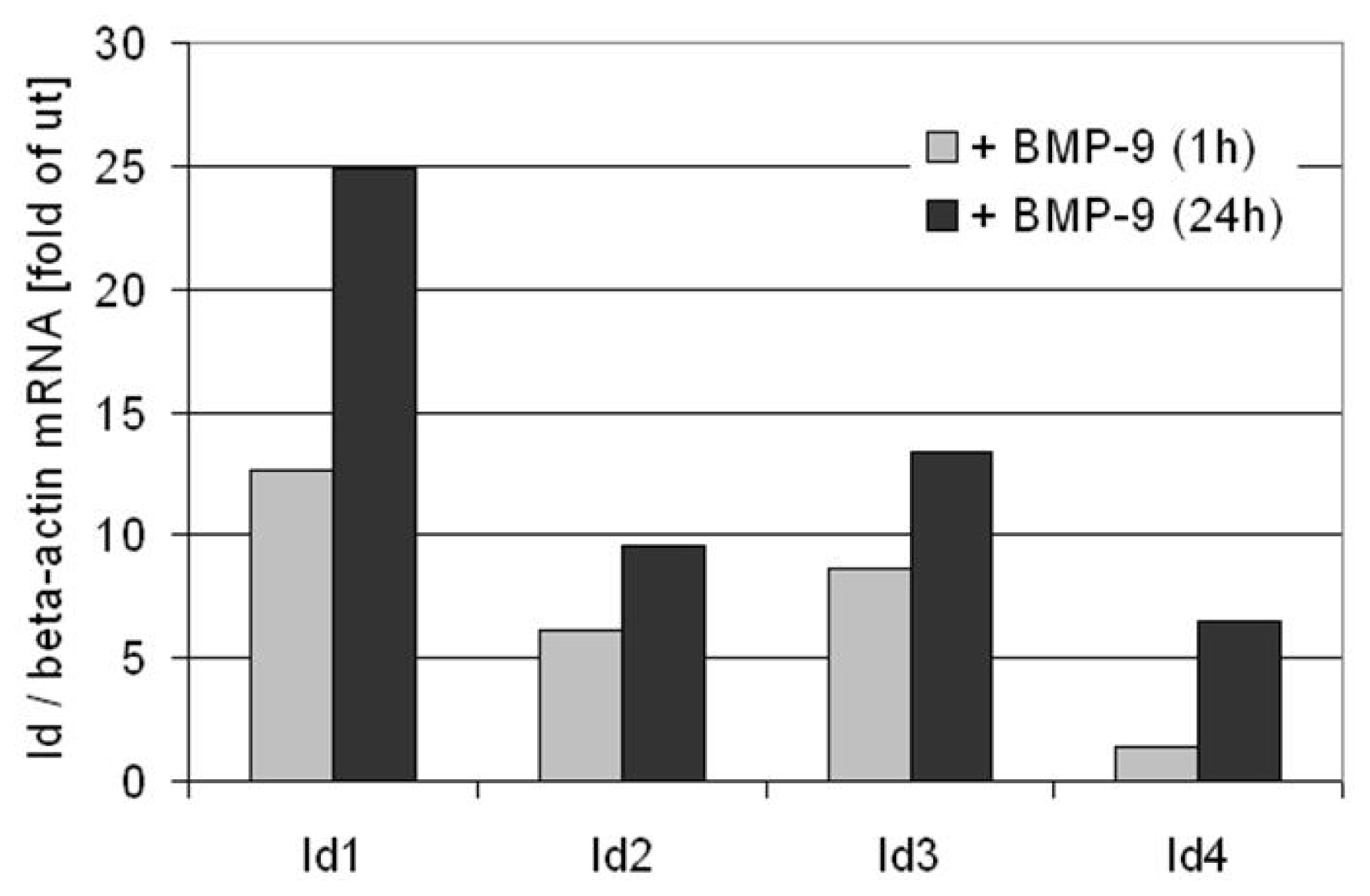
6. BMP-9 Mediated Regulation of Target Gene Expression
7. BMP-9 versus BMP-10
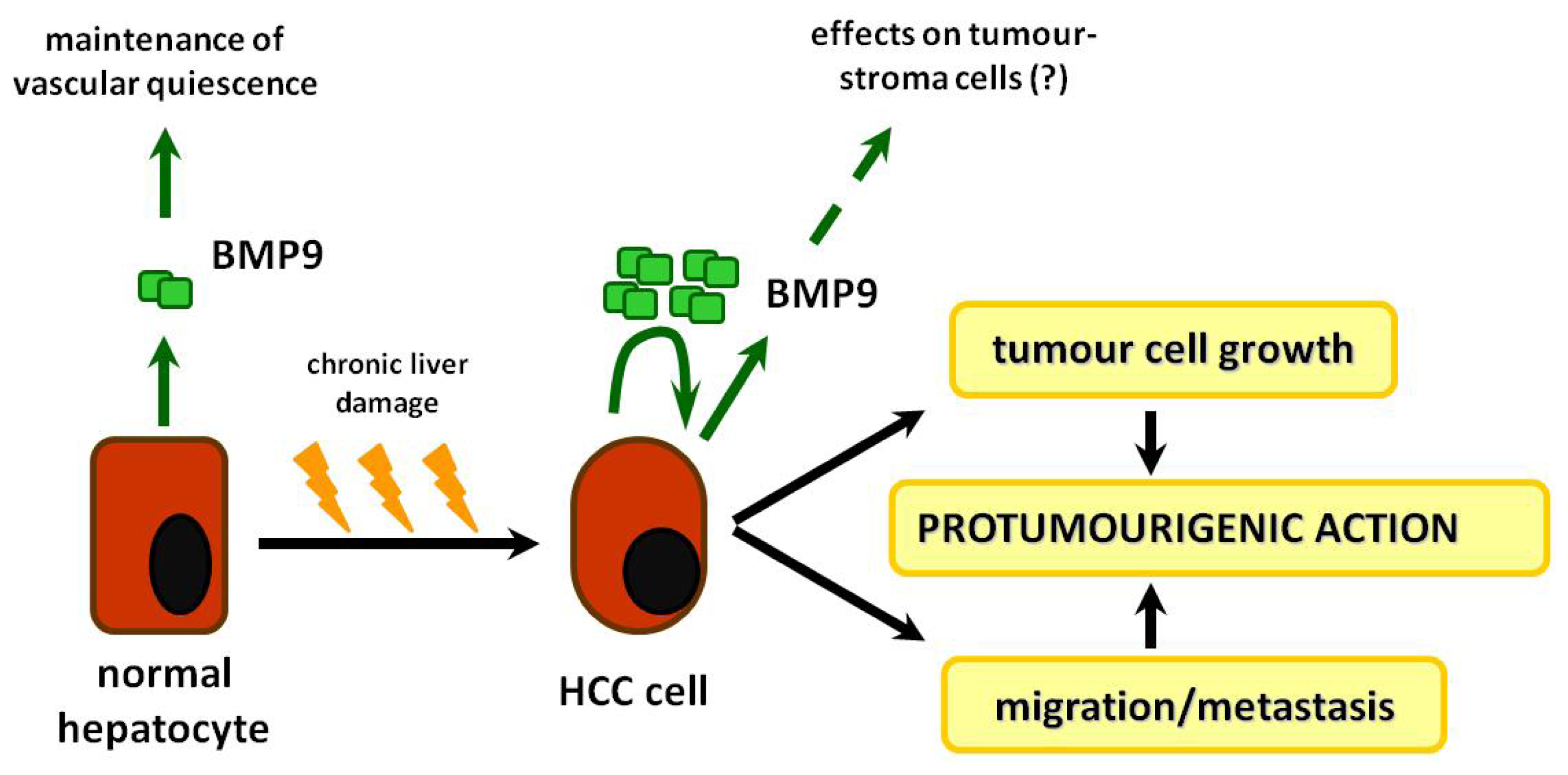
8. BMP-9: Role in Liver Physiology
9. BMP-9: Role in Neo-Angiogenesis
10. Roles of BMP-9 in HCC and Other Types of Cancer
11. Summary, Conclusions and Outlook
Acknowledgments
Conflicts of Interest
Abbreviations
| BMPs | Bone morphogenetic proteins |
| TGF | Transforming growth factor |
| HCC | Hepatocellular carcinoma |
| GDF | Growth and differentiation factor |
| EMT | Epithelial to mesenchymal transition |
| ALK | Activin like kinase |
| ActRIIA | Activin type II receptor A |
| ActRIIB | Activin type II receptor B |
| HSCs | Hepatic stellate cells |
| Smad | a portmanteau of “mothers against decapentaplegic” (Drosophila homologue) and the protein “SMA” (Caenorhabditis elegans homologue) |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| CV2 | Crossveinless2 |
| PI3K | Phosphatidylinositol-3-kinase |
| Akt | Protein kinase B |
| JNK | c-Jun N-terminal kinase |
| Erk | Extracellular signal regulated kinase |
| NF-κB | Nuclear factor kappa B |
| Wnt | Wingless (Drosophila homologue) |
| TAK 1 | TGF-β activated kinase 1 |
| TAB1 | TGF-β activated kinase 1/MAP3K7 binding protein 1 |
| XIAP | X-linked inhibitor of apoptosis |
| MAPK | Mitogen activated protein kinase |
| MPC | Mesenchymal progenitor cell |
| ColxA1/2 | Collagen type x, alpha-1 or -2 chain |
| Id | Inhibitor of differentiation |
| Hif1α | hypoxia inducible factor 1α |
| NGF | Nerve growth factor |
| IGF | Insulin like growth factor |
| VEGF | Vascular endothelial growth factor receptor |
| ET | Endothelin |
| TMEM100 | Transmembrane protein 100 |
| SREBP | Sterol regulatory element binding protein |
| BMPRII | BMP receptor type II |
| ActRIIA/B | Activin receptor type IIA/B |
| BRE | BMP response element |
| SV40 | Simian virus 40 |
| HHT | Hemorrhagic hereditary telangiectasia |
| ENG | Endoglin |
| FGF | fibroblast growth factor |
| CAM | Chick chorioallantoic membrane |
| MESECs | Embryonic-stem-cell-derived endothelial cells |
| TECs | Tumor associated endothelial cells |
| HMVECs | Human dermal microvascular endothelial cells |
References
- Mueller, T.D.; Nickel, J. Promiscuity and specificity in BMP receptor activation. FEBS Lett 2012, 586, 1846–1859. [Google Scholar]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar]
- Wozney, J.M.; Rosen, V.; Celeste, A.J.; Mitsock, L.M.; Whitters, M.J.; Kriz, R.W.; Hewick, R.M.; Wang, E.A. Novel regulators of bone formation: Molecular clones and activities. Science 1988, 242, 1528–1534. [Google Scholar]
- De Robertis, E.M. Spemann’s organizer and the self-regulation of embryonic fields. Mech. Dev 2009, 126, 925–941. [Google Scholar]
- Plouhinec, J.L.; Zakin, L.; de Robertis, E.M. Systems control of BMP morphogen flow in vertebrate embryos. Curr. Opin. Genet. Dev 2011, 21, 696–703. [Google Scholar]
- Reddi, A.H.; Reddi, A. Bone morphogenetic proteins (BMPs): From morphogens to metabologens. Cytokine Growth Factor Rev 2009, 20, 341–342. [Google Scholar]
- Wagner, D.O.; Sieber, C.; Bhushan, R.; Borgermann, J.H.; Graf, D.; Knaus, P. BMPs: From bone to body morphogenetic proteins. Sci. Signal 2010, 3, mr1. [Google Scholar] [CrossRef]
- Herrera, B.; Sanchez, A.; Fabregat, I. BMPs and liver: More questions than answers. Curr. Pharm. Des 2012, 18, 4114–4125. [Google Scholar]
- Finberg, K.E. Regulation of systemic iron homeostasis. Curr. Opin. Hematol 2013, 20, 208–214. [Google Scholar]
- Chayanupatkul, M.; Honsawek, S.; Vejchapipat, P.; Chongsrisawat, V.; Poovorawan, Y. Elevated serum bone morphogenetic protein 7 levels and clinical outcome in children with biliary atresia. Eur. J. Pediatr. Surg 2009, 19, 246–250. [Google Scholar]
- Chen, B.L.; Peng, J.; Li, Q.F.; Yang, M.; Wang, Y.; Chen, W. Exogenous bone morphogenetic protein-7 reduces hepatic fibrosis in Schistosoma japonicum-infected mice via transforming growth factor-beta/Smad signaling. World J. Gastroenterol 2013, 19, 1405–1415. [Google Scholar]
- Kinoshita, K.; Iimuro, Y.; Otogawa, K.; Saika, S.; Inagaki, Y.; Nakajima, Y.; Kawada, N.; Fujimoto, J.; Friedman, S.L.; Ikeda, K. Adenovirus-mediated expression of BMP-7 suppresses the development of liver fibrosis in rats. Gut 2007, 56, 706–714. [Google Scholar]
- Meurer, S.; Tihaa, L.; Borkham-Kamphorst, E.; Weiskirchen, R. Expression and functional analysis of endoglin in isolated liver cells and its involvement in fibrogenic Smad signalling. Cell Signal 2011, 23, 683–699. [Google Scholar]
- Tacke, F.; Gabele, E.; Bataille, F.; Schwabe, R.F.; Hellerbrand, C.; Klebl, F.; Straub, R.H.; Luedde, T.; Manns, M.P.; Trautwein, C.; et al. Bone morphogenetic protein 7 is elevated in patients with chronic liver disease and exerts fibrogenic effects on human hepatic stellate cells. Dig. Dis. Sci 2007, 52, 3404–3415. [Google Scholar]
- Wang, S.L.; Yang, C.Q.; Qi, X.L.; Yuan, M.; Chang, Y.Z.; Yang, L.; Gao, H.J. Inhibitory effect of bone morphogenetic protein-7 on hepatic fibrosis in rats. Int. J. Clin. Exp. Pathol 2013, 6, 897–903. [Google Scholar]
- Yang, T.; Chen, S.L.; Lu, X.J.; Shen, C.Y.; Liu, Y.; Chen, Y.P. Bone morphogenetic protein 7 suppresses the progression of hepatic fibrosis and regulates the expression of gremlin and transforming growth factor beta1. Mol. Med. Rep 2012, 6, 246–252. [Google Scholar]
- Zeisberg, M.; Yang, C.; Martino, M.; Duncan, M.B.; Rieder, F.; Tanjore, H.; Kalluri, R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J. Biol. Chem 2007, 282, 23337–23347. [Google Scholar]
- Sugimoto, H.; Yang, C.; LeBleu, V.S.; Soubasakos, M.A.; Giraldo, M.; Zeisberg, M.; Kalluri, R. BMP-7 functions as a novel hormone to facilitate liver regeneration. FASEB J 2007, 21, 256–264. [Google Scholar]
- Do, N.; Zhao, R.; Ray, K.; Ho, K.; Dib, M.; Ren, X.; Kuzontkoski, P.; Terwilliger, E.; Karp, S.J. BMP4 is a novel paracrine inhibitor of liver regeneration. Am. J. Physiol. Gastrointest. Liver Physiol 2012, 303, G1220–G1227. [Google Scholar]
- Brown, M.A.; Zhao, Q.; Baker, K.A.; Naik, C.; Chen, C.; Pukac, L.; Singh, M.; Tsareva, T.; Parice, Y.; Mahoney, A.; et al. Crystal structure of BMP-9 and functional interactions with pro-region and receptors. J. Biol. Chem 2005, 280, 25111–25118. [Google Scholar]
- Townson, S.A.; Martinez-Hackert, E.; Greppi, C.; Lowden, P.; Sako, D.; Liu, J.; Ucran, J.A.; Liharska, K.; Underwood, K.W.; Seehra, J.; et al. Specificity and structure of a high affinity activin receptor-like kinase 1 (ALK1) signaling complex. J. Biol. Chem 2012, 287, 27313–27325. [Google Scholar]
- Herrera, B.; van Dinther, M.; Ten Dijke, P.; Inman, G.J. Autocrine bone morphogenetic protein-9 signals through activin receptor-like kinase-2/Smad1/Smad4 to promote ovarian cancer cell proliferation. Cancer Res 2009, 69, 9254–9262. [Google Scholar]
- Scharpfenecker, M.; van Dinther, M.; Liu, Z.; van Bezooijen, R.L.; Zhao, Q.; Pukac, L.; Lowik, C.W.; ten Dijke, P. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J. Cell Sci 2007, 120 Pt 6, 964–972. [Google Scholar]
- David, L.; Mallet, C.; Mazerbourg, S.; Feige, J.J.; Bailly, S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood 2007, 109, 1953–1961. [Google Scholar]
- Gonzalez-Nunez, M.; Munoz-Felix, J.M.; Lopez-Novoa, J.M. The ALK-1/Smad1 pathway in cardiovascular physiopathology. A new target for therapy? Biochim. Biophys. Acta 2013, 1832, 1492–1510. [Google Scholar]
- Arndt, S.; Maegdefrau, U.; Dorn, C.; Schardt, K.; Hellerbrand, C.; Bosserhoff, A.K. Iron-induced expression of bone morphogenic protein 6 in intestinal cells is the main regulator of hepatic hepcidin expressionin vivo. Gastroenterology 2010, 138, 372–382. [Google Scholar]
- Xia, Y.; Babitt, J.L.; Sidis, Y.; Chung, R.T.; Lin, H.Y. Hemojuvelin regulates hepcidin expression via a selective subset of BMP ligands and receptors independently of neogenin. Blood 2008, 111, 5195–5204. [Google Scholar]
- Miller, A.F.; Harvey, S.A.; Thies, R.S.; Olson, M.S. Bone morphogenetic protein-9. An autocrine/paracrine cytokine in the liver. J. Biol. Chem 2000, 275, 17937–17945. [Google Scholar]
- Li, Q.; Gu, X.; Weng, H.; Ghafoory, S.; Liu, Y.; Feng, T.; Dzieran, J.; Li, L.; Ilkavets, I.; Kruithof-de Julio, M.; et al. Bone morphogenetic protein-9 (BMP-9) induces epithelial to mesenchymal transition (EMT) in hepatocellular carcinoma cells. Cancer Sci 2013, 104, 398–408. [Google Scholar]
- Zilberberg, L.; ten Dijke, P.; Sakai, L.Y.; Rifkin, D.B. A rapid and sensitive bioassay to measure bone morphogenetic protein activity. BMC Cell Biol 2007, 8, 41. [Google Scholar] [CrossRef]
- Wiercinska, E.; Wickert, L.; Denecke, B.; Said, H.M.; Hamzavi, J.; Gressner, A.M.; Thorikay, M.; ten Dijke, P.; Mertens, P.R.; Breitkopf, K.; et al. Id1 is a critical mediator in TGF-beta-induced transdifferentiation of rat hepatic stellate cells. Hepatology 2006, 43, 1032–1041. [Google Scholar]
- Ciuclan, L.; Ehnert, S.; Ilkavets, I.; Weng, H.L.; Gaitantzi, H.; Tsukamoto, H.; Ueberham, E.; Meindl-Beinker, N.M.; Singer, M.V.; Breitkopf, K.; et al. TGF-beta enhances alcohol dependent hepatocyte damage via down-regulation of alcohol dehydrogenase I. J. Hepatol 2010, 52, 407–416. [Google Scholar]
- Panchenko, M.P.; Williams, M.C.; Brody, J.S.; Yu, Q. Type I receptor serine-threonine kinase preferentially expressed in pulmonary blood vessels. Am. J. Physiol 1996, 270, L547–L558. [Google Scholar]
- Castonguay, R.; Werner, E.D.; Matthews, R.G.; Presman, E.; Mulivor, A.W.; Solban, N.; Sako, D.; Pearsall, R.S.; Underwood, K.W.; Seehra, J.; et al. Soluble endoglin specifically binds bone morphogenetic proteins 9 and 10 via its orphan domain, inhibits blood vessel formation, and suppresses tumor growth. J. Biol. Chem 2011, 286, 30034–30046. [Google Scholar]
- Upton, P.D.; Davies, R.J.; Trembath, R.C.; Morrell, N.W. Bone morphogenetic protein (BMP) and activin type II receptors balance BMP9 signals mediated by activin receptor-like kinase-1 in human pulmonary artery endothelial cells. J. Biol. Chem 2009, 284, 15794–15804. [Google Scholar]
- Nolan-Stevaux, O.; Zhong, W.; Culp, S.; Shaffer, K.; Hoover, J.; Wickramasinghe, D.; Ruefli-Brasse, A. Endoglin requirement for BMP9 signaling in endothelial cells reveals new mechanism of action for selective anti-endoglin antibodies. PLoS One 2012, 7, e50920. [Google Scholar]
- Young, K.; Conley, B.; Romero, D.; Tweedie, E.; O’Neill, C.; Pinz, I.; Brogan, L.; Lindner, V.; Liaw, L.; Vary, C.P. BMP9 regulates endoglin-dependent chemokine responses in endothelial cells. Blood 2012, 120, 4263–4273. [Google Scholar]
- Clemente, M.; Nunez, O.; Lorente, R.; Rincon, D.; Matilla, A.; Salcedo, M.; Catalina, M.V.; Ripoll, C.; Iacono, O.L.; Banares, R.; et al. Increased intrahepatic and circulating levels of endoglin, a TGF-beta1 co-receptor, in patients with chronic hepatitis C virus infection: Relationship to histological and serum markers of hepatic fibrosis. J. Viral Hepat 2006, 13, 625–632. [Google Scholar]
- Wu, Q.; Sun, C.C.; Lin, H.Y.; Babitt, J.L. Repulsive guidance molecule (RGM) family proteins exhibit differential binding kinetics for bone morphogenetic proteins (BMPs). PLoS One 2012, 7, e46307. [Google Scholar]
- Walsh, D.W.; Godson, C.; Brazil, D.P.; Martin, F. Extracellular BMP-antagonist regulation in development and disease: Tied up in knots. Trends Cell Biol 2010, 20, 244–256. [Google Scholar]
- Yao, Y.; Jumabay, M.; Ly, A.; Radparvar, M.; Wang, A.H.; Abdmaulen, R.; Bostrom, K.I. Crossveinless 2 regulates bone morphogenetic protein 9 in human and mouse vascular endothelium. Blood 2012, 119, 5037–5047. [Google Scholar]
- Seemann, P.; Brehm, A.; Konig, J.; Reissner, C.; Stricker, S.; Kuss, P.; Haupt, J.; Renninger, S.; Nickel, J.; Sebald, W.; et al. Mutations in GDF5 reveal a key residue mediating BMP inhibition by NOGGIN. PLoS Genet 2009, 5, e1000747. [Google Scholar]
- Wang, Y.; Hong, S.; Li, M.; Zhang, J.; Bi, Y.; He, Y.; Liu, X.; Nan, G.; Su, Y.; Zhu, G.; et al. Noggin resistance contributes to the potent osteogenic capability of BMP9 in mesenchymal stem cells. J. Orthop. Res 2013, 31, 1796–1803. [Google Scholar]
- David, L.; Mallet, C.; Keramidas, M.; Lamande, N.; Gasc, J.M.; Dupuis-Girod, S.; Plauchu, H.; Feige, J.J.; Bailly, S. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ. Res 2008, 102, 914–922. [Google Scholar]
- Herrera, B.; Garcia-Alvaro, M.; Cruz, S.; Walsh, P.; Fernandez, M.; Roncero, C.; Fabregat, I.; Sanchez, A.; Inman, G.J. BMP9 is a proliferative and survival factor for human hepatocellular carcinoma cells. PLoS One 2013, 8, e69535. [Google Scholar]
- Star, G.P.; Giovinazzo, M.; Langleben, D. Bone morphogenic protein-9 stimulates endothelin-1 release from human pulmonary microvascular endothelial cells: A potential mechanism for elevated ET-1 levels in pulmonary arterial hypertension. Microvasc. Res 2010, 80, 349–354. [Google Scholar]
- Sieber, C.; Kopf, J.; Hiepen, C.; Knaus, P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev 2009, 20, 343–355. [Google Scholar]
- Zhao, Y.F.; Xu, J.; Wang, W.J.; Wang, J.; He, J.W.; Li, L.; Dong, Q.; Xiao, Y.; Duan, X.L.; Yang, X.; et al. Activation of JNKs is essential for BMP9-induced osteogenic differentiation of mesenchymal stem cells. BMB Rep 2013, 46, 422–427. [Google Scholar]
- Xu, D.J.; Zhao, Y.Z.; Wang, J.; He, J.W.; Weng, Y.G.; Luo, J.Y. Smads, p38 and ERK1/2 are involved in BMP9-induced osteogenic differentiation of C3H10T1/2 mesenchymal stem cells. BMB Rep 2012, 45, 247–252. [Google Scholar]
- Zhao, Y.; Song, T.; Wang, W.; Wang, J.; He, J.; Wu, N.; Tang, M.; He, B.; Luo, J. P38 and ERK1/2 MAPKs act in opposition to regulate BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. PLoS One 2012, 7, e43383. [Google Scholar]
- Park, H.; Drevelle, O.; Daviau, A.; Senta, H.; Bergeron, E.; Faucheux, N. Preventing MEK1 activation influences the responses of human osteosarcoma cells to bone morphogenetic proteins 2 and 9. Anticancer Drugs 2013, 24, 278–290. [Google Scholar]
- Fong, D.; Bisson, M.; Laberge, G.; McManus, S.; Grenier, G.; Faucheux, N.; Roux, S. Bone morphogenetic protein-9 activates Smad and ERK pathways and supports human osteoclast function and survival in vitro. Cell Signal. 2013, 25, 717–728. [Google Scholar]
- Li, C.; Yang, X.; He, Y.; Ye, G.; Li, X.; Zhang, X.; Zhou, L.; Deng, F. Bone morphogenetic protein-9 induces osteogenic differentiation of rat dental follicle stem cells in P38 and ERK1/2 MAPK dependent manner. Int. J. Med. Sci 2012, 9, 862–871. [Google Scholar]
- Majumdar, M.K.; Wang, E.; Morris, E.A. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J. Cell. Physiol 2001, 189, 275–284. [Google Scholar]
- Peng, Y.; Kang, Q.; Cheng, H.; Li, X.; Sun, M.H.; Jiang, W.; Luu, H.H.; Park, J.Y.; Haydon, R.C.; He, T.C. Transcriptional characterization of bone morphogenetic proteins (BMPs)-mediated osteogenic signaling. J. Cell. Biochem 2003, 90, 1149–1165. [Google Scholar]
- Sharff, K.A.; Song, W.X.; Luo, X.; Tang, N.; Luo, J.; Chen, J.; Bi, Y.; He, B.C.; Huang, J.; Li, X.; et al. Hey1 basic helix-loop-helix protein plays an important role in mediating BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. J. Biol. Chem 2009, 284, 649–659. [Google Scholar]
- Wang, J.H.; Liu, Y.Z.; Yin, L.J.; Chen, L.; Huang, J.; Liu, Y.; Zhang, R.X.; Zhou, L.Y.; Yang, Q.J.; Luo, J.Y.; et al. BMP9 and COX-2 form an important regulatory loop in BMP9-induced osteogenic differentiation of mesenchymal stem cells. Bone 2013, 57, 311–321. [Google Scholar]
- Nakamura, T.; Shinohara, Y.; Momozaki, S.; Yoshimoto, T.; Noguchi, K. Co-stimulation with bone morphogenetic protein-9 and FK506 induces remarkable osteoblastic differentiation in rat dedifferentiated fat cells. Biochem. Biophys. Res. Commun 2013, 440, 289–294. [Google Scholar]
- Hu, N.; Jiang, D.; Huang, E.; Liu, X.; Li, R.; Liang, X.; Kim, S.H.; Chen, X.; Gao, J.L.; Zhang, H.; et al. BMP9-regulated angiogenic signaling plays an important role in the osteogenic differentiation of mesenchymal progenitor cells. J. Cell Sci 2013, 126 Pt 2, 532–541. [Google Scholar]
- Lopez-Coviella, I.; Follettie, M.T.; Mellott, T.J.; Kovacheva, V.P.; Slack, B.E.; Diesl, V.; Berse, B.; Thies, R.S.; Blusztajn, J.K. Bone morphogenetic protein 9 induces the transcriptome of basal forebrain cholinergic neurons. Proc. Natl. Acad. Sci. USA 2005, 102, 6984–6989. [Google Scholar]
- Schnitzler, A.C.; Mellott, T.J.; Lopez-Coviella, I.; Tallini, Y.N.; Kotlikoff, M.I.; Follettie, M.T.; Blusztajn, J.K. BMP9 (bone morphogenetic protein 9) induces NGF as an autocrine/paracrine cholinergic trophic factor in developing basal forebrain neurons. J. Neurosci 2010, 30, 8221–8228. [Google Scholar]
- Somekawa, S.; Imagawa, K.; Hayashi, H.; Sakabe, M.; Ioka, T.; Sato, G.E.; Inada, K.; Iwamoto, T.; Mori, T.; Uemura, S.; et al. Tmem100, an ALK1 receptor signaling-dependent gene essential for arterial endothelium differentiation and vascular morphogenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 12064–12069. [Google Scholar]
- Kim, J.H.; Peacock, M.R.; George, S.C.; Hughes, C.C. BMP9 induces EphrinB2 expression in endothelial cells through an Alk1-BMPRII/ActRII-ID1/ID3-dependent pathway: Implications for hereditary hemorrhagic telangiectasia type II. Angiogenesis 2012, 15, 497–509. [Google Scholar]
- Park, J.E.; Shao, D.; Upton, P.D.; Desouza, P.; Adcock, I.M.; Davies, R.J.; Morrell, N.W.; Griffiths, M.J.; Wort, S.J. BMP-9 induced endothelial cell tubule formation and inhibition of migration involves Smad1 driven endothelin-1 production. PLoS One 2012, 7, e30075. [Google Scholar]
- Levet, S.; Ciais, D.; Merdzhanova, G.; Mallet, C.; Zimmers, T.A.; Lee, S.J.; Navarro, F.P.; Texier, I.; Feige, J.J.; Bailly, S.; et al. Bone morphogenetic protein 9 (BMP9) controls lymphatic vessel maturation and valve formation. Blood 2013, 122, 598–607. [Google Scholar]
- Wang, R.H.; Li, C.; Xu, X.; Zheng, Y.; Xiao, C.; Zerfas, P.; Cooperman, S.; Eckhaus, M.; Rouault, T.; Mishra, L.; et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab 2005, 2, 399–409. [Google Scholar]
- Casanovas, G.; Mleczko-Sanecka, K.; Altamura, S.; Hentze, M.W.; Muckenthaler, M.U. Bone morphogenetic protein (BMP)-responsive elements located in the proximal and distal hepcidin promoter are critical for its response to HJV/BMP/SMAD. J. Mol. Med 2009, 87, 471–480. [Google Scholar]
- Zhang, A.S.; Enns, C.A. Iron homeostasis: Recently identified proteins provide insight into novel control mechanisms. J. Biol. Chem 2009, 284, 711–715. [Google Scholar]
- Vujic Spasic, M.; Sparla, R.; Mleczko-Sanecka, K.; Migas, M.C.; Breitkopf-Heinlein, K.; Dooley, S.; Vaulont, S.; Fleming, R.E.; Muckenthaler, M.U. Smad6 and Smad7 are co-regulated with hepcidin in mouse models of iron overload. Biochim. Biophys. Acta 2013, 1832, 76–84. [Google Scholar]
- Andriopoulos, B., Jr.; Corradini, E.; Xia, Y.; Faasse, S.A.; Chen, S.; Grgurevic, L.; Knutson, M.D.; Pietrangelo, A.; Vukicevic, S.; Lin, H.Y.; et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat. Genet 2009, 41, 482–487. [Google Scholar]
- Babitt, J.L.; Huang, F.W.; Wrighting, D.M.; Xia, Y.; Sidis, Y.; Samad, T.A.; Campagna, J.A.; Chung, R.T.; Schneyer, A.L.; Woolf, C.J.; et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat. Genet 2006, 38, 531–539. [Google Scholar]
- Lin, L.; Valore, E.V.; Nemeth, E.; Goodnough, J.B.; Gabayan, V.; Ganz, T. Iron transferrin regulates hepcidin synthesis in primary hepatocyte culture through hemojuvelin and BMP2/4. Blood 2007, 110, 2182–2189. [Google Scholar]
- Truksa, J.; Peng, H.; Lee, P.; Beutler, E. Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6. Proc. Natl. Acad. Sci. USA 2006, 103, 10289–10293. [Google Scholar]
- Chen, C.; Grzegorzewski, K.J.; Barash, S.; Zhao, Q.; Schneider, H.; Wang, Q.; Singh, M.; Pukac, L.; Bell, A.C.; Duan, R.; et al. An integrated functional genomics screening program reveals a role for BMP-9 in glucose homeostasis. Nat. Biotechnol 2003, 21, 294–301. [Google Scholar]
- Oh, S.P.; Seki, T.; Goss, K.A.; Imamura, T.; Yi, Y.; Donahoe, P.K.; Li, L.; Miyazono, K.; ten Dijke, P.; Kim, S.; et al. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc. Natl. Acad. Sci. USA 2000, 97, 2626–2631. [Google Scholar]
- Urness, L.D.; Sorensen, L.K.; Li, D.Y. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat. Genet 2000, 26, 328–331. [Google Scholar]
- Chen, H.; Brady Ridgway, J.; Sai, T.; Lai, J.; Warming, S.; Chen, H.; Roose-Girma, M.; Zhang, G.; Shou, W.; Yan, M. Context-dependent signaling defines roles of BMP9 and BMP10 in embryonic and postnatal development. Proc. Natl. Acad. Sci. USA 2013, 110, 11887–11892. [Google Scholar]
- Ricard, N.; Ciais, D.; Levet, S.; Subileau, M.; Mallet, C.; Zimmers, T.A.; Lee, S.J.; Bidart, M.; Feige, J.J.; Bailly, S. BMP9 and BMP10 are critical for postnatal retinal vascular remodeling. Blood 2012, 119, 6162–6171. [Google Scholar]
- Sengle, G.; Ono, R.N.; Sasaki, T.; Sakai, L.Y. Prodomains of transforming growth factor beta (TGFbeta) superfamily members specify different functions: Extracellular matrix interactions and growth factor bioavailability. J. Biol. Chem 2011, 286, 5087–5099. [Google Scholar]
- Bidart, M.; Ricard, N.; Levet, S.; Samson, M.; Mallet, C.; David, L.; Subileau, M.; Tillet, E.; Feige, J.J.; Bailly, S. BMP9 is produced by hepatocytes and circulates mainly in an active mature form complexed to its prodomain. Cell. Mol. Life Sci 2011, 69, 313–324. [Google Scholar]
- Song, J.J.; Celeste, A.J.; Kong, F.M.; Jirtle, R.L.; Rosen, V.; Thies, R.S. Bone morphogenetic protein-9 binds to liver cells and stimulates proliferation. Endocrinology 1995, 136, 4293–4297. [Google Scholar]
- Caperuto, L.C.; Anhe, G.F.; Cambiaghi, T.D.; Akamine, E.H.; do Carmo Buonfiglio, D.; Cipolla-Neto, J.; Curi, R.; Bordin, S. Modulation of bone morphogenetic protein-9 expression and processing by insulin, glucose, and glucocorticoids: Possible candidate for hepatic insulin-sensitizing substance. Endocrinology 2008, 149, 6326–6335. [Google Scholar]
- Herrera, B.; Inman, G.J. A rapid and sensitive bioassay for the simultaneous measurement of multiple bone morphogenetic proteins. Identification and quantification of BMP4, BMP6 and BMP9 in bovine and human serum. BMC Cell Biol 2009, 10, 20. [Google Scholar] [CrossRef]
- David, L.; Feige, J.J.; Bailly, S. Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine Growth Factor Rev 2009, 20, 203–212. [Google Scholar]
- McAllister, K.A.; Grogg, K.M.; Johnson, D.W.; Gallione, C.J.; Baldwin, M.A.; Jackson, C.E.; Helmbold, E.A.; Markel, D.S.; McKinnon, W.C.; Murrell, J.; et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat. Genet 1994, 8, 345–351. [Google Scholar]
- Johnson, D.W.; Berg, J.N.; Baldwin, M.A.; Gallione, C.J.; Marondel, I.; Yoon, S.J.; Stenzel, T.T.; Speer, M.; Pericak-Vance, M.A.; Diamond, A.; et al. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat. Genet 1996, 13, 189–195. [Google Scholar]
- Gallione, C.J.; Richards, J.A.; Letteboer, T.G.; Rushlow, D.; Prigoda, N.L.; Leedom, T.P.; Ganguly, A.; Castells, A.; Ploos van Amstel, J.K.; Westermann, C.J.; et al. SMAD4 mutations found in unselected HHT patients. J. Med. Genet 2006, 43, 793–797. [Google Scholar]
- Kozawa, O.; Matsuno, H.; Uematsu, T. Involvement of p70 S6 kinase in bone morphogenetic protein signaling: Vascular endothelial growth factor synthesis by bone morphogenetic protein-4 in osteoblasts. J. Cell. Biochem 2001, 81, 430–436. [Google Scholar]
- Yeh, L.C.; Lee, J.C. Osteogenic protein-1 increases gene expression of vascular endothelial growth factor in primary cultures of fetal rat calvaria cells. Mol. Cell. Endocrinol 1999, 153, 113–124. [Google Scholar]
- Suzuki, Y.; Ohga, N.; Morishita, Y.; Hida, K.; Miyazono, K.; Watabe, T. BMP-9 induces proliferation of multiple types of endothelial cells in vitro and in vivo. J. Cell Sci. 2010, 123, 1684–1692. [Google Scholar]
- Pourgholami, M.H.; Morris, D.L. Inhibitors of vascular endothelial growth factor in cancer. Cardiovasc. Hematol. Agents Med. Chem 2008, 6, 343–347. [Google Scholar]
- Bendell, J.C.; Gordon, M.S.; Hurwitz, H.I.; Jones, S.F.; Mendelson, D.S.; Blobe, G.C.; Agarwal, N.; Condon, C.H.; Wilson, D.; Pearsall, A.E.; et al. Safety, Pharmacokinetics, Pharmacodynamics, and Antitumor Activity of Dalantercept, an Activin Receptor-like Kinase-1 Ligand Trap, in Patients with Advanced Cancer. Clin. Cancer Res 2013, 20, 480–489. [Google Scholar]
- Hu-Lowe, D.D.; Chen, E.; Zhang, L.; Watson, K.D.; Mancuso, P.; Lappin, P.; Wickman, G.; Chen, J.H.; Wang, J.; Jiang, X.; et al. Targeting activin receptor-like kinase 1 inhibits angiogenesis and tumorigenesis through a mechanism of action complementary to anti-VEGF therapies. Cancer Res 2011, 71, 1362–1373. [Google Scholar]
- Van Meeteren, L.A.; Thorikay, M.; Bergqvist, S.; Pardali, E.; Stampino, C.G.; Hu-Lowe, D.; Goumans, M.J.; ten Dijke, P. Anti-human activin receptor-like kinase 1 (ALK1) antibody attenuates bone morphogenetic protein 9 (BMP9)-induced ALK1 signaling and interferes with endothelial cell sprouting. J. Biol. Chem 2012, 287, 18551–18561. [Google Scholar]
- Hanahan, D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature 1985, 315, 115–122. [Google Scholar]
- Cunha, S.I.; Pardali, E.; Thorikay, M.; Anderberg, C.; Hawinkels, L.; Goumans, M.J.; Seehra, J.; Heldin, C.H.; ten Dijke, P.; Pietras, K. Genetic and pharmacological targeting of activin receptor-like kinase 1 impairs tumor growth and angiogenesis. J. Exp. Med 2010, 207, 85–100. [Google Scholar]
- Sun, H.C.; Tang, Z.Y. Angiogenesis in hepatocellular carcinoma: The retrospectives and perspectives. J. Cancer Res. Clin. Oncol 2004, 130, 307–319. [Google Scholar]
- Maegdefrau, U.; Bosserhoff, A.K. BMP activated Smad signaling strongly promotes migration and invasion of hepatocellular carcinoma cells. Exp. Mol. Pathol 2012, 92, 74–81. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Herrera, B.; Dooley, S.; Breitkopf-Heinlein, K. Potential Roles of Bone Morphogenetic Protein (BMP)-9 in Human Liver Diseases. Int. J. Mol. Sci. 2014, 15, 5199-5220. https://doi.org/10.3390/ijms15045199
Herrera B, Dooley S, Breitkopf-Heinlein K. Potential Roles of Bone Morphogenetic Protein (BMP)-9 in Human Liver Diseases. International Journal of Molecular Sciences. 2014; 15(4):5199-5220. https://doi.org/10.3390/ijms15045199
Chicago/Turabian StyleHerrera, Blanca, Steven Dooley, and Katja Breitkopf-Heinlein. 2014. "Potential Roles of Bone Morphogenetic Protein (BMP)-9 in Human Liver Diseases" International Journal of Molecular Sciences 15, no. 4: 5199-5220. https://doi.org/10.3390/ijms15045199



