Dominant Repression by Arabidopsis Transcription Factor MYB44 Causes Oxidative Damage and Hypersensitivity to Abiotic Stress
Abstract
:1. Introduction
1.1. Transcription Factors
1.2. Transcription Factors Involved in Plant Stress Responses
1.3. Transcriptional Activators and Repressors
1.4. Arabidopsis MYB Transcription Factors—Subfamily S22
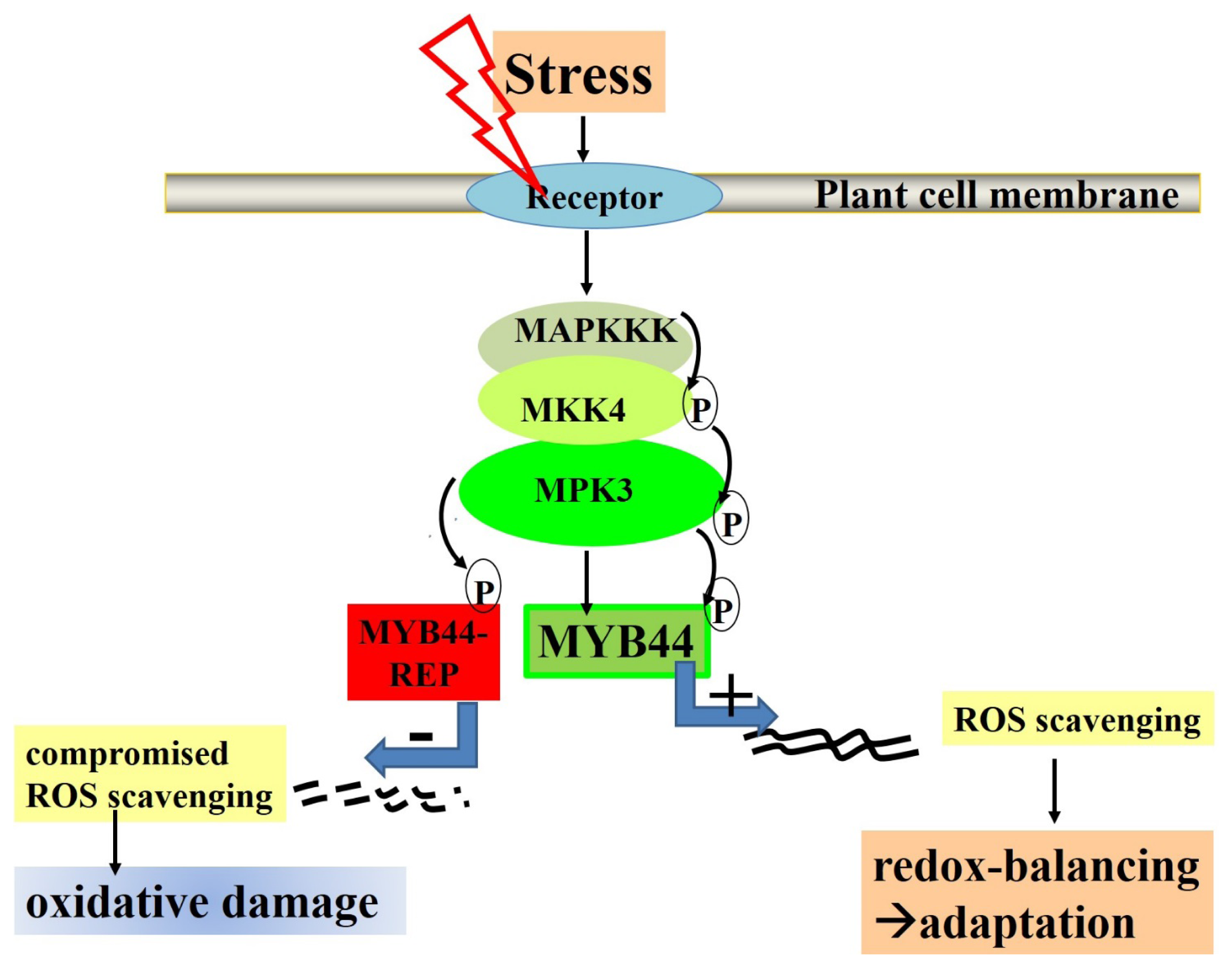
2. Results
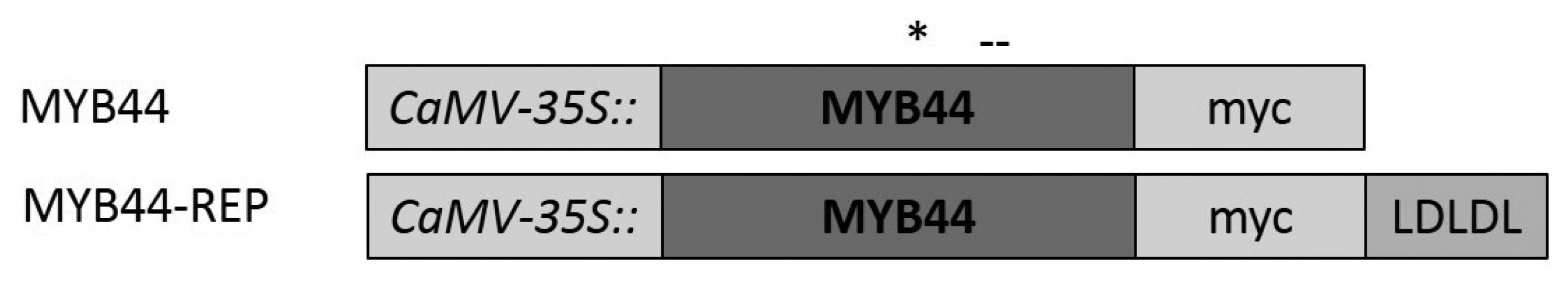
2.1. MYB S22 Family Members Carry a Putative Repressor Domain
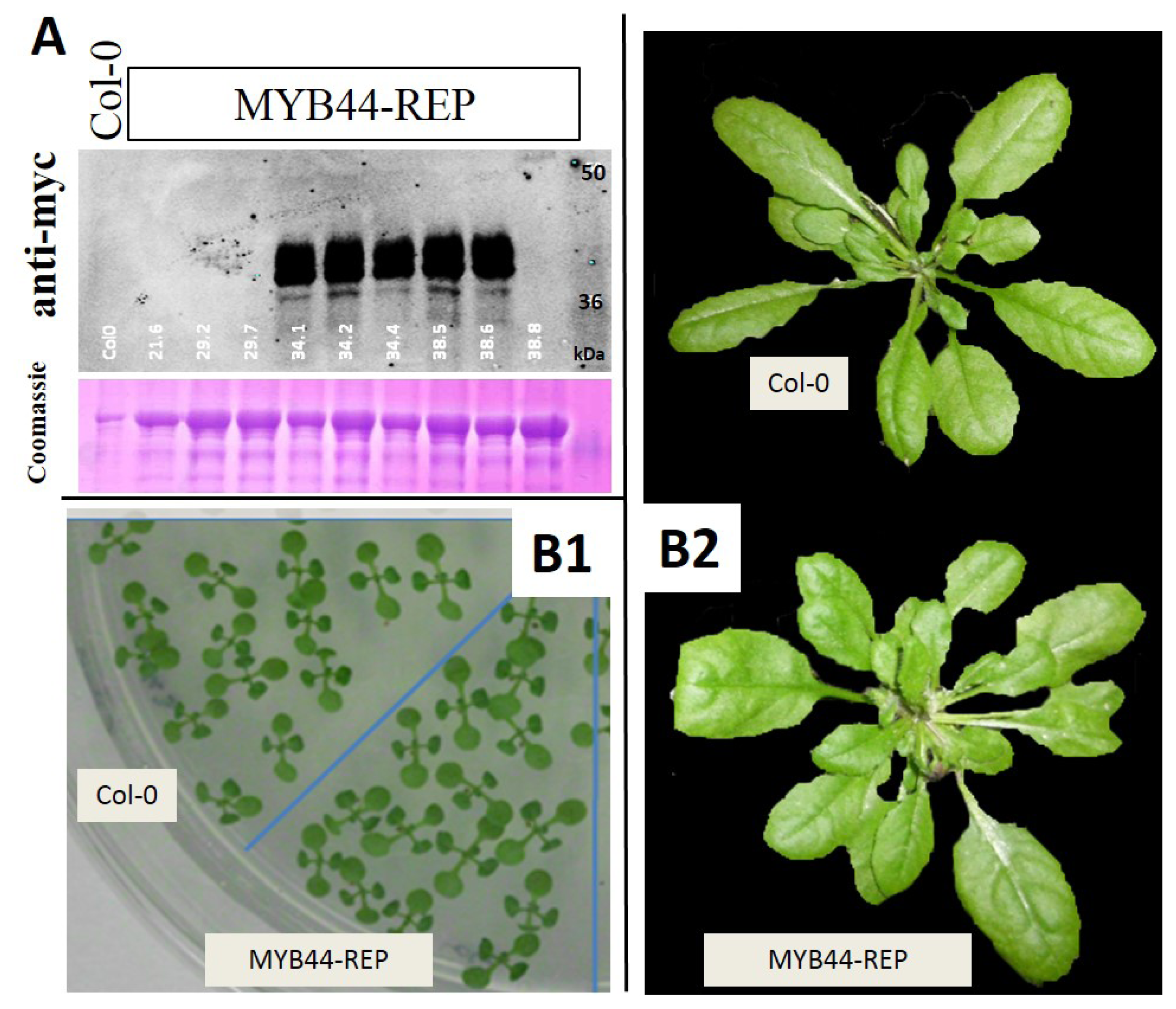
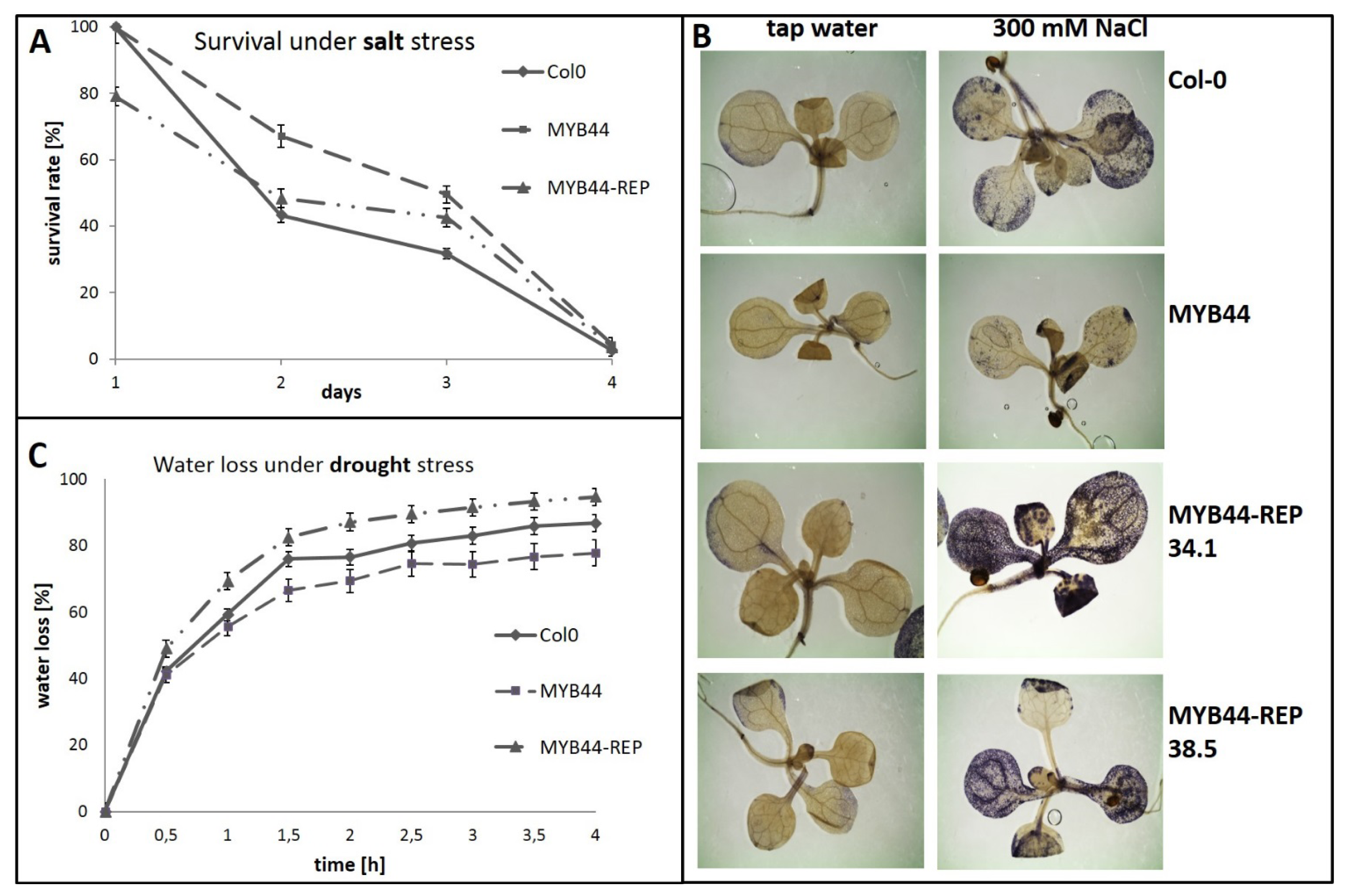
2.2. MYB44-REP Overexpression Interferes with Early Stress Responses and Antioxidative Defenses in Salt-Exposed Arabidopsis Seedlings
2.3. MYB44-REP Overexpression Reduces Tolerance to Drought Stress
2.4. MYB44 and MYB44-REP Transactivation Studies in Vivo
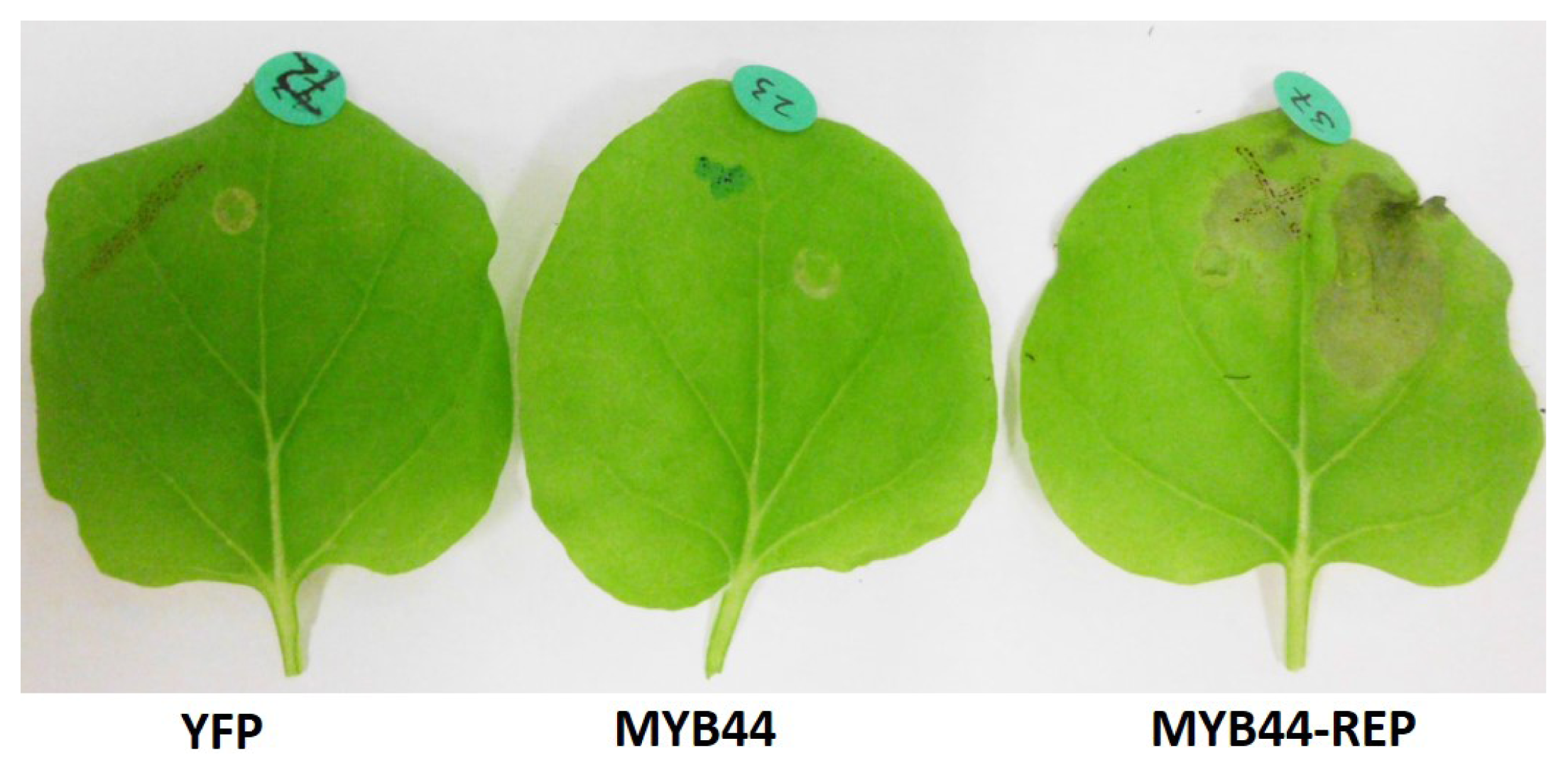
2.5. Ectopic Expression of MYB44-REP Causes Tissue Collapse in N. benthamiana
3. Discussion
3.1. MYB44-REP-Induced Tissue Collapse in N. benthamiana
3.2. Lack of MBSII-Related Transactivation/Repression in Co-Transfection Assays
3.3. Narrowing down the MYB44 Targetome
3.4. Effects of MYB44 and MYB44-REP on Osmotic Stress Tolerance in Arabidopsis
4. Experimental Section
4.1. Plant Material, Osmotic Stress and Drought Tolerance Test
4.2. Water Loss Determination
4.3. Detection of Superoxide
4.4. Constructs
4.5. β-Glucuronidase Activity Assay, Histological
4.6. β-Glucuronidase Activity Assay in Protein Extracts
4.7. Transient Expression in Nicotinana benthamiana
4.8. UV Microscopy
4.9. Protein Extraction and Immunoblot Analysis
5. Final Conclusions: MYB44 Manipulation for Optimizing Plant Stress Tolerance
Supplementary Information
ijms-15-02517-s001.pdfAcknowledgments
Conflicts of Interest
- Author ContributionsPlanned the experiments: A.P. and H.P.; conducted the experiments: H.P. and A.P.; contributed figures: H.P. and A.P.; wrote the manuscript: A.P.
References
- Pitzschke, A. From bench to barn: Plant model research and its applications in agriculture. Adv. Genet. Eng 2013. [Google Scholar] [CrossRef]
- Pitzschke, A. Make Your Best—MYB Transcription Factors for Improving Abiotic Stress Tolerance in Crops. In Improving Crop Resistance to Abiotic Stress; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 481–506. [Google Scholar]
- Shinozaki, K.; Yamaguchi-Shinozaki, K.; Seki, M. Regulatory network of gene expression in the drought and cold stress responses. Curr. Opin. Plant Biol 2003, 6, 410–417. [Google Scholar]
- Lindemose, S.; O’Shea, C.; Jensen, M.K.; Skriver, K. Structure, function and networks of transcription factors involved in abiotic stress responses. Int. J. Mol. Sci 2013, 14, 5842–5878. [Google Scholar]
- Singh, K.; Foley, R.C.; Oñate-Sánchez, L. Transcription factors in plant defense and stress responses. Curr. Opin. Plant Biol 2002, 5, 430–436. [Google Scholar]
- Park, J.M.; Park, C.J.; Lee, S.B.; Ham, B.K.; Shin, R.; Paek, K.H. Overexpression of the tobacco Tsi1 gene encoding an EREBP/AP2-type transcription factor enhances resistance against pathogen attack and osmotic stress in tobacco. Plant Cell 2001, 13, 1035–1046. [Google Scholar]
- Kasuga, M.; Liu, Q.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat. Biotech 1999, 17, 287–291. [Google Scholar]
- Polizel, A.M.; Medri, M.E.; Nakashima, K.; Yamanaka, N.; Farias, J.R.; de Oliveira, M.C.; Marin, S.R.; Abdelnoor, R.V.; Marcelino-Guimaraes, F.C.; Fuganti, R.; et al. Molecular, anatomical and physiological properties of a genetically modified soybean line transformed with rd29A:AtDREB1A for the improvement of drought tolerance. Genet. Mol. Res 2011, 10, 3641–3656. [Google Scholar]
- Ravikumar, G.; Manimaran, P.; Voleti, S.R.; Subrahmanyam, D.; Sundaram, R.M.; Bansal, K.C.; Viraktamath, B.C.; Balachandran, S.M. Stress-inducible expression of AtDREB1A transcription factor greatly improves drought stress tolerance in transgenic indica rice. Transgenic Res 2014. [Google Scholar] [CrossRef]
- Djamei, A.; Pitzschke, A.; Nakagami, H.; Rajh, I.; Hirt, H. Trojan horse strategy in Agrobacterium transformation: Abusing MAPK defense signaling. Science 2007, 318, 453–456. [Google Scholar]
- Pitzschke, A.; Djamei, A.; Teige, M.; Hirt, H. VIP1 response elements mediate mitogen-activated protein kinase 3-induced stress gene expression. Proc. Natl. Acad. Sci. USA 2009, 106, 18414–18419. [Google Scholar]
- Tsugama, D.; Liu, S.; Takano, T. A bZIP protein, VIP1, is a regulator of osmosensory signaling in Arabidopsis. Plant Physiol 2012, 159, 144–155. [Google Scholar]
- Li, G.; Meng, X.; Wang, R.; Mao, G.; Han, L.; Liu, Y.; Zhang, S. Dual-level regulation of ACC synthase activity by MPK3/MPK6 cascade and its downstream WRKY transcription factor during ethylene induction in Arabidopsis. PLoS Genet 2012, 8, e1002767. [Google Scholar]
- Hsu, F.C.; Chou, M.Y.; Chou, S.J.; Li, Y.R.; Peng, H.P.; Shih, M.C. Submergence confers immunity mediated by the WRKY22 transcription factor in Arabidopsis. Plant Cell 2013, 25, 2699–2713. [Google Scholar]
- Mao, G.; Meng, X.; Liu, Y.; Zheng, Z.; Chen, Z.; Zhang, S. Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 2011, 23, 1639–1653. [Google Scholar]
- Meng, X.; Xu, J.; He, Y.; Yang, K.Y.; Mordorski, B.; Liu, Y.; Zhang, S. Phosphorylation of an ERF transcription factor by Arabidopsis MPK3/MPK6 regulates plant defense gene induction and fungal resistance. Plant Cell 2013, 25, 1126–1142. [Google Scholar]
- Nguyen, X.C.; Kim, S.H.; Lee, K.; Kim, K.E.; Liu, X.M.; Han, H.J.; Hoang, M.H.; Lee, S.W.; Hong, J.C.; Moon, Y.H.; et al. Identification of a C2H2-type zinc finger transcription factor (ZAT10) from Arabidopsis as a substrate of MAP kinase. Plant Cell Rep 2012, 31, 737–745. [Google Scholar]
- Kagale, S.; Links, M.G.; Rozwadowski, K. Genome-wide analysis of ethylene-responsive element binding factor-associated amphiphilic repression motif-containing transcriptional regulators in Arabidopsis. Plant Physiol 2010, 152, 1109–1134. [Google Scholar]
- Kagale, S.; Rozwadowski, K. EAR motif-mediated transcriptional repression in plants: An underlying mechanism for epigenetic regulation of gene expression. Epigenetics 2011, 6, 141–146. [Google Scholar]
- Ohta, M.; Matsui, K.; Hiratsu, K.; Shinshi, H.; Ohme-Takagi, M. Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. Plant Cell 2001, 13, 1959–1968. [Google Scholar]
- Tsukagoshi, H.; Saijo, T.; Shibata, D.; Morikami, A.; Nakamura, K. Analysis of a sugar response mutant of Arabidopsis identified a novel B3 domain protein that functions as an active transcriptional repressor. Plant Physiol 2005, 138, 675–685. [Google Scholar]
- Tsukagoshi, H.; Morikami, A.; Nakamura, K. Two B3 domain transcriptional repressors prevent sugar-inducible expression of seed maturation genes in Arabidopsis seedlings. Proc. Natl. Acad. Sci. USA 2007, 104, 2543–2547. [Google Scholar]
- Hill, K.; Wang, H.; Perry, S.E. A transcriptional repression motif in the MADS factor AGL15 is involved in recruitment of histone deacetylase complex components. Plant J 2008, 53, 172–185. [Google Scholar]
- Tiwari, S.B.; Hagen, G.; Guilfoyle, T.J. Aux/IAA proteins contain a potent transcriptional repression domain. Plant Cell 2004, 16, 533–543. [Google Scholar]
- Weigel, R.R.; Pfitzner, U.M.; Gatz, C. Interaction of NIMIN1 with NPR1 modulates PR gene expression in Arabidopsis. Plant Cell 2005, 17, 1279–1291. [Google Scholar]
- Preston, J.; Wheeler, J.; Heazlewood, J.; Li, S.F.; Parish, R.W. AtMYB32 is required for normal pollen development in Arabidopsis thaliana. Plant J 2004, 40, 979–995. [Google Scholar]
- Dubos, C.; le Gourrierec, J.; Baudry, A.; Huep, G.; Lanet, E.; Debeaujon, I.; Routaboul, J.M.; Alboresi, A.; Weisshaar, B.; Lepiniec, L. MYBL2 is a new regulator of flavonoid biosynthesis in Arabidopsis thaliana. Plant J 2008, 55, 940–953. [Google Scholar]
- Jin, H.; Cominelli, E.; Bailey, P.; Parr, A.; Mehrtens, F.; Jones, J.; Tonelli, C.; Weisshaar, B.; Martin, C. Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis. EMBO J 2000, 19, 6150–6161. [Google Scholar]
- Szemenyei, H.; Hannon, M.; Long, J.A. TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 2008, 319, 1384–1386. [Google Scholar]
- Hiratsu, K.; Matsui, K.; Koyama, T.; Ohme-Takagi, M. Dominant repression of target genes by chimeric repressors that include the EAR motif, a repression domain, in Arabidopsis. Plant J 2003, 34, 733–739. [Google Scholar]
- Galis, I.; Simek, P.; Narisawa, T.; Sasaki, M.; Horiguchi, T.; Fukuda, H.; Matsuoka, K. A novel R2R3 MYB transcription factor NtMYBJS1 is a methyl jasmonate-dependent regulator of phenylpropanoid-conjugate biosynthesis in tobacco. Plant J 2006, 46, 573–592. [Google Scholar]
- Deluc, L.; Barrieu, F.; Marchive, C.; Lauvergeat, V.; Decendit, A.; Richard, T.; Carde, J.P.; Merillon, J.M.; Hamdi, S. Characterization of a grapevine R2R3-MYB transcription factor that regulates the phenylpropanoid pathway. Plant Physiol 2006, 140, 499–511. [Google Scholar]
- Uimari, A.; Strommer, J. Myb26: A MYB-like protein of pea flowers with affinity for promoters of phenylpropanoid genes. Plant J 1997, 12, 1273–1284. [Google Scholar]
- Sablowski, R.W.; Moyano, E.; Culianez-Macia, F.A.; Schuch, W.; Martin, C.; Bevan, M. A flower-specific Myb protein activates transcription of phenylpropanoid biosynthetic genes. EMBO J 1994, 13, 128–137. [Google Scholar]
- Stracke, R.; Werber, M.; Weisshaar, B. The R2R3-MYB gene family in Arabidopsis thaliana. Curr. Opin. Plant Biol 2001, 4, 447–456. [Google Scholar]
- Romero, I.; Fuertes, A.; Benito, M.J.; Malpica, J.M.; Leyva, A.; Paz-Ares, J. More than 80R2R3-MYB regulatory genes in the genome of Arabidopsis thaliana. Plant J 1998, 14, 273–284. [Google Scholar]
- Shin, R.; Burch, A.Y.; Huppert, K.A.; Tiwari, S.B.; Murphy, A.S.; Guilfoyle, T.J.; Schachtman, D.P. The Arabidopsis transcription factor MYB77 modulates auxin signal transduction. Plant Cell 2007, 19, 2440–2453. [Google Scholar]
- Lu, B.; Sun, W.; Zhang, S.; Zhang, C.; Qian, J.; Wang, X.; Gao, R.; Dong, H. HrpN Ea-induced deterrent effect on phloem feeding of the green peach aphid Myzus persicae requires AtGSL5 and AtMYB44 genes in Arabidopsis thaliana. J. Biosci 2011, 36, 123–137. [Google Scholar]
- Jung, C.; Seo, J.S.; Han, S.W.; Koo, Y.J.; Kim, C.H.; Song, S.I.; Nahm, B.H.; Choi, Y.D.; Cheong, J.J. Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiol 2008, 146, 623–635. [Google Scholar]
- Persak, H.; Pitzschke, A. Tight interconnection and multi-level control of Arabidopsis MYB44 in MAPK cascade signalling. PLoS One 2013, 8, e57547. [Google Scholar]
- Seo, J.; Sohn, H.; Noh, K.; Jung, C.; An, J.; Donovan, C.; Somers, D.; Kim, D.; Jeong, S.-C.; Kim, C.-G.; et al. Expression of the Arabidopsis AtMYB44 gene confers drought/salt-stress tolerance in transgenic soybean. Mol. Breed 2012, 29, 601–608. [Google Scholar]
- Wenke, K.; Wanke, D.; Kilian, J.; Berendzen, K.; Harter, K.; Piechulla, B. Volatiles of two growth-inhibiting rhizobacteria commonly engage AtWRKY18 function. Plant J 2012, 70, 445–459. [Google Scholar]
- Colcombet, J.; Hirt, H. Arabidopsis MAPKs: A complex signalling network involved in multiple biological processes. Biochem. J 2008, 413, 217–226. [Google Scholar]
- Krishna, M.; Narang, H. The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cell. Mol. Life Sci 2008, 65, 3525–3544. [Google Scholar]
- Nakagami, H.; Pitzschke, A.; Hirt, H. Emerging MAP kinase pathways in plant stress signalling. Trends Plant Sci 2005, 10, 339–346. [Google Scholar]
- Pitzschke, A.; Schikora, A.; Hirt, H. MAPK cascade signalling networks in plant defence. Curr. Opin. Plant Biol 2009, 12, 421–426. [Google Scholar]
- Pitzschke, A.; Forzani, C.; Hirt, H. Reactive oxygen species signaling in plants. Antioxid. Redox Signal 2006, 8, 1757–1764. [Google Scholar]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant 2008, 133, 481–489. [Google Scholar]
- Kirik, V.; Kolle, K.; Misera, S.; Baumlein, H. Two novel MYB homologues with changed expression in late embryogenesis-defective Arabidopsis mutants. Plant Mol. Biol 1998, 37, 819–827. [Google Scholar]
- Shikata, M.; Ohme-Takagi, M. The utility of transcription factors for manipulation of floral traits. Plant Biotech 2008, 25, 31–36. [Google Scholar]
- Tamagnone, L.; Merida, A.; Parr, A.; Mackay, S.; Culianez-Macia, F.A.; Roberts, K.; Martin, C. The AmMYB308 and AmMYB330 transcription factors from antirrhinum regulate phenylpropanoid and lignin biosynthesis in transgenic tobacco. Plant Cell 1998, 10, 135–154. [Google Scholar]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci 2010, 15, 573–581. [Google Scholar]
- Ogata, K.; Morikawa, S.; Nakamura, H.; Hojo, H.; Yoshimura, S.; Zhang, R.; Aimoto, S.; Ametani, Y.; Hirata, Z.; Sarai, A.; et al. Comparison of the free and DNA-complexed forms of the DNA-binding domain from c-Myb. Nat. Struct. Biol 1995, 2, 309–320. [Google Scholar]
- Myrset, A.H.; Bostad, A.; Jamin, N.; Lirsac, P.N.; Toma, F.; Gabrielsen, O.S. DNA and redox state induced conformational changes in the DNA-binding domain of the Myb oncoprotein. EMBO J 1993, 12, 4625–4633. [Google Scholar]
- Heim, M.A.; Jakoby, M.; Werber, M.; Martin, C.; Weisshaar, B.; Bailey, P.C. The basic helix-loop-helix transcription factor family in plants: A genome-wide study of protein structure and functional diversity. Mol. Biol. Evol 2003, 20, 735–747. [Google Scholar]
- Baudry, A.; Heim, M.A.; Dubreucq, B.; Caboche, M.; Weisshaar, B.; Lepiniec, L. TT2, TT8, and TTG1 synergistically specify the expression of banyuls and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J 2004, 39, 366–380. [Google Scholar]
- Zimmermann, I.M.; Heim, M.A.; Weisshaar, B.; Uhrig, J.F. Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/B-like BHLH proteins. Plant J 2004, 40, 22–34. [Google Scholar]
- Grotewold, E.; Sainz, M.B.; Tagliani, L.; Hernandez, J.M.; Bowen, B.; Chandler, V.L. Identification of the residues in the Myb domain of maize C1 that specify the interaction with the bHLH cofactor R. Proc. Natl. Acad. Sci. USA 2000, 97, 13579–13584. [Google Scholar]
- Wester, K.; Digiuni, S.; Geier, F.; Timmer, J.; Fleck, C.; Hulskamp, M. Functional diversity of R3 single-repeat genes in trichome development. Development 2009, 136, 1487–1496. [Google Scholar]
- Li, L.; Yu, X.; Thompson, A.; Guo, M.; Yoshida, S.; Asami, T.; Chory, J.; Yin, Y. Arabidopsis MYB30 is a direct target of BES1 and cooperates with BES1 to regulate brassinosteroid-induced gene expression. Plant J 2009, 58, 275–286. [Google Scholar]
- Yang, S.W.; Jang, I.C.; Henriques, R.; Chua, N.H. FAR-RED ELONGATED HYPOCOTYL1 and FHY1-like associate with the Arabidopsis transcription factors LAF1 and HFR1 to transmit phytochrome A signals for inhibition of hypocotyl elongation. Plant Cell 2009, 21, 1341–1359. [Google Scholar]
- Wang, P.; Du, Y.; Zhao, X.; Miao, Y.; Song, C.P. The MPK6-ERF6-ROS-responsive cis-acting Element7/GCC box complex modulates oxidative gene transcription and the oxidative response in Arabidopsis. Plant Physiol 2013, 161, 1392–1408. [Google Scholar]
- Smart, R.E. Rapid estimates of relative water content. Plant Physiol 1974, 53, 258–260. [Google Scholar]
- Hellens, R.P.; Edwards, E.A.; Leyland, N.R.; Bean, S.; Mullineaux, P.M. pGreen: A versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol. Biol 2000, 42, 819–832. [Google Scholar]
- Pitzschke, A.; Persak, H. Poinsettia protoplasts—A simple, robust and efficient system for transient gene expression studies. Plant Methods 2012, 8, 1–14. [Google Scholar]
- Wu, F.H.; Shen, S.C.; Lee, L.Y.; Lee, S.H.; Chan, M.T.; Lin, C.S. Tape-Arabidopsis Sandwich—A simpler Arabidopsis protoplast isolation method. Plant Methods 2009, 5, 1–16. [Google Scholar]
- Hellens, R.P.; Allan, A.C.; Friel, E.N.; Bolitho, K.; Grafton, K.; Templeton, M.D.; Karunairetnam, S.; Gleave, A.P.; Laing, W.A. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 2005, 1, 1–13. [Google Scholar]


© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Persak, H.; Pitzschke, A. Dominant Repression by Arabidopsis Transcription Factor MYB44 Causes Oxidative Damage and Hypersensitivity to Abiotic Stress. Int. J. Mol. Sci. 2014, 15, 2517-2537. https://doi.org/10.3390/ijms15022517
Persak H, Pitzschke A. Dominant Repression by Arabidopsis Transcription Factor MYB44 Causes Oxidative Damage and Hypersensitivity to Abiotic Stress. International Journal of Molecular Sciences. 2014; 15(2):2517-2537. https://doi.org/10.3390/ijms15022517
Chicago/Turabian StylePersak, Helene, and Andrea Pitzschke. 2014. "Dominant Repression by Arabidopsis Transcription Factor MYB44 Causes Oxidative Damage and Hypersensitivity to Abiotic Stress" International Journal of Molecular Sciences 15, no. 2: 2517-2537. https://doi.org/10.3390/ijms15022517
APA StylePersak, H., & Pitzschke, A. (2014). Dominant Repression by Arabidopsis Transcription Factor MYB44 Causes Oxidative Damage and Hypersensitivity to Abiotic Stress. International Journal of Molecular Sciences, 15(2), 2517-2537. https://doi.org/10.3390/ijms15022517




