Cellular Behavior of Human Adipose-Derived Stem Cells on Wettable Gradient Polyethylene Surfaces
Abstract
:1. Introduction
2. Results
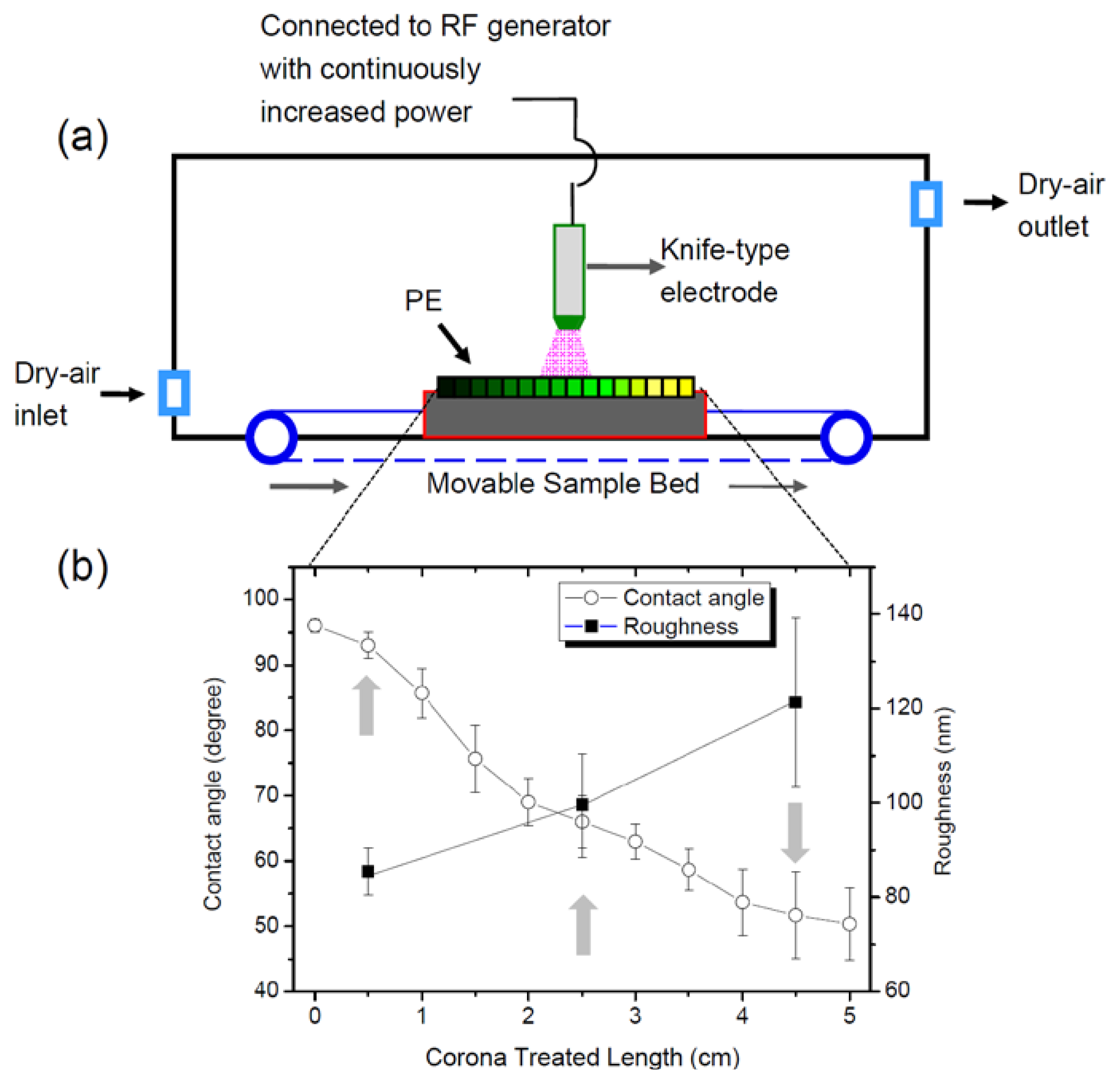
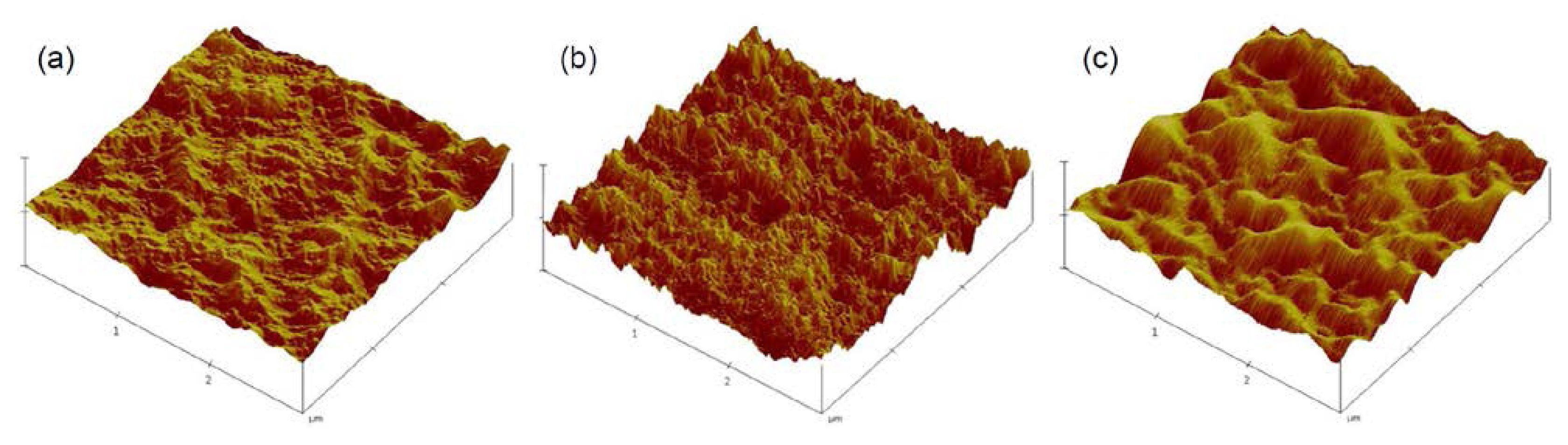
2.1. Characterization of Gradient PE Surfaces
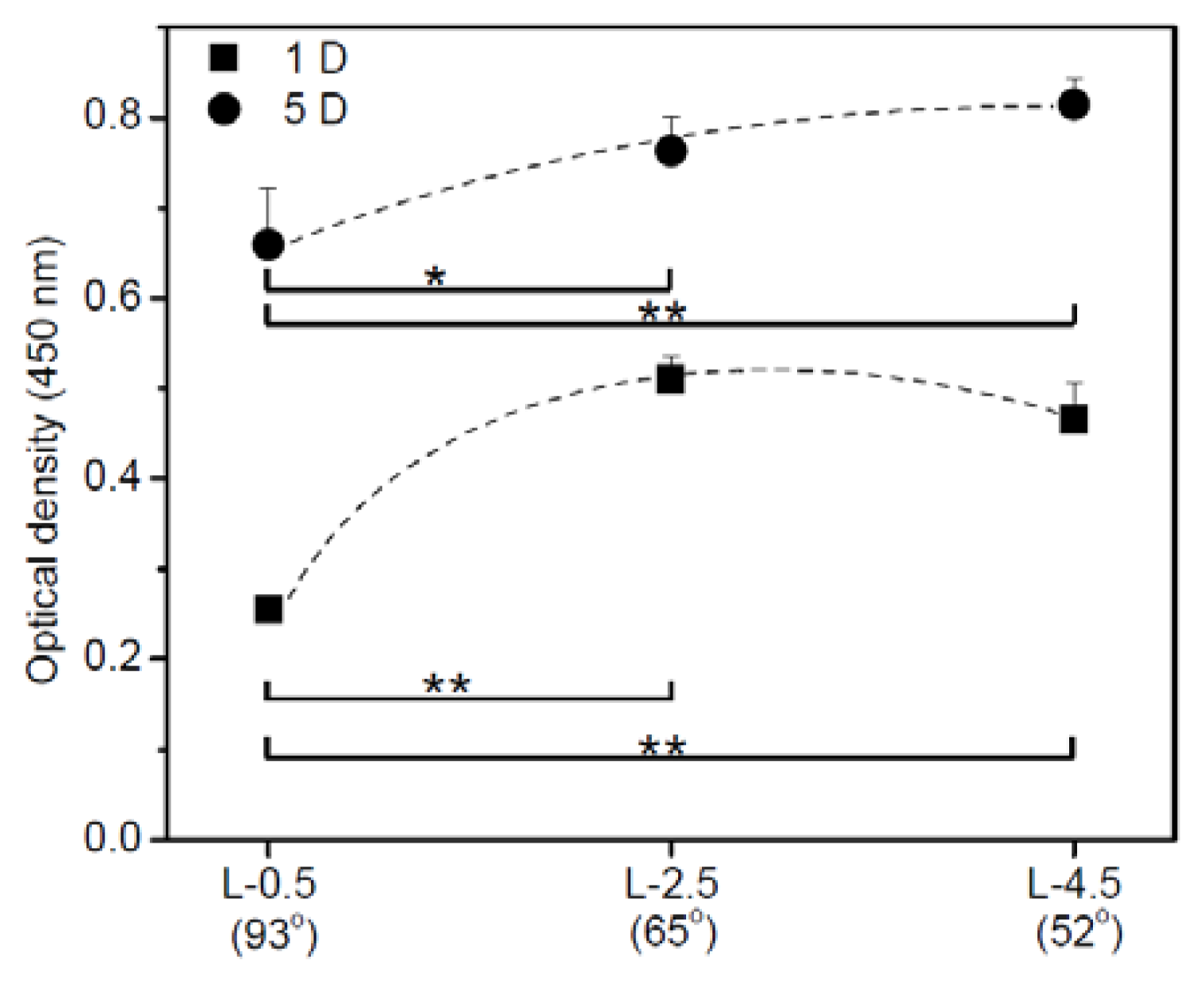
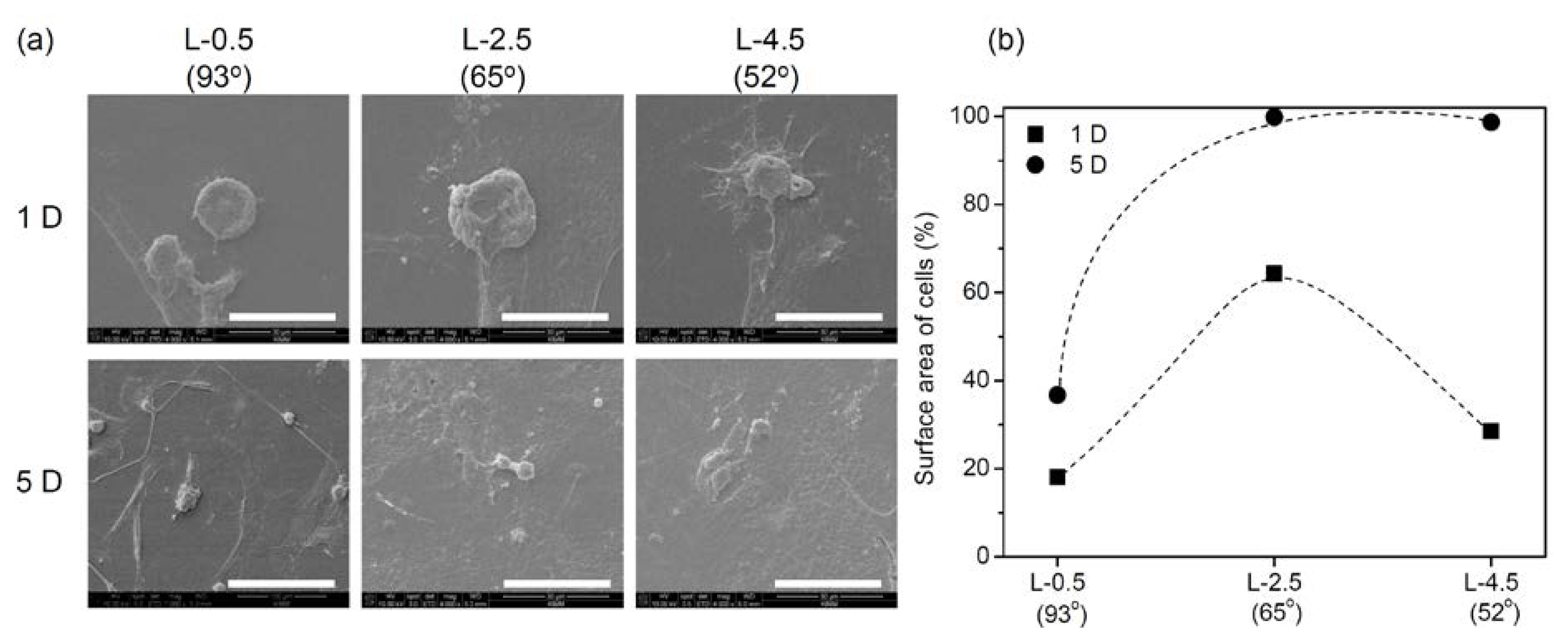
2.2. Cell Adhesion and Proliferation on Gradient PE Surfaces
2.3. IGb mRNA
2.4. HSP70 mRNA
3. Discussion
4. Experimental Section
4.1. Preparation and Characterization of Gradient Polyethylene (PE) Surfaces
4.2. Isolation and Characterization of hADSCs
4.3. hADSCs on Gradient Surface
4.4. Cell Viability Assay
4.5. RNA Extraction and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Gerberich, B.G.; Bhatia, S.K. Tissue scaffold surface patterning for clinical applications. Biotechnol. J 2013, 8, 73–84. [Google Scholar]
- Jayakumar, R.; Chennazhi, K.P.; Srinivasan, S.; Nair, S.V.; Furuike, T.; Hiroshi, T. Chitin scaffolds in tissue engineering. Int. J. Mol. Sci 2011, 12, 1876–1887. [Google Scholar]
- Thitiset, T.; Damrongsakkul, S.; Bunaprasert, T.; Leeanansaksiri, W.; Honsawek, S. Development of collagen/demineralized bone powder scaffolds and periosteum-derived cells for bone tissue engineering application. Int. J. Mol. Sci 2013, 14, 2056–2071. [Google Scholar]
- Fu, Y.; Xu, K.; Zheng, X.; Giacomin, A.J.; Mix, A.W.; Kao, W.J. 3D cell entrapment in crosslinked thiolated gelatin-poly(ethylene glycol) diacrylate hydrogels. Biomaterials 2012, 33, 48–58. [Google Scholar]
- Kim, M.S.; Kim, J.H.; Min, B.H.; Chun, H.J.; Han, D.K.; Lee, H.B. Polymeric scaffolds for regenerative medicine. Polym. Rev 2011, 51, 1–30. [Google Scholar]
- Kim, S.E.; Yun, Y.P.; Suh, D.H.; Kim, Y.R.; Park, K.; Kwon, Y.D.; Suh, J.H.; Chung, J.Y.; Lee, D.W. Recombinant human bone morphogenic protein-2 (rhbmp-2) immobilization onto the surface of apatite-coated titanium significantly promotes osteoblast function and mineralization. Tissue Eng. Regen. Med 2012, 9, 216–223. [Google Scholar]
- Yin, Y.; Dong, Z.; Luo, Q.; Liu, J. Biomimetic catalysts designed on macromolecula scaffold. Prog. Polym. Sci 2012, 37, 1476–1509. [Google Scholar]
- Kwon, J.S.; Kim, G.H.; Kim, D.Y.; Yoon, S.M.; Seo, H.W.; Kim, J.H.; Min, B.H.; Kim, M.S. Chitosan-based hydrogels to induce neuronal differentiation of rat muscle-derived stem cells. Int. J. Biol. Macromol 2012, 51, 974–979. [Google Scholar]
- Zhou, M.; Smith, A.M.; Das, A.K.; Hodson, N.W.; Collins, R.F.; Ulijn, R.V.; Gough, J.E. Self-assembled peptide-based hydrogels as scaffolds for anchorage-dependent cells. Biomaterials 2009, 30, 2523–2530. [Google Scholar]
- Weon, J.I.; Lee, S.Y. Improvement of wettability and removal of skin layer on Ar-plasma-treated polypropylene blend surface. Polymer (Korea) 2012, 36, 461–469. [Google Scholar]
- Kim, M.S.; Khang, G.; Lee, H.B. Gradient polymer surface for biomedical applications. Prog. Polym. Sci 2008, 33, 138–164. [Google Scholar]
- Galvin, C.J.; Genzer, J. Applications of surface-grafted macromolecules derived from post-polymerization modification reactions. Prog. Polym. Sci 2012, 37, 871–906. [Google Scholar]
- Yu, X.; Wang, Z.; Jiang, Y.; Zhang, X. Surface gradient material: From superhydrophobicity to superhydrophilicity. Langmuir 2006, 22, 4483–4486. [Google Scholar]
- Karabanova, L.V.; Mikhalovsky, S.V.; Lloyd, A.W. Gradient semi-interpenetrating polymer networks based on polyurethane and poly(2-hydroxyethyl methacrylate) for biomedical applications. J. Mater. Chem 2012, 22, 7919–7928. [Google Scholar]
- Kim, M.S.; Shin, Y.N.; Cho, M.H.; Kim, S.H.; Kim, S.K.; Cho, Y.H.; Khang, G.; Lee, I.W.; Lee, H.B. Adhesion behavior of human bone marrow stromal cells on differentially wettable polymer surfaces. Tissue Eng 2007, 13, 2095–2103. [Google Scholar]
- Kim, M.S.; Seo, K.S.; Khang, G.; Lee, H.B. Preparation of a gradient biotinylated polyethylene surface to bind streptavidin−FITC. Bioconjug. Chem 2005, 16, 245–249. [Google Scholar]
- Kim, M.S.; Seo, K.S.; Khang, G.; Lee, H.B. The first preparation of biotinylated gradient polyethylene surface to bind photoactive caged streptavidin. Langmuir 2005, 21, 4066–4070. [Google Scholar]
- Kim, B.S.; Lee, S.Y.; Cho, Y.H.; Kim, M.S.; Youn, J.Y.; Khang, G.; Lee, H.B. Preparation of a polymer surface coated with a gradient of quantum dots. Nanotechnology 2008, 19, 045301. [Google Scholar]
- Shin, Y.N.; Kim, B.S.; Ahn, H.H.; Cho, M.H.; Kim, M.S.; Khang, G.; Lee, H.B. Adhesion comparison of human bone marrow stem cells on a gradient wettable surface prepared by corona treatment. Appl. Surf. Sci 2008, 255, 293–296. [Google Scholar]
- Lee, S.J.; Khang, G.; Lee, Y.M.; Lee, H.B. The effect of surface wettability on induction and growth of neurites from the PC-12 cell on a polymer surface. J. Colloid Interface Sci 2003, 259, 228–235. [Google Scholar]
- Lee, J.H.; Lee, J.W.; Khang, G.; Lee, H.B. Interaction of cells on chargeable functional group gradient surfaces. Biomaterials 1997, 18, 351–358. [Google Scholar]
- Chen, F.M.; Sun, H.H.; Lu, H.; Yu, Q. Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials 2012, 33, 6320–6344. [Google Scholar]
- Ahn, H.H.; Kim, K.S.; Lee, J.H.; Lee, J.Y.; Lee, I.W.; Chun, H.J.; Kim, J.H.; Lee, H.B.; Kim, M.S. In vivo osteogenic differentiation of human adipose-derived stem cells in an injectable in situ-forming gel scaffold. Tissue Eng. Part A 2009, 15, 1821–1832. [Google Scholar]
- Casteilla, L.; Planat-Benard, V.; Laharrague, P.; Cousin, B. Adipose-derived stromal cells: Their identity and uses in clinical trials, an update. World J. Stem Cells 2011, 3, 25–33. [Google Scholar]
- Okura, H.; Saga, A.; Soeda, M.; Ichinose, A.; Matsuyama, A. Adipose tissue-derived multi-lineage progenitor cells as a promising tool for in situ stem cell therapy. Curr. Tissue Eng 2012, 1, 54–62. [Google Scholar]
- García, A.J. Get a grip: Integrins in cell-biomaterial interactions. Biomaterials 2005, 26, 7525–7529. [Google Scholar]
- Hu, Z.; Gao, S.; Gao, S.; Hou, R.; Liu, C.; Liu, J.; Li, B.; Liu, D.; Zhang, S.; Lin, B. Elevated levels of lewis Y and integrin α5β1 correlate with chemotherapeutic drug resistance in epithelial ovarian carcinoma. Int. J. Mol. Sci 2012, 13, 15588–15600. [Google Scholar]
- Jäättelä, M.; Wissing, D.; Kokholm, K.; Kallunki, T.; Egeblad, M. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J 1998, 17, 6124–6134. [Google Scholar]
- Nylandsted, J.; Gyrd-Hansen, M.; Danielewicz, A.; Fehrenbacher, N.; Lademann, U.; Høyer-Hansen, M.; Weber, E.; Multhoff, G.; Rohde, M.; Jäättelä, M. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J. Exp. Med 2004, 200, 425–435. [Google Scholar]
- Schakenraad, J.M.; Busscher, H.J.; Wildevuur, C.R.; Arends, J. The influence of substratum surface free energy on growth and spreading of human fibroblasts in the presence and absence of serum proteins. J. Biomed. Mater. Res 1986, 20, 773–784. [Google Scholar]
- Absolom, D.R.; Hawthorn, L.A.; Chang, G. Endothelialization of polymer surfaces. J. Biomed. Mater. Res 1988, 22, 271–285. [Google Scholar]
- Tamada, Y.; Ikada, Y. Effect of preadsorbed proteins on cell adhesion to polymer surfaces. J. Colloid Interface Sci 1993, 155, 334–339. [Google Scholar]



© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ahn, H.H.; Lee, I.W.; Lee, H.B.; Kim, M.S. Cellular Behavior of Human Adipose-Derived Stem Cells on Wettable Gradient Polyethylene Surfaces. Int. J. Mol. Sci. 2014, 15, 2075-2086. https://doi.org/10.3390/ijms15022075
Ahn HH, Lee IW, Lee HB, Kim MS. Cellular Behavior of Human Adipose-Derived Stem Cells on Wettable Gradient Polyethylene Surfaces. International Journal of Molecular Sciences. 2014; 15(2):2075-2086. https://doi.org/10.3390/ijms15022075
Chicago/Turabian StyleAhn, Hyun Hee, Il Woo Lee, Hai Bang Lee, and Moon Suk Kim. 2014. "Cellular Behavior of Human Adipose-Derived Stem Cells on Wettable Gradient Polyethylene Surfaces" International Journal of Molecular Sciences 15, no. 2: 2075-2086. https://doi.org/10.3390/ijms15022075



