Carvacrol and trans-Cinnamaldehyde Reduce Clostridium difficile Toxin Production and Cytotoxicity in Vitro
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
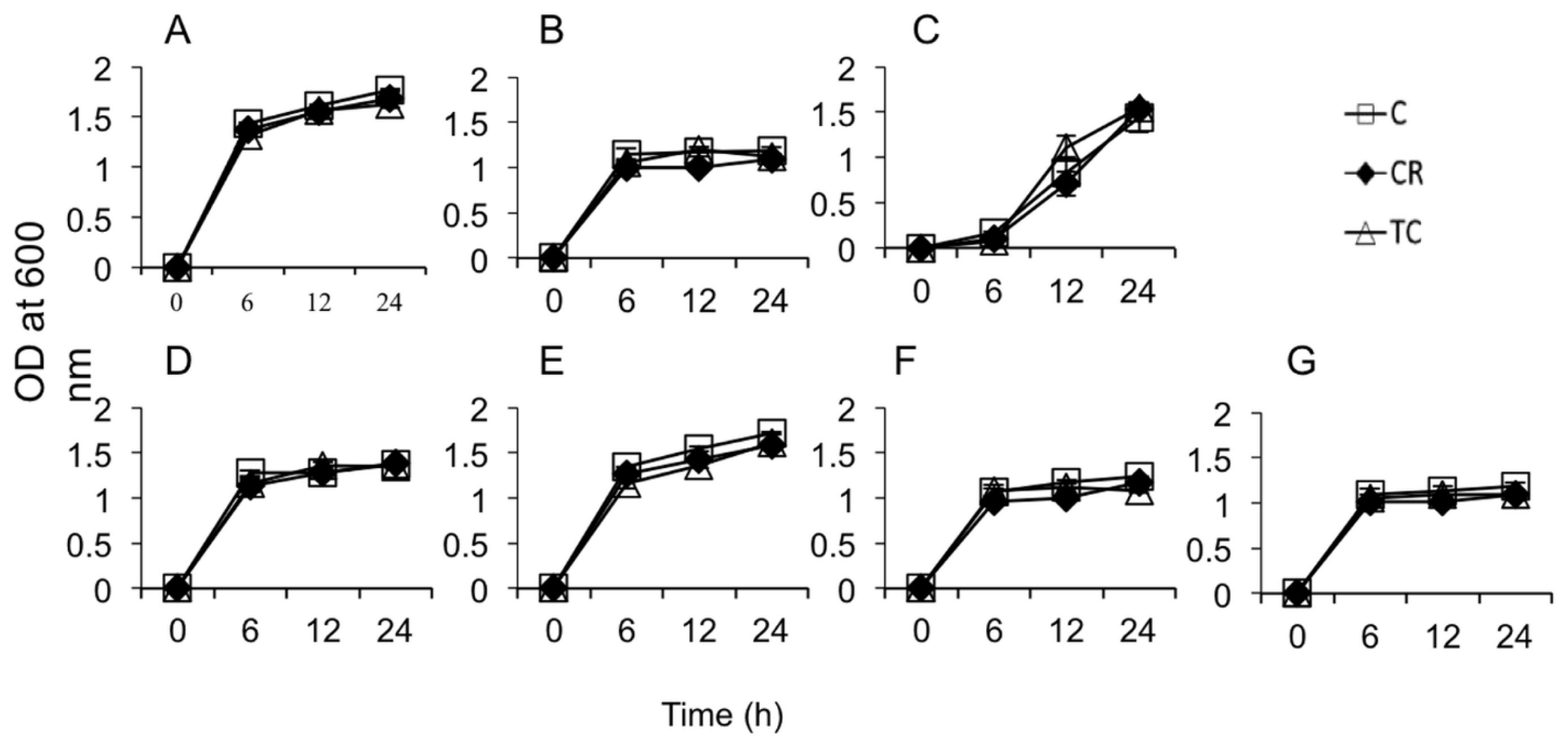
2.1.1. Sub-Inhibitory Concentrations of CR and TC
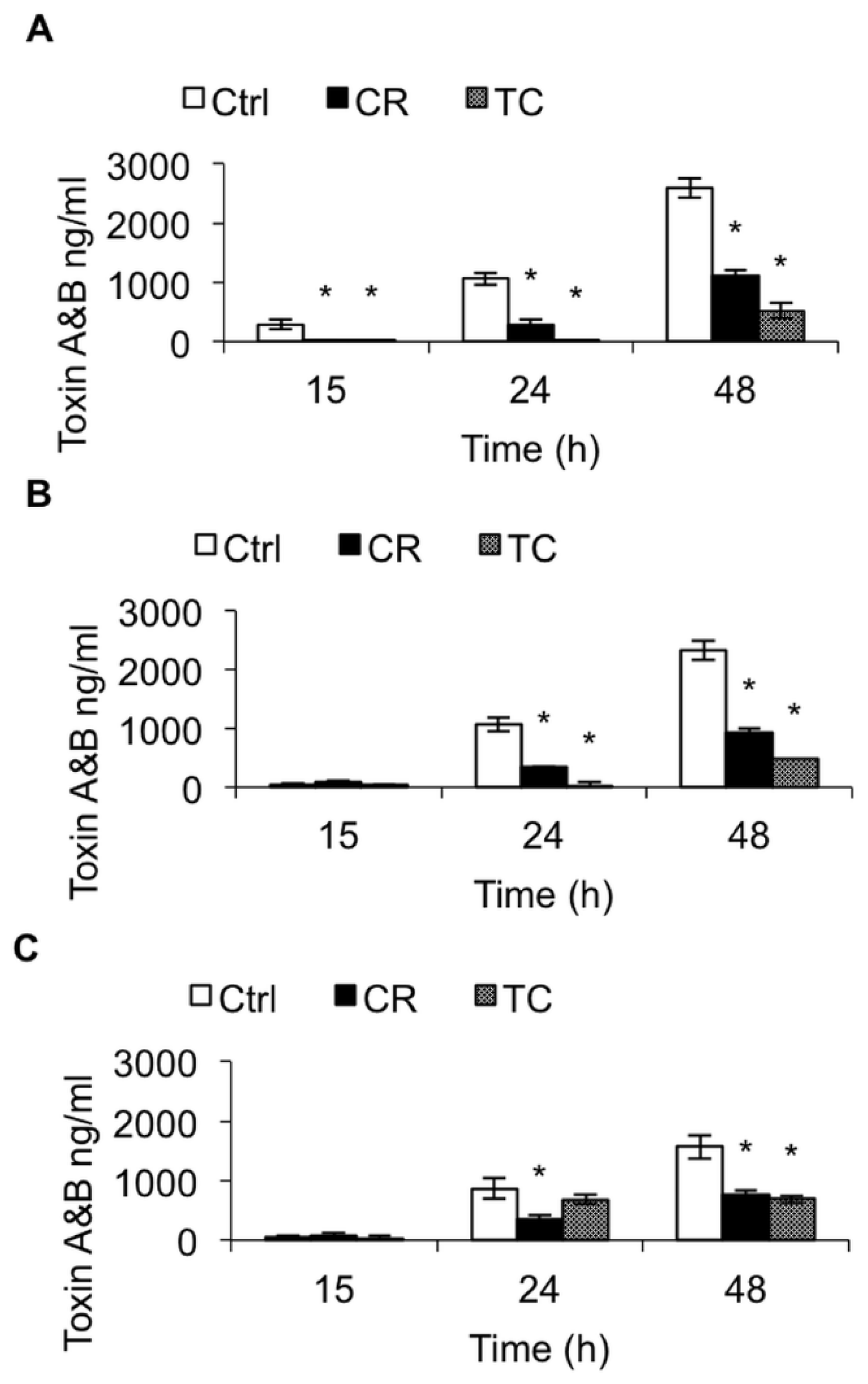
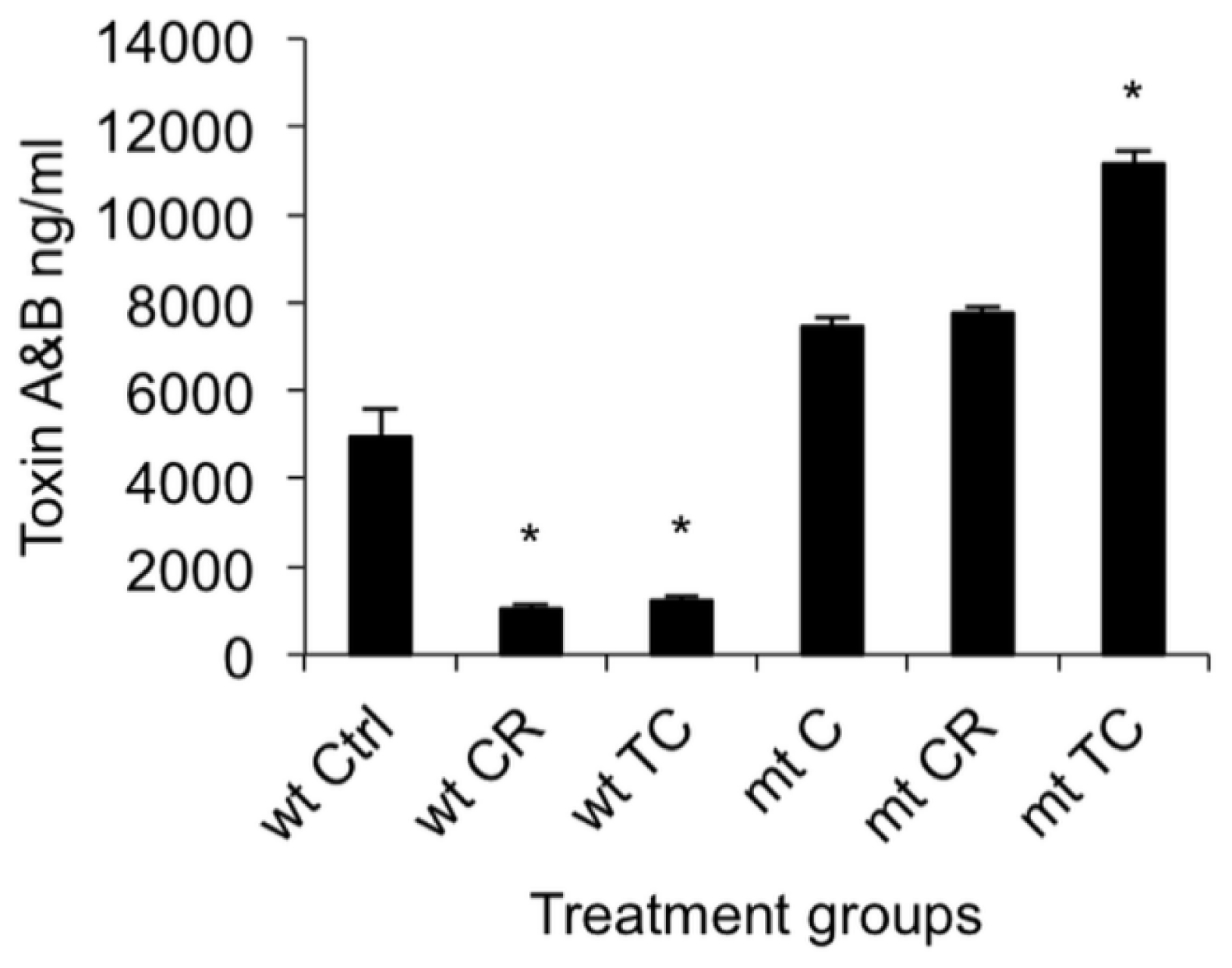
2.1.2. Effect of CR and TC on C. difficile Toxin Production
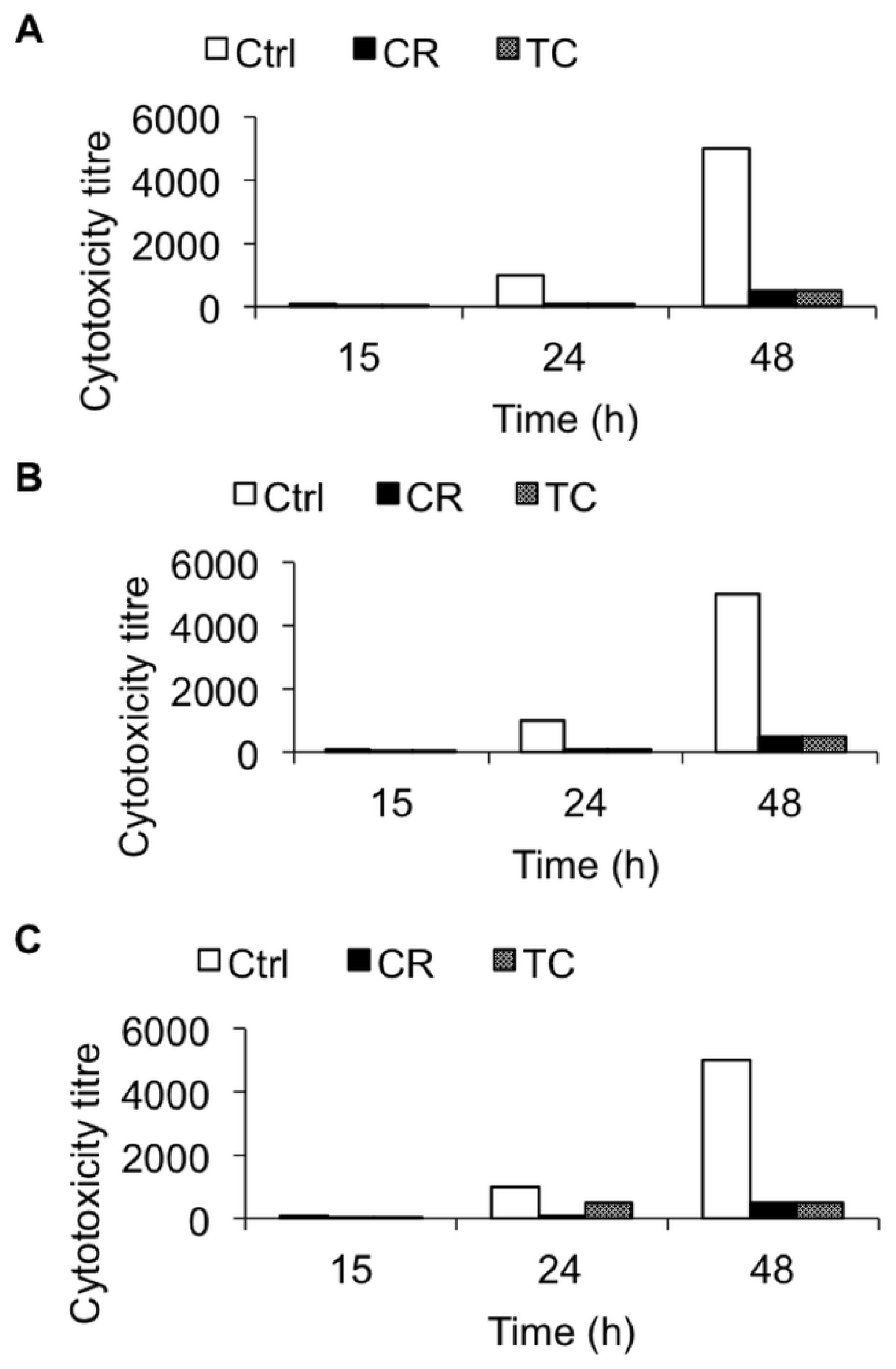
2.1.3. Effect of CR and TC on C. difficile Toxin-Mediated Cytotoxicity
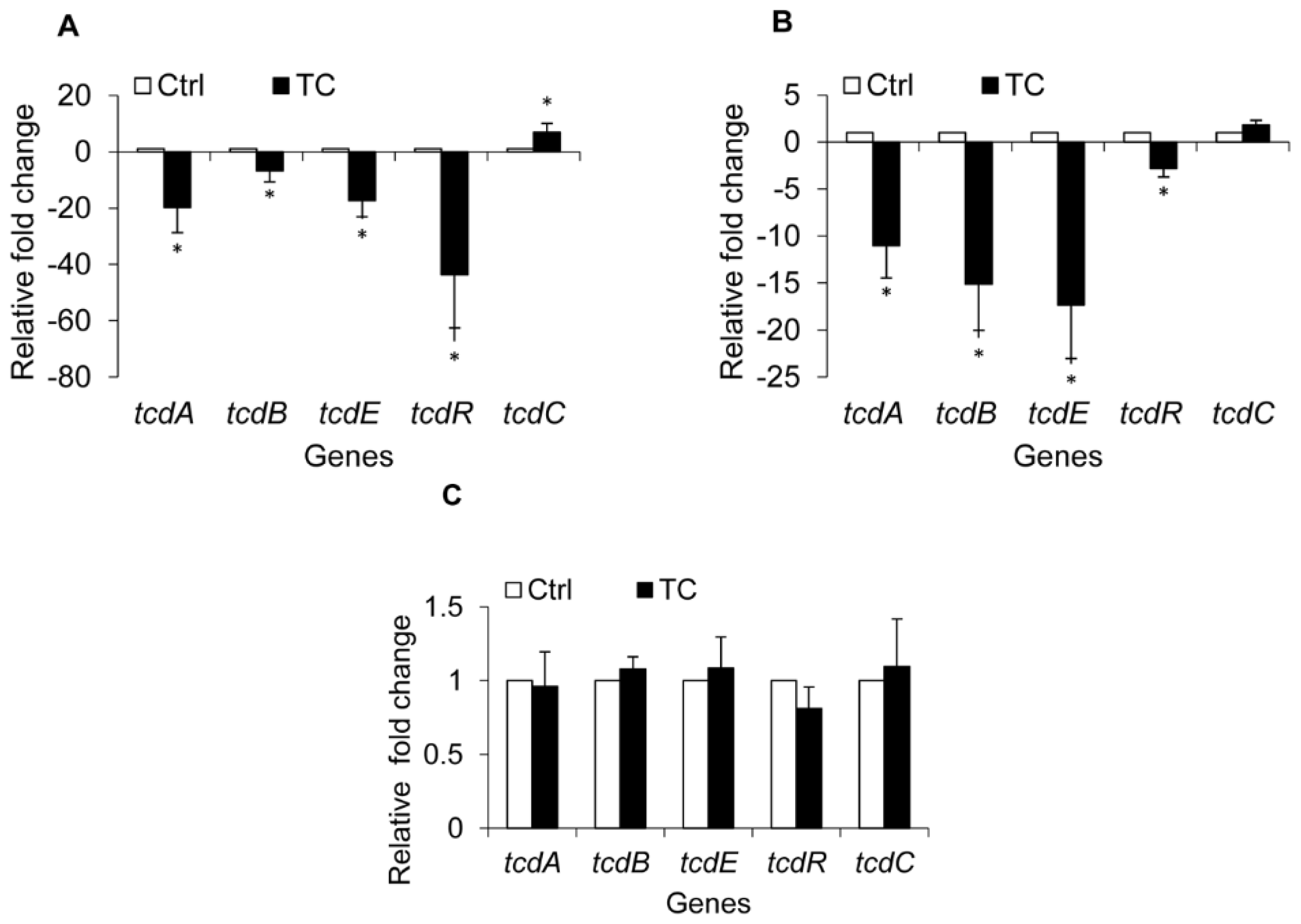
2.1.4. Effect of CR and TC on C. difficile Toxin Genes
2.1.5. Effect of CR and TC on a codY Mutant of C. difficile
2.2. Discussion
3. Experimental Section
3.1. Bacterial Strains and Culture Conditions
3.2. Plant Compounds and SIC Determination
3.3. Effect of Plant Compounds on C. difficile Toxin Production and Cytotoxicity
3.4. ELISA for Total Toxin A and B
3.5. Cytotoxicity Assay
3.6. Real Time Quantitative PCR (RT-qPCR)
3.7. Construction of a codY Mutant of C. difficile Strain UK-1 and the Effect of Plant Compounds on codY Mutant and Parental Strains
3.8. Statistical Analysis
4. Conclusions
Supplementary Information
ijms-15-04415-s001.pdfAcknowledgments
Conflicts of Interest
- Author ContributionsKumar Venkitanarayanan conceived the idea. Kumar Venkitanarayanan and Shankumar Mooyottu designed the experiment. Shankumar Mooyottu, Anup Kollanoor-Johny, Genevieve Flock and Abhinav Upadhyay performed the experiment. Laurent Bouillaut and Abraham Sonenshein designed and constructed the mutant. Anup Kollanoor-Johny and Shankumar Mooyottu analysed the data. Shankumar Mooyottu and Kumar Venkitanarayanan wrote the manuscript.
References
- Bartlett, J.G. Clostridium difficile infection: Pathophysiology and diagnosis. Semin. Gastrointest. Dis. 1997, 8, 12–21. [Google Scholar]
- Weese, J.S. Clostridium difficile in food—Innocent bystander or serious threat? Clin. Microbiol. Infect. 2010, 16, 3–10. [Google Scholar]
- Ghose, C.; Kalsy, A.; Sheikh, A.; Rollenhagen, J.; John, M.; Young, J.; Rollins, S.M.; Qadri, F.; Calderwood, S.B.; Kelly, C.P.; et al. Transcutaneous immunization with Clostridium difficile toxoid A induces systemic and mucosal immune responses and toxin a-neutralizing antibodies in mice. Infect. Immun. 2007, 75, 2826–2832. [Google Scholar]
- Wilkins, T.D.; Lyerly, D.M. Clostridium difficile testing: After 20 years still challenging. J. Clin. Microbiol. 2003, 41, 531–534. [Google Scholar]
- Blossom, D.B.; McDonald, L.C. The challenges posed by reemerging Clostridium difficile infection. Clin. Infect. Dis. 2007, 45, 222–227. [Google Scholar]
- Hookman, P.; Barkin, J.S. Clostridium difficile associated infection diarrhea and colitis. World J. Gastroenterol. 2009, 15, 1554–1580. [Google Scholar]
- Sunenshine, R.H.; McDonald, L.C. Clostridium difficile-associated disease: New challenges from an established pathogen. Clevel. Clin. J. Med. 2006, 73, 187–197. [Google Scholar]
- Dial, S.; Delaney, J.A.; Barkun, A.N.; Suissa, S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA 2005, 294, 2989–2995. [Google Scholar]
- Keel, M.K.; Songer, J.G. The comparative pathology of Clostridium difficile-associated disease. Vet. Pathol. 2006, 43, 225–240. [Google Scholar]
- Von Eichel-Streiber, C.; Zec-Pirnat, I.; Grabnar, M.; Rupnik, M. A nonsense mutation abrogates production of a functional enterotoxin A in Clostridium difficile toxinotype VIII strains of serogroups F and X. FEMS Microbiol. Lett. 1999, 178, 163–168. [Google Scholar]
- McDonald, L.C.; Owings, M.; Jernigan, D.B. Clostridium difficile infection in patients discharged from US short-stay hospitals 1996–2003. Emerg. Infect. Dis. 2006, 12, 409–415. [Google Scholar]
- Antunes, A.; Martin-Verstraete, I.; Dupuy, B. CcpA-mediated repression of Clostridium difficile toxin gene expression. Mol. Microbiol. 2011, 79, 882–899. [Google Scholar]
- Antunes, A.; Camiade, E.; Monot, M.; Courtois, E.; Barbut, F.; Sernova, N.V.; Rodionov, D.A.; Martin-Verstraete, I.; Dupuy, B. Global transcriptional control by glucose and carbon regulator CcpA in Clostridium difficile. Nucleic Acids Res. 2012, 40, 10701–10718. [Google Scholar]
- Dineen, S.S.; McBride, S.M.; Sonenshein, A.L. Integration of metabolism and virulence by Clostridium difficile CodY. J. Bacteriol. 2010, 192, 5350–5362. [Google Scholar]
- Dineen, S.S.; Villapakkam, A.C.; Nordman, J.T.; Sonenshein, A.L. Repression of Clostridium difficile toxin gene expression by CodY. Mol. Microbiol. 2007, 66, 206–219. [Google Scholar]
- Bartlett, J.G. Antibiotic-associated diarrhea. Clin. Infect. Dis. 1992, 15, 573–581. [Google Scholar]
- O’Connor, K.A.; Kingston, M.; O’Donovan, M.; Cryan, B.; Twomey, C.; O’Mahony, D. Antibiotic prescribing policy and Clostridium difficile diarrhoea. QJM 2004, 97, 423–429. [Google Scholar]
- Prabaker, K.; Weinstein, R.A. Trends in antimicrobial resistance in intensive care units in the United States. Curr. Opin. Crit. Care 2011, 17, 472–479. [Google Scholar]
- Spigaglia, P.; Barbanti, F.; Mastrantonio, P. European study group on Clostridium difficile (ESGCD) Multidrug resistance in European Clostridium difficile clinical isolates. J. Antimicrob. Chemother. 2011, 66, 2227–2234. [Google Scholar]
- Johny, A.K.; Hoagland, T.; Venkitanarayanan, K. Effect of Subinhibitory concentrations of plant-derived molecules in increasing the sensitivity of multidrug-resistant salmonella enterica serovar typhimurium DT104 to antibiotics. Foodborne Pathog. Dis. 2010, 7, 1165–1170. [Google Scholar]
- Baser, K.H. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des. 2008, 14, 3106–3119. [Google Scholar]
- Amalaradjou, M.A.; Narayanan, A.; Venkitanarayanan, K. Trans-cinnamaldehyde decreases attachment and invasion of uropathogenic escherichia coli in urinary tract epithelial cells by modulating virulence gene expression. J. Urol. 2011, 185, 1526–1531. [Google Scholar]
- Amalaradjou, M.A.; Narayanan, A.; Baskaran, S.A.; Venkitanarayanan, K. Antibiofilm effect of trans-cinnamaldehyde on uropathogenic Escherichia coli. J. Urol. 2010, 184, 358–363. [Google Scholar]
- Merrigan, M.; Venugopal, A.; Mallozzi, M.; Roxas, B.; Viswanathan, V.K.; Johnson, S.; Gerding, D.N.; Vedantam, G. Human hypervirulent Clostridium difficile strains exhibit increased sporulation as well as robust toxin production. J. Bacteriol. 2010, 192, 4904–4911. [Google Scholar]
- Baines, S.D.; Freeman, J.; Wilcox, M.H. Effects of piperacillin/tazobactam on Clostridium difficile growth and toxin production in a human gut model. J. Antimicrob. Chemother. 2005, 55, 974–982. [Google Scholar]
- He, D.; Sougioultzis, S.; Hagen, S.; Liu, J.; Keates, S.; Keates, A.C.; Pothoulakis, C.; Lamont, J.T. Clostridium difficile toxin A triggers human colonocyte IL-8 release via mitochondrial oxygen radical generation. Gastroenterology 2002, 122, 1048–1057. [Google Scholar]
- Feltis, B.A.; Wiesner, S.M.; Kim, A.S.; Erlandsen, S.L.; Lyerly, D.L.; Wilkins, T.D.; Wells, C.L. Clostridium difficile toxins A and B can alter epithelial permeability and promote bacterial paracellular migration through HT-29 enterocytes. Shock 2000, 14, 629–634. [Google Scholar]
- Castagliuolo, I.; Keates, A.C.; Wang, C.C.; Pasha, A.; Valenick, L.; Kelly, C.P.; Nikulasson, S.T.; LaMont, J.T.; Pothoulakis, C. Clostridium difficile toxin A stimulates macrophage-inflammatory protein-2 production in rat intestinal epithelial cells. J. Immunol. 1998, 160, 6039–6045. [Google Scholar]
- Kelly, C.P.; Becker, S.; Linevsky, J.K.; Joshi, M.A.; O’Keane, J.C.; Dickey, B.F.; LaMont, J.T.; Pothoulakis, C. Neutrophil recruitment in Clostridium difficile toxin A enteritis in the rabbit. J. Clin. Investig. 1994, 93, 1257–1265. [Google Scholar]
- Rasko, D.A.; Sperandio, V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 2010, 9, 117–128. [Google Scholar]
- Cegelski, L.; Marshall, G.R.; Eldridge, G.R.; Hultgren, S.J. The biology and future prospects of antivirulence therapies. Nat. Rev. Microbiol. 2008, 6, 17–27. [Google Scholar]
- Hung, D.T.; Shakhnovich, E.A.; Pierson, E.; Mekalanos, J.J. Small-molecule inhibitor of vibrio cholerae virulence and intestinal colonization. Science 2005, 310, 670–674. [Google Scholar]
- Hughes, D.T.; Sperandio, V. Inter-Kingdom signalling: Communication between bacteria and their hosts. Nat. Rev. Microbiol. 2008, 6, 111–120. [Google Scholar]
- Tsui, W.H.; Yim, G.; Wang, H.H.; McClure, J.E.; Surette, M.G.; Davies, J. Dual effects of MLS antibiotics: Transcriptional modulation and interactions on the ribosome. Chem. Biol. 2004, 11, 1307–1316. [Google Scholar]
- Rupa, P.; Mine, Y. Recent advances in the role of probiotics in human inflammation and gut health. J. Agric. Food Chem. 2012, 60, 8249–8256. [Google Scholar]
- Jamroz, D.; Wiliczkiewicz, A.; Wertelecki, T.; Orda, J.; Skorupinska, J. Use of active substances of plant origin in chicken diets based on maize and locally grown cereals. Br. Poult. Sci. 2005, 46, 485–493. [Google Scholar]
- Si, W.; Gong, J.; Chanas, C.; Cui, S.; Yu, H.; Caballero, C.; Friendship, R.M. In Vitro assessment of antimicrobial activity of carvacrol thymol and cinnamaldehyde towards salmonella serotype typhimurium DT104: Effects of pig diets and emulsification in hydrocolloids. J. Appl. Microbiol. 2006, 101, 1282–1291. [Google Scholar]
- Ultee, A.; Smid, E.J. Influence of carvacrol on growth and toxin production by bacillus cereus. Int. J. Food Microbiol. 2001, 64, 373–378. [Google Scholar]
- Upadhyay, A.; Johny, A.K.; Amalaradjou, M.A.; Ananda Baskaran, S.; Kim, K.S.; Venkitanarayanan, K. Plant-derived antimicrobials reduce listeria monocytogenes virulence factors in vitro and down-regulate expression of virulence genes. Int. J. Food Microbiol. 2012, 157, 88–94. [Google Scholar]
- Mani, N.; Lyras, D.; Barroso, L.; Howarth, P.; Wilkins, T.; Rood, J.I.; Sonenshein, A.L.; Dupuy, B. Environmental response and autoregulation of Clostridium difficile TxeR a sigma factor for toxin gene expression. J. Bacteriol. 2002, 184, 5971–5978. [Google Scholar]
- Guedon, E.; Serror, P.; Ehrlich, S.D.; Renault, P.; Delorme, C. Pleiotropic transcriptional repressor CodY senses the intracellular pool of branched-chain amino acids in lactococcus lactis. Mol. Microbiol. 2001, 40, 1227–1239. [Google Scholar]
- Pohl, K.; Francois, P.; Stenz, L.; Schlink, F.; Geiger, T.; Herbert, S.; Goerke, C.; Schrenzel, J.; Wolz, C. CodY in staphylococcus aureus: A regulatory link between metabolism and virulence gene expression. J. Bacteriol. 2009, 191, 2953–2963. [Google Scholar]
- Stenz, L.; Francois, P.; Whiteson, K.; Wolz, C.; Linder, P.; Schrenzel, J. The CodY pleiotropic repressor controls virulence in gram-positive pathogens. FEMS Immunol. Med. Microbiol. 2011, 62, 123–139. [Google Scholar]
- Rivera, F.E.; Miller, H.K.; Kolar, S.L.; Stevens, S.M., Jr; Shaw, L.N. The impact of CodY on virulence determinant production in community-associated methicillin-resistant staphylococcus aureus. Proteomics 2012, 12, 263–268. [Google Scholar]
- Montgomery, C.P.; Boyle-Vavra, S.; Roux, A.; Ebine, K.; Sonenshein, A.L.; Daum, R.S. CodY deletion enhances in vivo virulence of community-associated methicillin-resistant staphylococcus aureus clone USA300. Infect. Immun. 2012, 80, 2382–2389. [Google Scholar]
- Vohra, P.; Poxton, I.R. Comparison of toxin and spore production in clinically relevant strains of Clostridium difficile. Microbiology 2011, 157, 1343–1353. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar]
- Francis, M.B.; Allen, C.A.; Shrestha, R.; Sorg, J.A. Bile acid recognition by the Clostridium difficile germinant receptor CspC is important for establishing infection. PLoS Pathog. 2013, 9, e1003356. [Google Scholar]
- Bouillaut, L.; Self, W.T.; Sonenshein, A.L. Proline-dependent regulation of Clostridium difficile stickland metabolism. J. Bacteriol. 2013, 195, 844–854. [Google Scholar]
- Carter, G.P.; Douce, G.R.; Govind, R.; Howarth, P.M.; Mackin, K.E.; Spencer, J.; Buckley, A.M.; Antunes, A.; Kotsanas, D.; Jenkin, G.A.; et al. The anti-sigma factor TcdC modulates hypervirulence in an epidemic BI/NAP1/027 clinical isolate of Clostridium difficile. PLoS Pathog. 2011, 7, e1002317. [Google Scholar]


© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mooyottu, S.; Kollanoor-Johny, A.; Flock, G.; Bouillaut, L.; Upadhyay, A.; Sonenshein, A.L.; Venkitanarayanan, K. Carvacrol and trans-Cinnamaldehyde Reduce Clostridium difficile Toxin Production and Cytotoxicity in Vitro. Int. J. Mol. Sci. 2014, 15, 4415-4430. https://doi.org/10.3390/ijms15034415
Mooyottu S, Kollanoor-Johny A, Flock G, Bouillaut L, Upadhyay A, Sonenshein AL, Venkitanarayanan K. Carvacrol and trans-Cinnamaldehyde Reduce Clostridium difficile Toxin Production and Cytotoxicity in Vitro. International Journal of Molecular Sciences. 2014; 15(3):4415-4430. https://doi.org/10.3390/ijms15034415
Chicago/Turabian StyleMooyottu, Shankumar, Anup Kollanoor-Johny, Genevieve Flock, Laurent Bouillaut, Abhinav Upadhyay, Abraham L. Sonenshein, and Kumar Venkitanarayanan. 2014. "Carvacrol and trans-Cinnamaldehyde Reduce Clostridium difficile Toxin Production and Cytotoxicity in Vitro" International Journal of Molecular Sciences 15, no. 3: 4415-4430. https://doi.org/10.3390/ijms15034415
APA StyleMooyottu, S., Kollanoor-Johny, A., Flock, G., Bouillaut, L., Upadhyay, A., Sonenshein, A. L., & Venkitanarayanan, K. (2014). Carvacrol and trans-Cinnamaldehyde Reduce Clostridium difficile Toxin Production and Cytotoxicity in Vitro. International Journal of Molecular Sciences, 15(3), 4415-4430. https://doi.org/10.3390/ijms15034415



