Antidiabetic Activity of Zinc Oxide and Silver Nanoparticles on Streptozotocin-Induced Diabetic Rats
Abstract
:1. Introduction
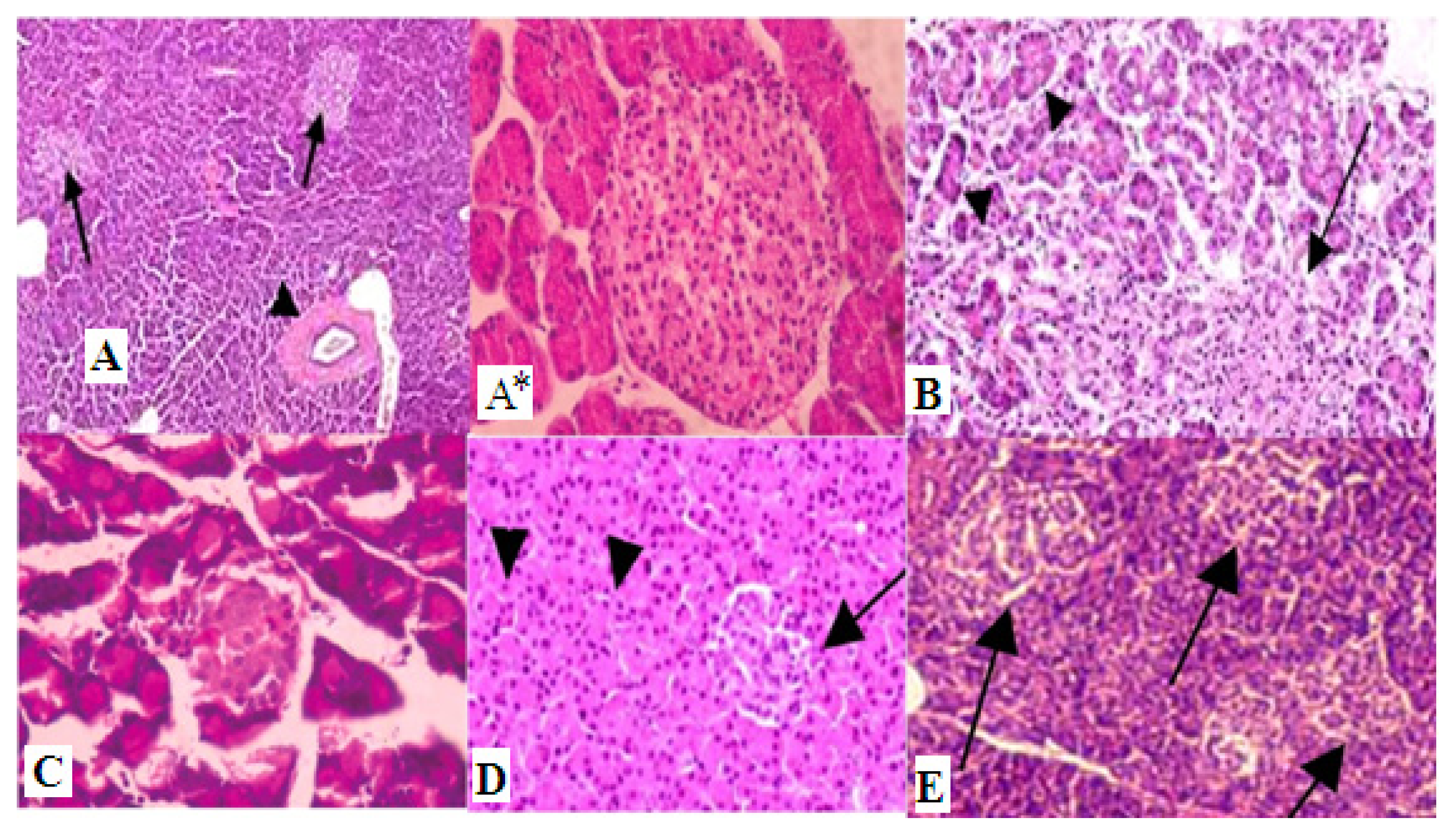
2. Results and Discussion
3. Experimental Section
3.1. Animal Selection and Grouping
3.2. Animal Management
3.3. Ethical Statement
3.4. Induction of Experimental Diabetes
3.5. Sampling Protocol
3.6. Biochemical Determinations
3.7. Molecular Biological Determinations
3.8. Histopathological Examinations
3.9. Statistical Analyses
4. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsA.K.: Participated in the design of the study and helped to draft the manuscript; A.M.A.: Carried out the molecular genetic studies and, participated in the design of the study and helped to draft the manuscript; M.A.: Design and coordination of the study, analysis and interpretation of data, helped to draft the manuscript.
References
- Lin, Y.; Sun, Z. Current views on Type 2 diabetes. J. Endocrinol 2010, 204, 1–11. [Google Scholar]
- Thompson, K.H.; Lichter, J.; LeBel, C.; Scaife, M.C.; McNeill, J.H.; Orvig, C. Vanadium treatment of Type 2 diabetes: A view to the future. J. Inorg. Biochem 2009, 103, 554–558. [Google Scholar]
- Wang, Z.Q.; Cefalu, W.T. Current concepts about chromium supplementation in Type 2 diabetes and insulin resistance. Curr. Diabetes Rep 2010, 10, 145–151. [Google Scholar]
- Wells, I.C. Evidence that the etiology of the syndrome containing Type 2 diabetes mellitus results from abnormal magnesium metabolism. Can. J. Physiol. Pharmacol 2008, 86, 16–24. [Google Scholar]
- Chausmer, A.B. Zinc, insulin and diabetes. J. Am. Coll. Nutr 1998, 17, 109–115. [Google Scholar]
- Haase, H.; Overbeck, S.; Rink, L. Zinc supplementation for the treatment or prevention of disease: Current status and future perspectives. Exp. Gerontol 2008, 43, 394–408. [Google Scholar]
- Jansen, J.; Karges, W.; Rink, L. Zinc and diabetes—Clinical links and molecular mechanisms. J. Nutr. Biochem 2009, 20, 399–417. [Google Scholar]
- Sun, Q.; van Dam, R.M.; Willett, W.C.; Hu, F.B. Prospective study of zinc intake and risk of Type 2 diabetes in women. Diabetes Care 2009, 32, 629–634. [Google Scholar]
- Smidt, K.; Jessen, N.; Petersen, A.B. SLC30A3 responds to glucose- and zinc variations in b-cells and is critical for insulin production and in vivo glucose-metabolism during b-cell stress. PLoS One 2009, 4, e5684–e5691. [Google Scholar]
- Rungby, J. Zinc, zinc transporters and diabetes. Diabetologia 2010, 53, 1549–1551. [Google Scholar]
- Ukperoro, J.U.; Offiah, N.; Idris, T.; Awogoke, D. Antioxidant effect of zinc, selenium and their combination on the liver and kidney of alloxan-induced diabetes in rats. Med. J. Nutr. Metab 2010, 3, 25–30. [Google Scholar]
- Yih, T.C.; Al-Fandi, M.J. Engineered nanoparticles as precise drug delivery system. J. Cell. Biochem 2006, 97, 1184–1190. [Google Scholar]
- Hirst, S.M.; Karakoti, A.S.; Tyler, R.D.; Sriranganathan, N.; Seal, S.; Reilly, C.M. Anti-inflammatory properties of cerium oxide nanoparticles. Small 2009, 5, 2848–2856. [Google Scholar]
- Richards-Williams, C.; Contreras, J.L.; Berecek, K.H.; Schwiebert, E.M. Extracellular ATP and zinc are co-secreted with insulin and activate multiple P2X purinergic receptor channels expressed by islet β-cells to potentiate insulin secretion. Purinergic Signal 2008, 4, 393–405. [Google Scholar]
- Umrani, D.R.; Paknikar, K.M. Zinc oxide nanoparticles show antidiabetic activity in streptozotocin-induced Types 1 and 2 diabetic rats. Nanomedicine 2014, 9, 89–104. [Google Scholar]
- Wijesekara, N.; Dai, F.F.; Hardy, A.B. Beta cell-specific ZnT8 deletion in mice causes marked defects in insulin processing, crystallization and secretion. Diabetologia 2010, 53, 1656–1658. [Google Scholar]
- Mocchegiani, E.; Giacconi, R.; Malavolta, M. Zinc signalling and subcellular distribution: Emerging targets in Type 2 diabetes. Trends Mol. Med 2008, 14, 419–428. [Google Scholar]
- White, M.F.; Yenush, L. The IRS-signaling system: A network of docking proteins that mediate insulin and cytokine action. Curr. Top. Microbiol. Immunol 1998, 228, 179–208. [Google Scholar]
- Matschinsky, F.M.; Magnuson, M.A.; Zelent, D.; Jetton, T.L.; Doliba, N.; Han, Y.; Taub, R.; Grimsby, J. The network of Glucokinase-expressing cells in glucose homeostasis and the potential of Glucokinase activators for diabetes therapy. Diabetes 2006, 55, 1–12. [Google Scholar]
- Zhang, X.; Wenbo, L.; Yiqing, M.; Hui, L.; Yang, Y.; Huanran, T. Hepatic glucokinase activity is the primary defect in alloxan-induced diabetes of mice. Biomed. Pharmacother 2009, 63, 180–186. [Google Scholar]
- Tahrani, A.; Milan, K.; Amy, K.; Anthony, H. Glycaemic control in type 2 diabetes: Targets and new therapies. Pharmacol. Ther 2010, 12, 328–361. [Google Scholar]
- Van, S.E.; Veiga-da-Cunha, M.; Niculescu, L. The regulatory protein of glucokinase. Biochem. Soc. Trans 1997, 25, 136–140. [Google Scholar]
- Orci, L.; Unger, R.H.; Ravazzola, M. Reduced β-cell glucose transporter in new onset diabetic BB rats. J. Clin. Invest 1990, 86, 1615–1622. [Google Scholar]
- Brown, G.K. Glucose transporters: Structure, function and consequences of deficiency. J. Inherit. Metab. Dis 2000, 23, 237–246. [Google Scholar]
- Lee, K.N.; In, C.J.; Song, J.L.; Sang, H.O.; Moo, Y.C. Regulation of leptin gene expression by insulin and growth hormone in mouse adipocytes. Exp. Mol. Med 2001, 33, 234–239. [Google Scholar]
- Pakoskey, A.M.; Lesher, E.C.; Scott, D.B. Hexokinase of Escherichia coli. assay of enzyme activity and adaptation to growth in various media. J. Gen. Microbiol 1965, 38, 73–80. [Google Scholar]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Technique, 4th Ed. ed; Churchill: Livingston, NY, USA, 2008. [Google Scholar]


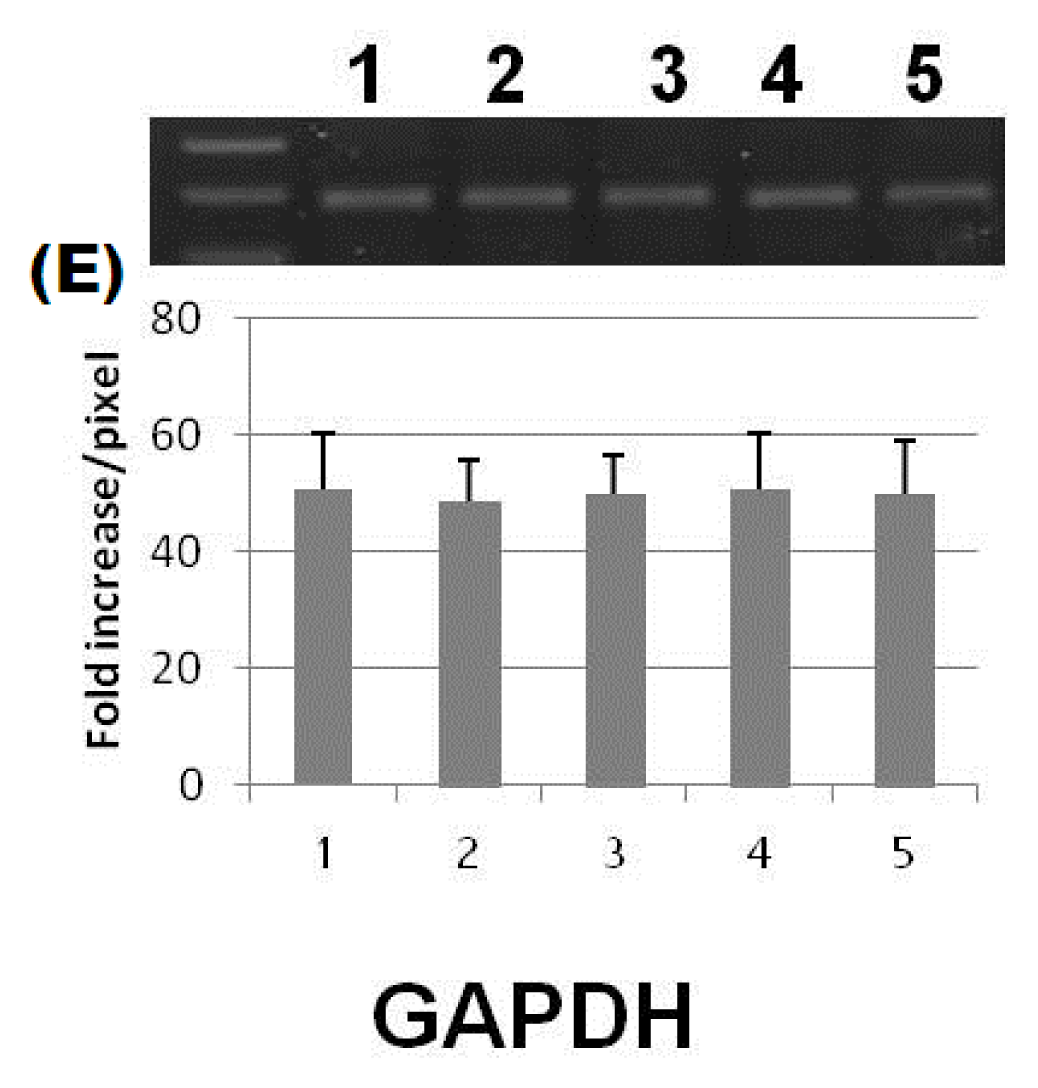
| Parameters | Control | Diabetic | Diabetic + ZnONPs | Diabetic + SNP | Diabetic + insulin |
|---|---|---|---|---|---|
| Blood glucose (mg/dL) | 76.4 ± 2.48 d | 505 ±2 1.04 a | 122.2 ± 6.62 c | 160.6 ± 10.56 b | 80.2 ± 6.36 d |
| Insulin (pg/mL) | 512 ± 36.93 a | 199.9 ± 4.93 c | 358.6 ± 16.46 b | 206.2 ± 3.33 c | 394.4 ± 7.21 b |
| Glucokinase (U/L) | 121 ± 1.51 a | 63.6 ± 3.83 d | 97 ± 1.58 b | 80.2 ± 3.86 c | 92.8 ± 1.48 b |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Alkaladi, A.; Abdelazim, A.M.; Afifi, M. Antidiabetic Activity of Zinc Oxide and Silver Nanoparticles on Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2014, 15, 2015-2023. https://doi.org/10.3390/ijms15022015
Alkaladi A, Abdelazim AM, Afifi M. Antidiabetic Activity of Zinc Oxide and Silver Nanoparticles on Streptozotocin-Induced Diabetic Rats. International Journal of Molecular Sciences. 2014; 15(2):2015-2023. https://doi.org/10.3390/ijms15022015
Chicago/Turabian StyleAlkaladi, Ali, Aaser Mohamed Abdelazim, and Mohamed Afifi. 2014. "Antidiabetic Activity of Zinc Oxide and Silver Nanoparticles on Streptozotocin-Induced Diabetic Rats" International Journal of Molecular Sciences 15, no. 2: 2015-2023. https://doi.org/10.3390/ijms15022015




