Involvement of Hydrogen Peroxide in Safingol-Induced Endonuclease G-Mediated Apoptosis of Squamous Cell Carcinoma Cells
Abstract
:1. Introduction
2. Results
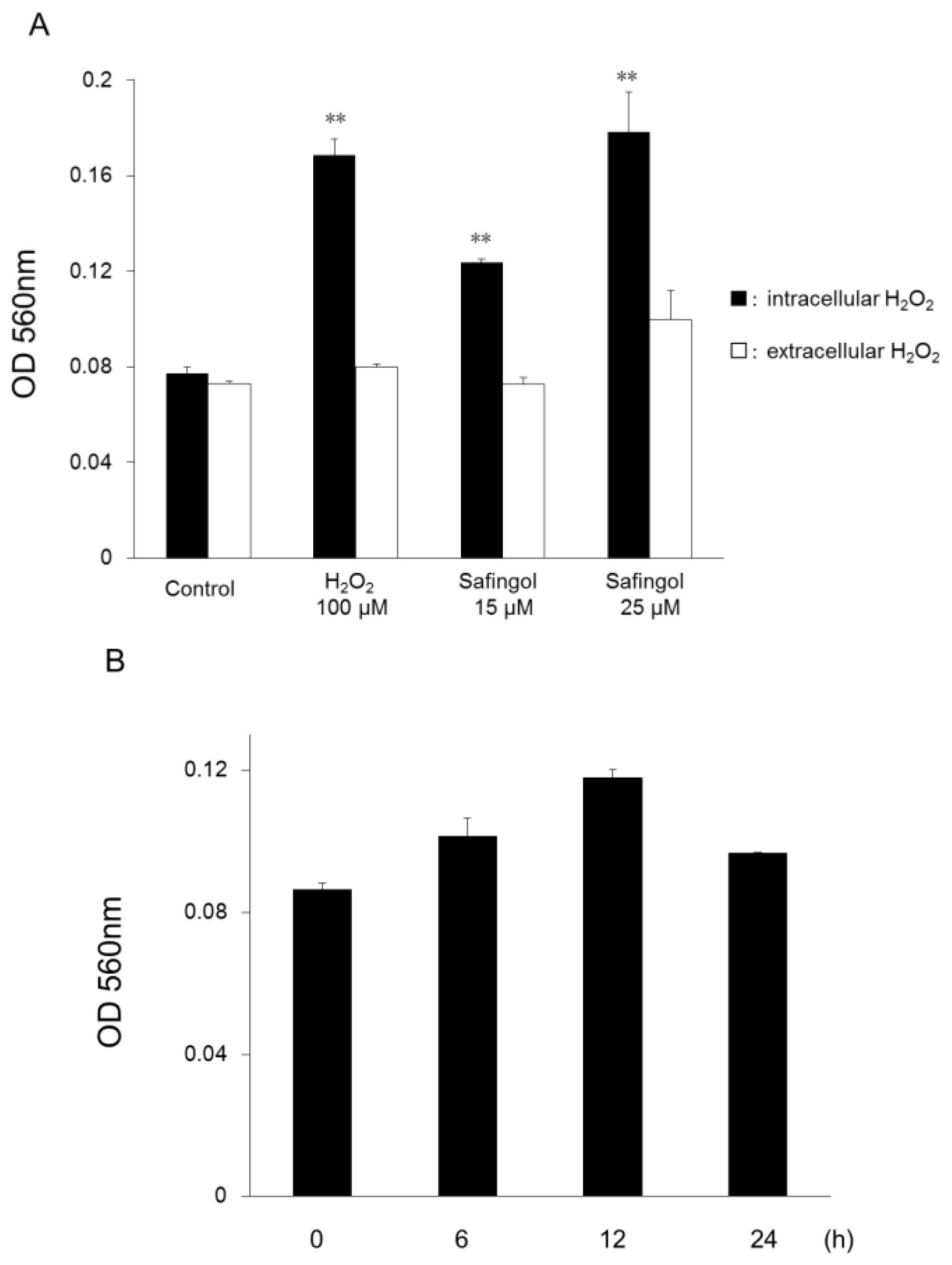
2.1. Production of ROS in SCC Cells by Treatment with Safingol
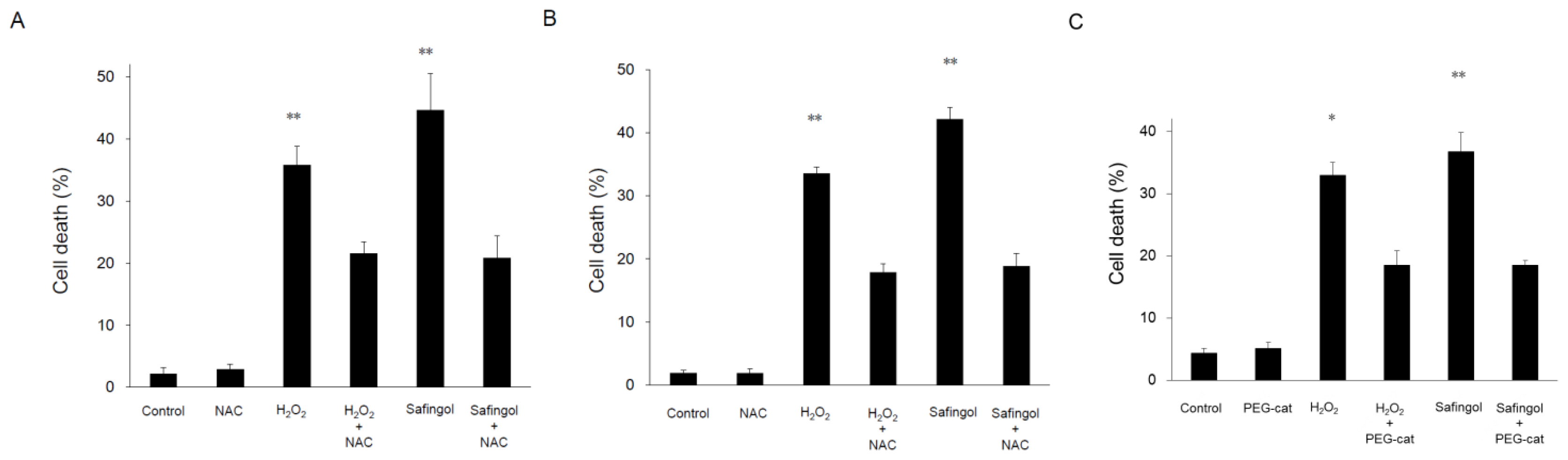
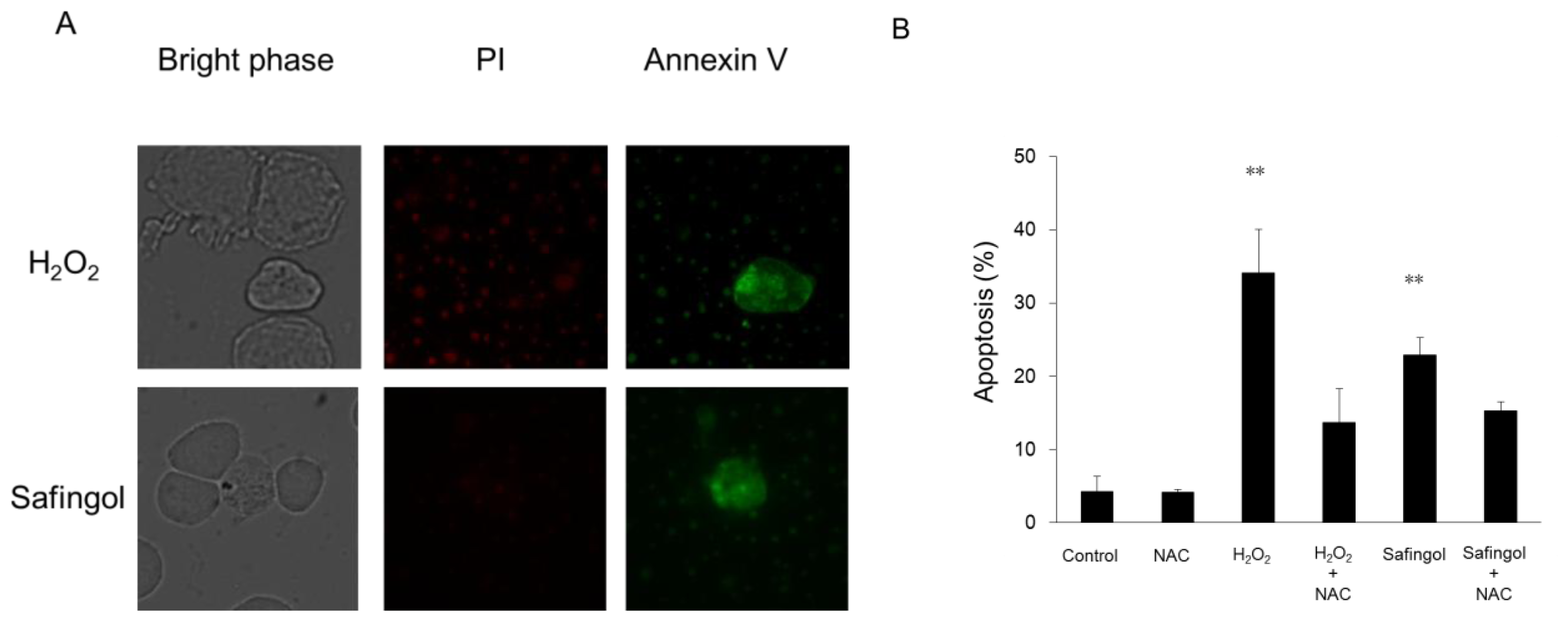
2.2. Induction of Cell Death by H2O2 and Safingol
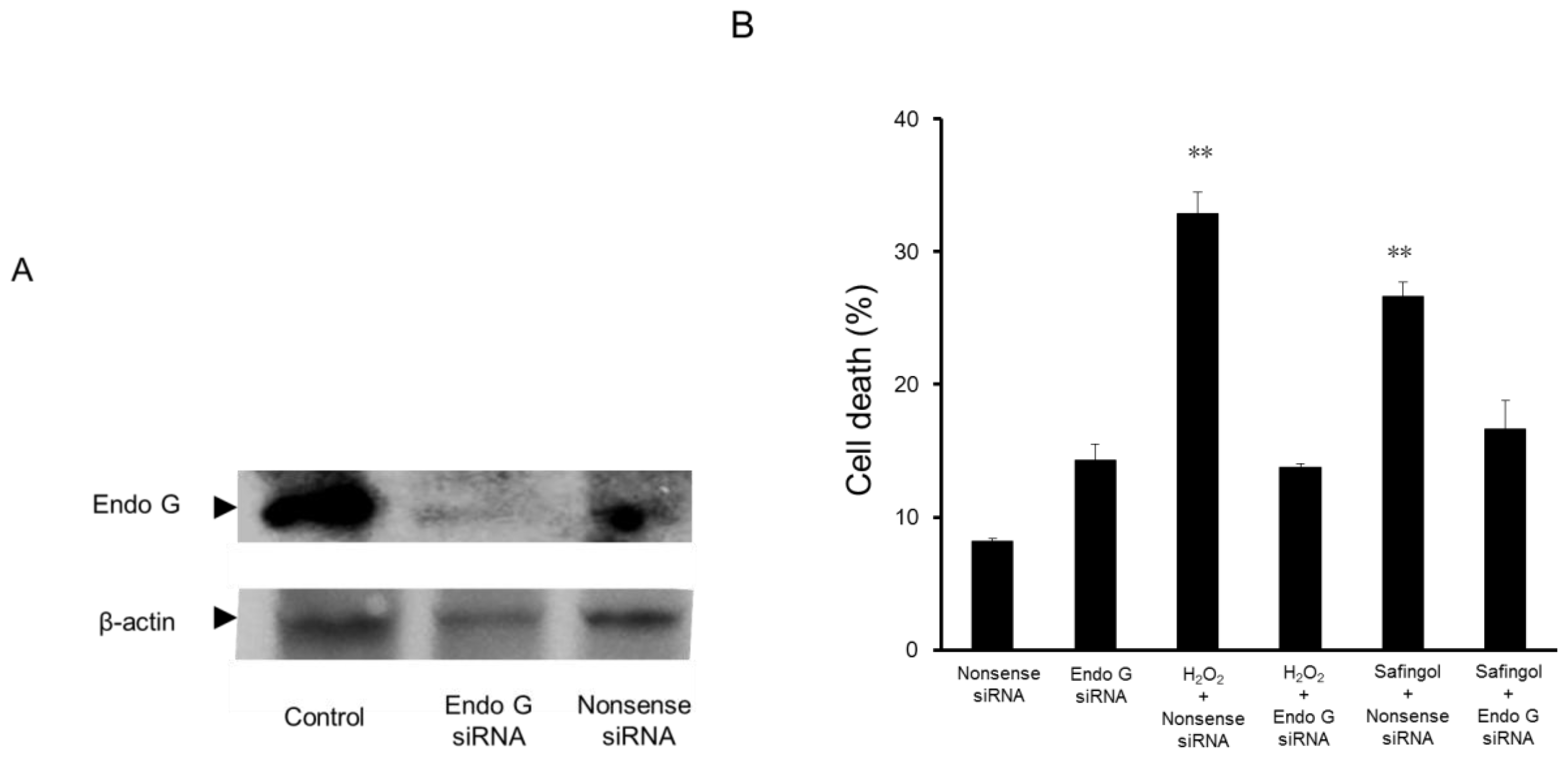
2.3. Effect of Endo G Small Interfering RNA (siRNA) on the Cell Death Caused by H2O2 and Safingol
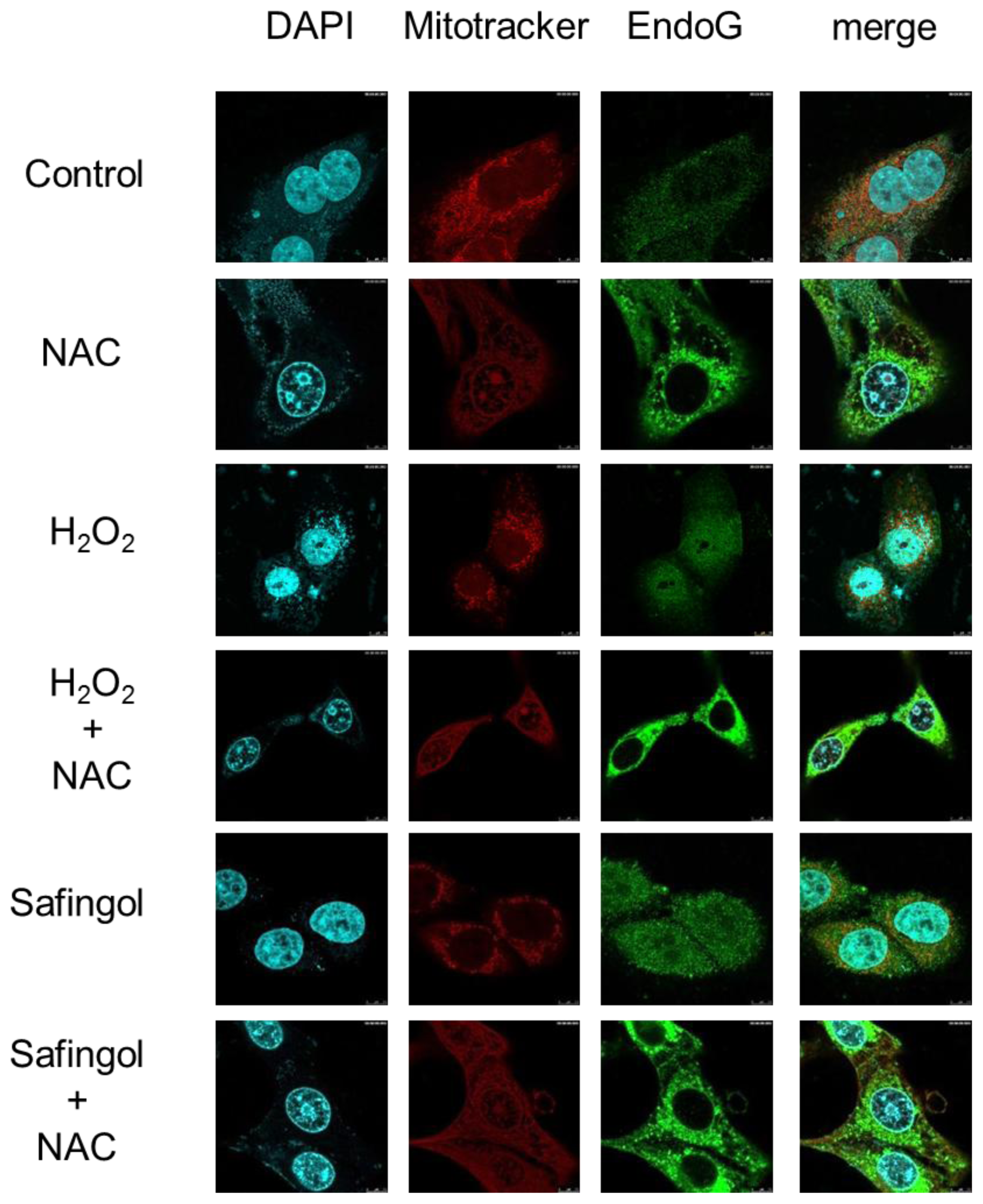
2.4. Translocation of Endo G by H2O2 and Safingol
3. Discussion
4. Experimental Section
4.1. Cell Culture
4.2. Reagents
4.3. Measurement of H2O2
4.4. Trypan Blue Staining
4.5. Annexin V and PI Staining
4.6. Immunoblot Analysis
4.7. siRNA Transfection
4.8. Confocal Laser Microscopic Analysis
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kroemer, G.; Galluzzi, L.; Vandenabeele, P.; Abrams, J.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; El-Deiry, W.S.; Golstein, P.; Green, D.R.; et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell. Death Differ 2009, 16, 3–11. [Google Scholar]
- Ghobrial, I.M.; Witzig, T.E.; Adjei, A.A. Targeting apoptosis pathways in cancer therapy. CA: A Cancer J. Clin 2005, 55, 178–194. [Google Scholar]
- Sakahira, H.; Enari, M.; Nagata, S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature 1998, 391, 96–99. [Google Scholar]
- Li, L.Y.; Luo, X.; Wang, X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 2001, 412, 95–99. [Google Scholar]
- Lorenzo, H.K.; Susin, S.A. Mitochondrial effectors in caspase-independent cell death. FEBS Lett 2004, 557, 14–20. [Google Scholar]
- Li, J.; Zhou, J.; Li, Y.; Qin, D.; Li, P. Mitochondrial fission controls DNA fragmentation by regulating endonuclease G. Free Radic. Biol. Med 2010, 49, 622–631. [Google Scholar]
- Hannun, Y.A.; Loomis, C.R.; Merrill, A.H., Jr.; Bell, R.M. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J. Biol. Chem 1986, 261, 12604–12609. [Google Scholar]
- Kedderis, L.B.; Bozigian, H.P.; Kleeman, J.M.; Hall, R.L.; Palmer, T.E.; Harrison, S.D., Jr.; Susick, R.L., Jr. Toxicity of the protein kinase C inhibitor safingol administered alone and in combination with chemotherapeutic agents. Fundam. Appl. Toxicol 1995, 25, 201–217. [Google Scholar]
- Schwartz, G.K.; Haimovitz-Friedman, A.; Dhupar, S.K.; Ehleiter, D.; Maslak, P.; Lai, L.; Loganzo, F., Jr.; Kelsen, D.P.; Fuks, Z.; Albino, A.P. Potentiation of apoptosis by treatment with the protein kinase C-specific inhibitor safingol in mitomycin C-treated gastric cancer cells. J. Natl. Cancer Inst 1995, 87, 1394–1399. [Google Scholar]
- Hoffmann, T.K.; Leenen, K.; Hafner, D.; Balz, V.; Gerharz, C.D.; Grund, A.; Ballo, H.; Hauser, U.; Bier, H. Antitumor activity of protein kinase C inhibitors and cisplatin in human head and neck squamous cell carcinoma lines. Anticancer Drugs 2002, 13, 93–100. [Google Scholar]
- Choe, Y.; Jung, H.; Khang, I.; Kim, K. Selective roles of protein kinase C isoforms on cell motility of GT1 immortalized hypothalamic neurones. J. Neuroendocrinol 2003, 15, 508–515. [Google Scholar]
- Uemura, K.; Aki, T.; Yamaguchi, K.; Yoshida, K. Protein kinase C-epsilon protects PC12 cells against methamphetamine-induced death: Possible involvement of suppression of glutamate receptor. Life Sci 2003, 72, 1595–1607. [Google Scholar]
- Buehrer, B.M.; Bell, R.M. Sphingosine kinase: Properties and cellular functions. Adv. Lipid Res 1993, 26, 59–67. [Google Scholar]
- Olivera, A.; Kohama, T.; Tu, Z.; Milstien, S.; Spiegel, S. Purification and characterization of rat kidney sphingosine kinase. J. Biol. Chem 1998, 273, 12576–12583. [Google Scholar]
- Pyne, N.J.; Pyne, S. Sphingosine 1-phosphate and cancer. Nat. Rev. Cancer 2010, 10, 489–503. [Google Scholar]
- Dickson, M.A.; Carvajal, R.D.; Merrill, A.H., Jr.; Gonen, M.; Cane, L.M.; Schwartz, G.K. A phase I clinical trial of safingol in combination with cisplatin in advanced solid tumors. Clin. Cancer Res 2011, 17, 2484–2492. [Google Scholar]
- Hamada, M.; Sumi, T.; Iwai, S.; Nakazawa, M.; Yura, Y. Induction of endonuclease G-mediated apopotosis in human oral squamous cell carcinoma cells by protein kinase C inhibitor safingol. Apoptosis 2006, 11, 47–56. [Google Scholar]
- Azad, M.B.; Chen, Y.; Gibson, S.B. Regulation of autophagy by reactive oxygen species (ROS): Implications for cancer progression and treatment. Antioxid Redox. Signal 2009, 11, 777–790. [Google Scholar]
- Jiang, Z.Y.; Woollard, A.C.; Wolff, S.P. Hydrogen peroxide production during experimental protein glycation. FEBS Lett 1990, 268, 69–71. [Google Scholar]
- Jiang, Z.Y.; Hunt, J.V.; Wolff, S.P. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal. Biochem 1992, 202, 384–389. [Google Scholar]
- Zafarullah, M.; Li, W.Q.; Sylvester, J.; Ahmad, M. Molecular mechanisms of N-acetylcysteine actions. Cell. Mol. Life Sci 2003, 60, 6–20. [Google Scholar]
- Aruoma, O.I.; Halliwell, B.; Hoey, B.M.; Butler, J. The antioxidant action of N-acetylcysteine: Its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med 1989, 6, 593–597. [Google Scholar]
- Noda, T.; Iwai, S.; Hamada, M.; Fujita, Y.; Yura, Y. Induction of apoptosis of detached oral squamous cell carcinoma cells by safingol. Possible role of Bim, focal adhesion kinase and endonuclease G. Apoptosis 2009, 14, 287–297. [Google Scholar]
- Cai, J.; Jones, D.P. Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J. Biol. Chem 1998, 273, 11401–11404. [Google Scholar]
- Gewirtz, D.A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol 1999, 57, 727–741. [Google Scholar]
- Varbiro, G.; Veres, B.; Gallyas, F., Jr.; Sumegi, B. Direct effect of taxol on free radical formation and mitochondrial permeability transition. Free Radic. Biol. Med 2001, 31, 548–558. [Google Scholar]
- Mizutani, H.; Tada-Oikawa, S.; Hiraku, Y.; Kojima, M.; Kawanishi, S. Mechanism of apoptosis induced by doxorubicin through the generation of hydrogen peroxide. Life Sci 2005, 76, 1439–1453. [Google Scholar]
- Ling, L.U.; Tan, K.B.; Lin, H.; Chiu, G.N. The role of reactive oxygen species and autophagy in safingol-induced cell death. Cell Death Dis 2011, 2, e129. [Google Scholar]
- Zamzami, N.; Kroemer, G. Methods to measure membrane potential and permeability transition in the mitochondria during apoptosis. Methods Mol. Biol 2004, 282, 103–115. [Google Scholar]
- Jourdain, A.; Martinou, J.C. Mitochondrial outer-membrane permeabilization and remodelling in apoptosis. Int. J. Biochem. Cell Biol 2009, 41, 1884–1889. [Google Scholar]
- Higgins, G.C.; Beart, P.M.; Nagley, P. Oxidative stress triggers neuronal caspase-independent death: endonuclease G involvement in programmed cell death-type III. Cell. Mol. Life Sci 2009, 66, 2773–2787. [Google Scholar]
- Kim, J.S.; Lee, J.H.; Jeong, W.W.; Choi, D.H.; Cha, H.J.; Kim, D.H.; Kwon, J.K.; Park, S.E.; Park, J.H.; Cho, H.R.; et al. Reactive oxygen species-dependent EndoG release mediates cisplatin-induced caspase-independent apoptosis in human head and neck squamous carcinoma cells. Int. J. Cancer 2008, 122, 672–680. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hamada, M.; Wakabayashi, K.; Masui, A.; Iwai, S.; Imai, T.; Yura, Y. Involvement of Hydrogen Peroxide in Safingol-Induced Endonuclease G-Mediated Apoptosis of Squamous Cell Carcinoma Cells. Int. J. Mol. Sci. 2014, 15, 2660-2671. https://doi.org/10.3390/ijms15022660
Hamada M, Wakabayashi K, Masui A, Iwai S, Imai T, Yura Y. Involvement of Hydrogen Peroxide in Safingol-Induced Endonuclease G-Mediated Apoptosis of Squamous Cell Carcinoma Cells. International Journal of Molecular Sciences. 2014; 15(2):2660-2671. https://doi.org/10.3390/ijms15022660
Chicago/Turabian StyleHamada, Masakazu, Ken Wakabayashi, Atsushi Masui, Soichi Iwai, Tomoaki Imai, and Yoshiaki Yura. 2014. "Involvement of Hydrogen Peroxide in Safingol-Induced Endonuclease G-Mediated Apoptosis of Squamous Cell Carcinoma Cells" International Journal of Molecular Sciences 15, no. 2: 2660-2671. https://doi.org/10.3390/ijms15022660



