The Anticoagulant Effect of PGI2S and tPA in Transgenic Umbilical Vein Endothelial Cells Is Linked to Up-Regulation of PKA and PKC
Abstract
:1. Introduction
2. Results
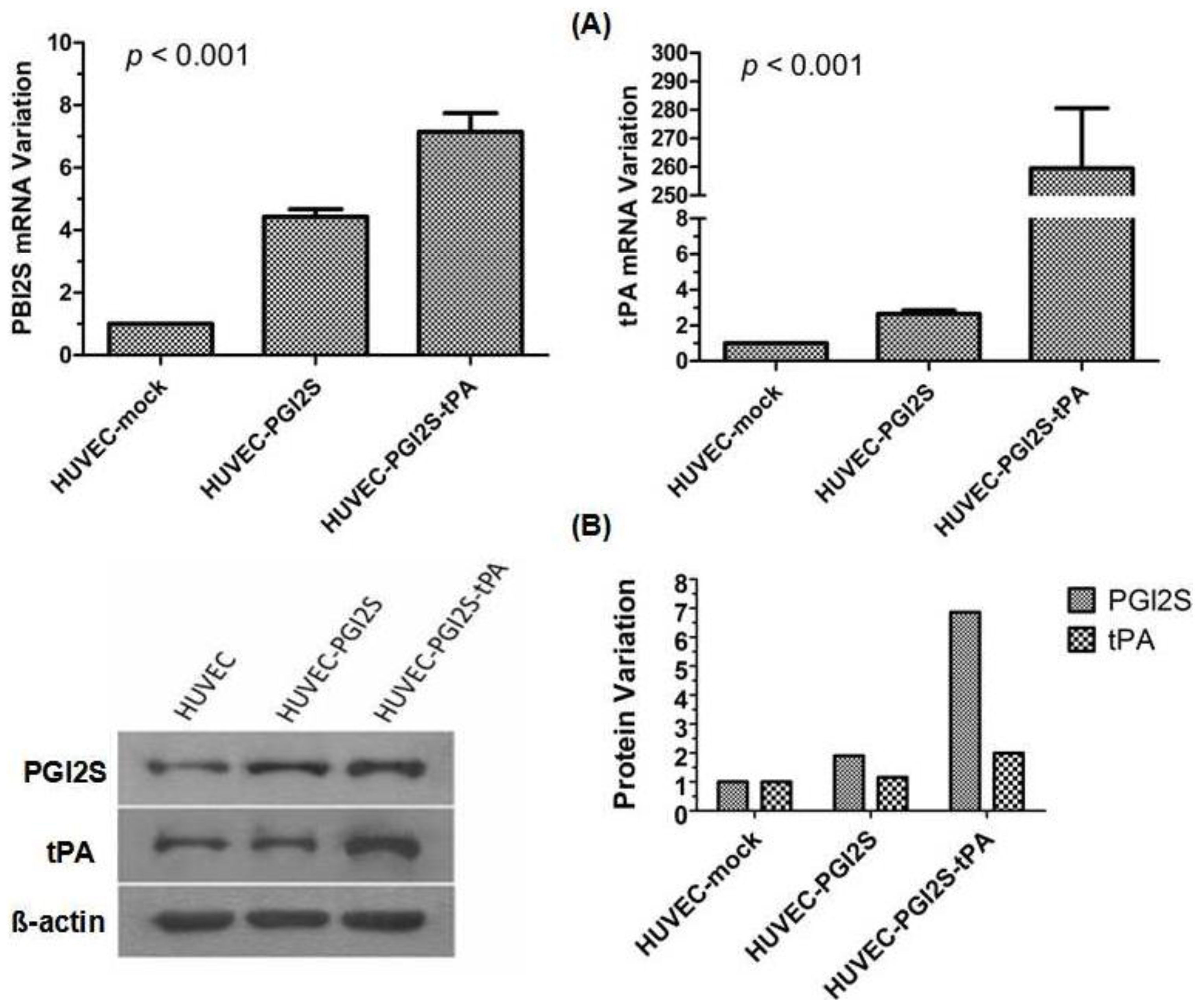
2.1. PGI2S and tPA Are Over-Expressed in HUVEC-PGI2S and HUVEC-PGI2S-tPA Cell Lines
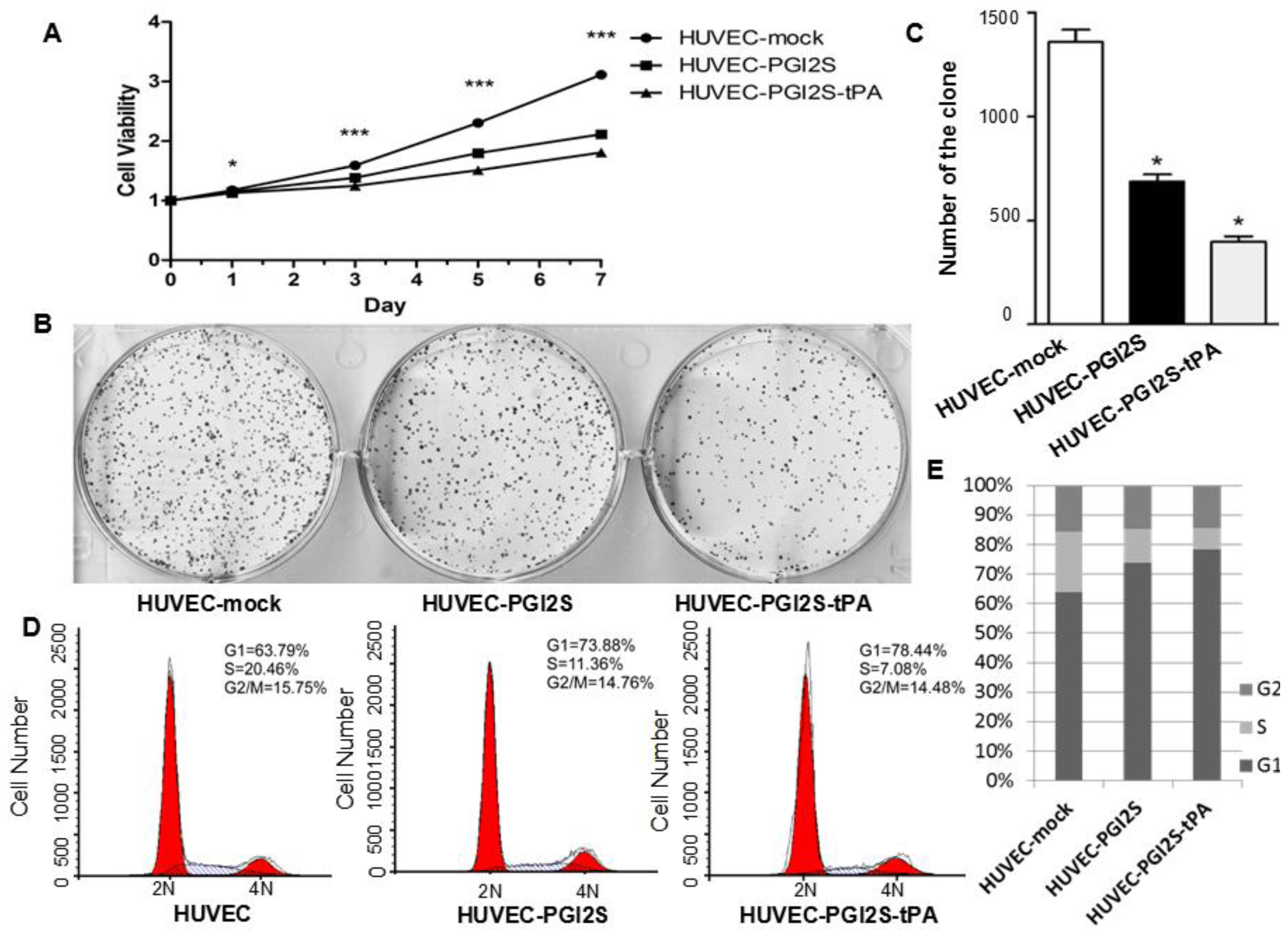
2.2. Cell Growth Is Restricted in HUVEC-PGI2S and HUVEC-PGI2S-tPA Cell Lines
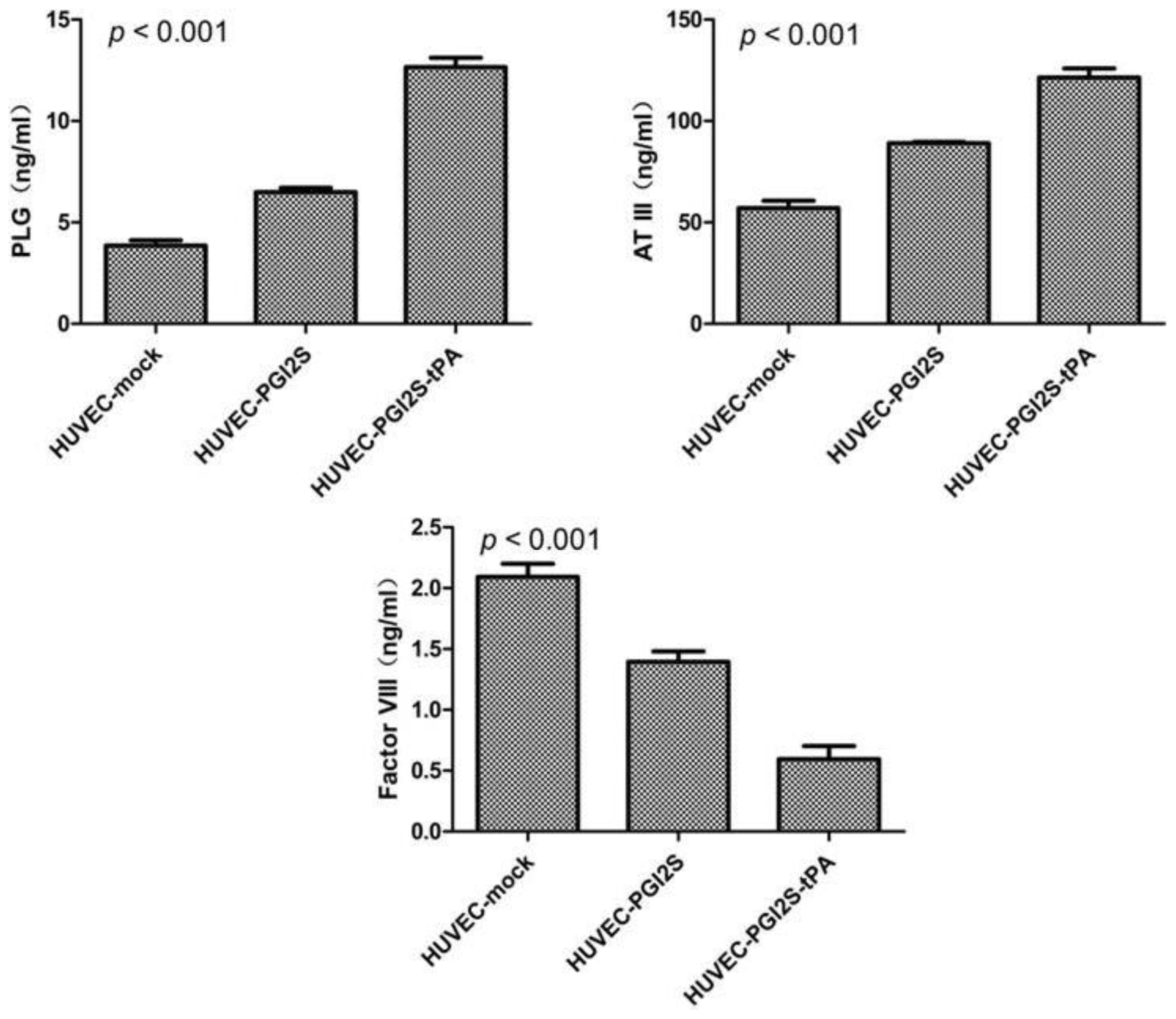
2.3. HUVEC-PGI2S and HUVEC-PGI2S-tPA Cell Lines Expressed Higher Levels of Anticoagulation Factors
2.4. Signaling Pathways Involved in Vasodilation and Platelet Disaggregation Are Activated in HUVEC-PGI2S and HUVEC-PGI2S-tPA Cell Lines
3. Discussion
4. Experimental Section
4.1. Cell Culture
4.2. Plasmid Construction
4.3. RNA Preparation and Quantitative Real-Time PCR (qRT-PCR)
4.4. Western Blotting
4.5. Cell Proliferation Assays
4.6. Plate Colony Formation Assays
4.7. Cell Cycle Analysis
4.8. Enzyme-Linked Immunosorbent Assays
5. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsJian-Hua Wang contributed to the study design, data collection and analysis, manuscript writing and revisions to the manuscript. Lin-Jing Yuan and Zhi-Min Zhong contributed to the study design, experimentation and manuscript writing. Zhe-Sheng Wen contributed to the study design, data collection and analysis. Jian-Ming Deng and Rong-Xin Liang contributed to the experimentation. Min Zheng had the idea of the study, was responsible for its design and coordination, contributed to analysis and interpretation of the data, as well as manuscript writing and revisions to the manuscript. All authors read and approved the final manuscript.
References
- Mehta, N.J.; Khan, I.A. Cardiology’s 10 greatest discoveries of the 20th century. Tex. Heart Inst. J 2002, 29, 164–171. [Google Scholar]
- Goetz, R.H.; Rohman, M.; Haller, J.D.; Dee, R.; Rosenak, S.S. Internal mammary-coronary artery anastomosis: A nonsuture method employing tantalum rings. J. Thorac. Cardiovasc. Surg 1961, 41, 378–386. [Google Scholar]
- Jeremy, J.Y.; Zacharowski, K.; Shukla, N.; Wan, S. Pharmacological strategies aimed at reducing complications associated with coronary artery bypass graft surgery. Curr. Opin. Pharmacol 2012, 12, 111–113. [Google Scholar]
- Englberger, L.; Noti, J.; Immer, F.F.; Stalder, M.; Eckstein, F.S.; Carrel, T.P. The shelhigh no-react bovine internal mammary artery: A questionable alternative conduit in coronary bypass surgery? Eur. J. Cardiothorac. Surg 2008, 33, 222–224. [Google Scholar]
- Cleary, M.A.; Geiger, E.; Grady, C.; Best, C.; Naito, Y.; Breuer, C. Vascular tissue engineering: The next generation. Trends Mol. Med 2012, 18, 394–404. [Google Scholar]
- Kim, F.Y.; Marhefka, G.; Ruggiero, N.J.; Adams, S.; Whellan, D.J. Saphenous vein graft disease: Review of pathophysiology, prevention, and treatment. Cardiol. Rev 2013, 21, 101–109. [Google Scholar]
- Herring, M.; Gardner, A.; Glover, J. Seeding endothelium onto canine arterial prostheses: The effects of graft design. Arch. Surg 1979, 114, 679–682. [Google Scholar]
- Herring, M.B.; Dilley, R.; Jersild, R.A., Jr.; Boxer, L.; Gardner, A.; Glover, J. Seeding arterial prostheses with vascular endothelium: The nature of the lining. Ann. Surg 1979, 190, 84–90. [Google Scholar]
- LaFayette, N.G.; Skrzypchak, A.M.; Merz, S.; Bartlett, R.H.; Annich, G.M. An in vitro method for assessing biomaterial-associated platelet activation. ASAIO J 2007, 53, 159–162. [Google Scholar]
- McGuigan, A.P.; Sefton, M.V. The influence of biomaterials on endothelial cell thrombogenicity. Biomaterials 2007, 28, 2547–2571. [Google Scholar]
- Bednar, F.; Tencer, T.; Plasil, P.; Paluch, Z.; Sadilkova, L.; Prucha, M.; Kopa, M. Evaluation of aspirin’s effect on platelet function early after coronary artery bypass grafting. J. Cardiothorac. Vasc. Anesth 2012, 26, 575–580. [Google Scholar]
- Balyasnikova, I.V.; Danilov, S.M.; Muzykantov, V.R.; Fisher, A.B. Modulation of angiotensin-converting enzyme in cultured human vascular endothelial cells. In Vitro Cell. Dev. Biol 1998, 34, 545–554. [Google Scholar]
- Dichek, D.A.; Neville, R.F.; Zwiebel, J.A.; Freeman, S.M.; Leon, M.B.; Anderson, W.F. Seeding of intravascular stents with genetically engineered endothelial cells. Circulation 1989, 80, 1347–1353. [Google Scholar]
- Degen, S.J.; Rajput, B.; Reich, E. The human tissue plasminogen activator gene. J. Biol. Chem 1986, 261, 6972–6985. [Google Scholar]
- MacDonald, M.E.; van Zonneveld, A.J.; Pannekoek, H. Functional analysis of the human tissue-type plasminogen activator protein: The light chain. Gene 1986, 42, 59–67. [Google Scholar]
- Wu, K.K.; Liou, J.Y. Cellular and molecular biology of prostacyclin synthase. Biochem. Biophys. Res. Commun 2005, 338, 45–52. [Google Scholar]
- Nakayama, T. Prostacyclin analogues: Prevention of cardiovascular diseases. Cardiovasc. Hematol. Agents Med. Chem 2006, 4, 351–359. [Google Scholar]
- Brash, A.R. Mechanistic aspects of CYP74 allene oxide synthases and related cytochrome P450 enzymes. Phytochemistry 2009, 70, 1522–1531. [Google Scholar]
- Nakayama, T.; Soma, M.; Watanabe, Y.; Hasimu, B.; Sato, M.; Aoi, N.; Kosuge, K.; Kansmatsuse, K.; Kokubun, S.; Marrow, J.D.; et al. Splicing mutation of the prostacyclin synthase gene in a family associated with hypertension. Biochem. Biophys. Res. Commun 2002, 297, 1135–1139. [Google Scholar]
- Suhara, H.; Sawa, Y.; Fukushima, N.; Kagisaki, K.; Yokoyama, C.; Tanabe, T.; Ohtake, S.; Matsuda, H. Gene transfer of human prostacyclin synthase into the liver is effective for the treatment of pulmonary hypertension in rats. J. Thorac. Cardiovasc. Surg 2002, 123, 855–861. [Google Scholar]
- Dengler, T.J.; Johnson, D.R.; Pober, J.S. Human vascular endothelial cells stimulate a lower frequency of alloreactive CD8+ pre-CTL and induce less clonal expansion than matching B lymphoblastoid cells: Development of a novel limiting dilution analysis method based on CFSE labeling of lymphocytes. J. Immunol 2001, 166, 3846–3854. [Google Scholar]
- Waldman, W.J.; Knight, D.A.; Adams, P.W.; Orosz, C.G.; Sedmak, D.D. In vitro induction of endothelial HLA class II antigen expression by cytomegalovirus-activated CD4+ T cells. Transplantation 1993, 56, 1504–1512. [Google Scholar]
- Grundy, C.B.; Thomas, F.; Millar, D.S.; Krawczak, M.; Melissari, E.; Lindo, V.; Moffat, E.; Kakkar, V.V.; Cooper, D.N. Recurrent deletion in the human antithrombin III gene. Blood 1991, 78, 1027–1032. [Google Scholar]
- Blajchman, M.A.; Austin, R.C.; Fernandez-Rachubinski, F.; Sheffield, W.P. Molecular basis of inherited human antithrombin deficiency. Blood 1992, 80, 2159–2171. [Google Scholar]
- Labarrere, C.A.; Pitts, D.; Halbrook, H.; Faulk, W.P. Natural anticoagulant pathways in normal and transplanted human hearts. J. Heart Lung Transplant 1992, 11, 342–347. [Google Scholar]
- Aoki, N.; Moroi, M.; Sakata, Y.; Yoshida, N.; Matsuda, M. Abnormal plasminogen: A hereditary molecular abnormality found in a patient with recurrent thrombosis. J. Clin. Invest 1978, 61, 1186–1195. [Google Scholar]
- Petersen, T.E.; Martzen, M.R.; Ichinose, A.; Davie, E.W. Characterization of the gene for human plasminogen, a key proenzyme in the fibrinolytic system. J. Biol. Chem 1990, 265, 6104–6111. [Google Scholar]
- Della-Morte, D.; Beecham, A.; Dong, C.; Wang, L.; McClendon, M.S.; Gardener, H.; Blanton, S.H.; Sacco, R.L.; Rundek, T. Association between variations in coagulation system genes and carotid plaque. J. Neurol. Sci 2012, 323, 93–98. [Google Scholar]
- Starke, R.D.; Paschalaki, K.E.; Dyer, C.E.; Harrison-Lavoie, K.J.; Cutler, J.A.; McKinnon, T.A.J.; Millar, C.M.; Cutler, D.F.; Laffan, M.A.; Randi, A.M. Cellular and molecular basis of von Willebrand disease: Studies on blood outgrowth endothelial cells. Blood 2013, 121, 2773–2784. [Google Scholar]
- Gliki, G.; Abu-Ghazaleh, R.; Jezequel, S.; Wheeler-Jones, C.; Zachary, I. Vascular endothelial growth factor-induced prostacyclin production is mediated by a protein kinase C (PKC)-dependent activation of extracellular signal-regulated protein kinases 1 and 2 involving PKC-δ and by mobilization of intracellular Ca2+. Biochem. J 2001, 353, 503–512. [Google Scholar]
- Mustonen, P.; van Willigen, G.; Lassila, R. Epinephrine—Via activation of p38-MAPK—Abolishes the effect of aspirin on platelet deposition to collagen. Thromb. Res 2001, 104, 439–449. [Google Scholar]
- Webb, J.G.; Tan, Y.; Jaffa, M.A.; Jaffa, A.A. Evidence for prostacyclin and cAMP up-regulation by bradykinin and insulin-like growth factor 1 in vascular smooth muscle cells. J. Recept. Signal Transduct. Res 2010, 30, 61–71. [Google Scholar]
- Muto, A.; Nishibe, T.; Miyauchi, Y.; Kondo, Y.; Yamamoto, Y.; Dardik, A.; Shigematsu, H. Prostaglandin receptors EP2 and IP are detectable in atherosclerotic arteries and plaques. Int. Angiol 2010, 29, 43–48. [Google Scholar]

© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, J.-H.; Yuan, L.-J.; Zhong, Z.-M.; Wen, Z.-S.; Deng, J.-M.; Liang, R.-X.; Zheng, M. The Anticoagulant Effect of PGI2S and tPA in Transgenic Umbilical Vein Endothelial Cells Is Linked to Up-Regulation of PKA and PKC. Int. J. Mol. Sci. 2014, 15, 2826-2839. https://doi.org/10.3390/ijms15022826
Wang J-H, Yuan L-J, Zhong Z-M, Wen Z-S, Deng J-M, Liang R-X, Zheng M. The Anticoagulant Effect of PGI2S and tPA in Transgenic Umbilical Vein Endothelial Cells Is Linked to Up-Regulation of PKA and PKC. International Journal of Molecular Sciences. 2014; 15(2):2826-2839. https://doi.org/10.3390/ijms15022826
Chicago/Turabian StyleWang, Jian-Hua, Lin-Jing Yuan, Zhi-Min Zhong, Zhe-Sheng Wen, Jian-Ming Deng, Rong-Xin Liang, and Min Zheng. 2014. "The Anticoagulant Effect of PGI2S and tPA in Transgenic Umbilical Vein Endothelial Cells Is Linked to Up-Regulation of PKA and PKC" International Journal of Molecular Sciences 15, no. 2: 2826-2839. https://doi.org/10.3390/ijms15022826
APA StyleWang, J.-H., Yuan, L.-J., Zhong, Z.-M., Wen, Z.-S., Deng, J.-M., Liang, R.-X., & Zheng, M. (2014). The Anticoagulant Effect of PGI2S and tPA in Transgenic Umbilical Vein Endothelial Cells Is Linked to Up-Regulation of PKA and PKC. International Journal of Molecular Sciences, 15(2), 2826-2839. https://doi.org/10.3390/ijms15022826



