Chronic Exposure to Rhodobacter Sphaeroides Extract Lycogen™ Prevents UVA-Induced Malondialdehyde Accumulation and Procollagen I Down-Regulation in Human Dermal Fibroblasts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Toxicity Testing of Lycogen™ in Human Fibroblast Lines
2.2. UVA Irradiation-Induced Cell Death Accompanied by Downregulation of Type 1 Procollagen in Human Fibroblast Lines
2.3. Lycogen™ Ameliorated Cell Death Induced by UVA Irradiation in Human Fibroblast Lines
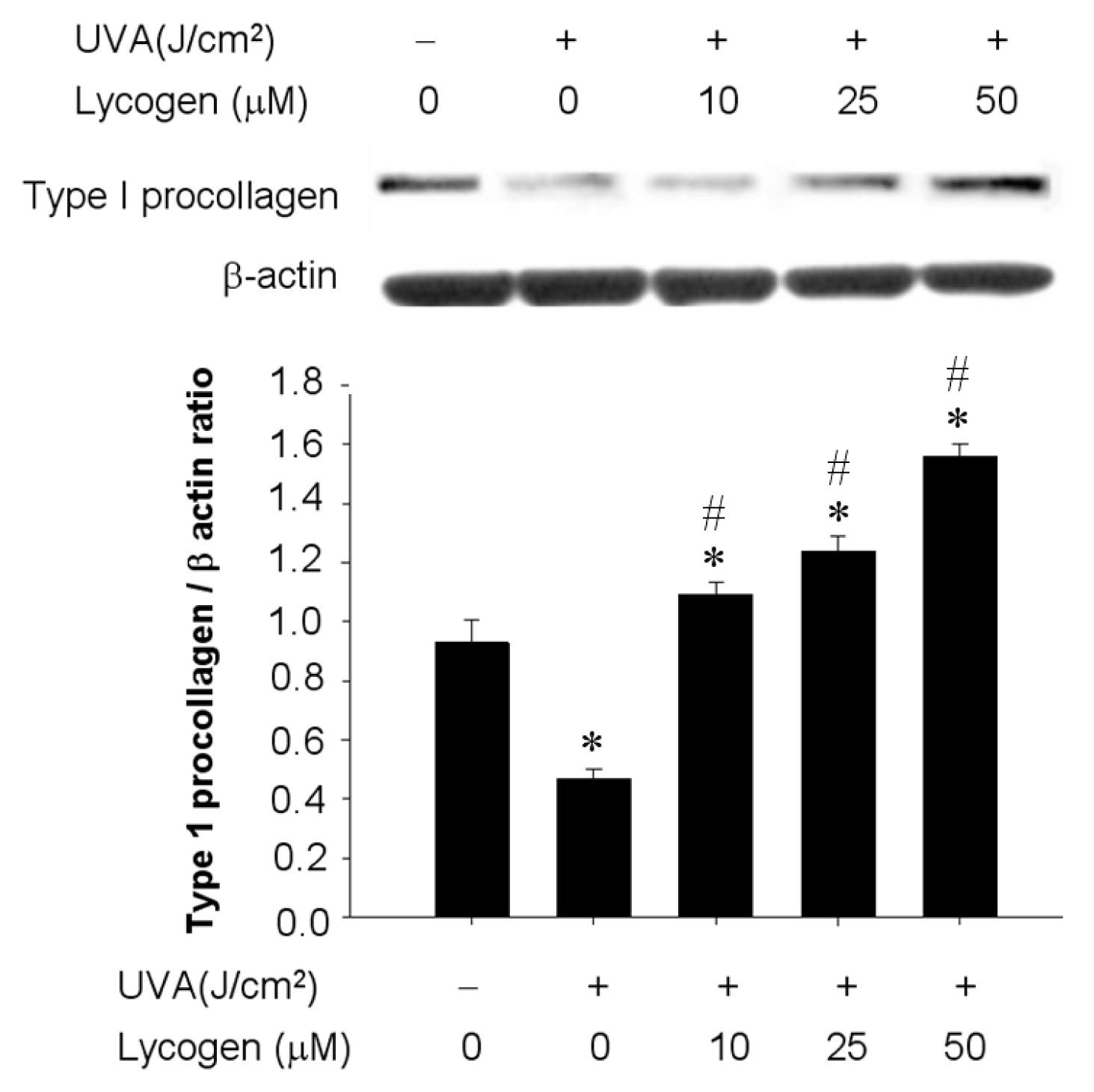
2.4. Chronic Exposure to Lycogen™ Prevented UVA-Induced Downregulation of Type I Procollagen in Human Dermal Fibroblasts
2.5. Chronic Exposure to Lycogen™ Prevented the UVA-Induced Upregulation of MMP-1 in Human Dermal Fibroblasts
2.6. Chronic Exposure to Lycogen™ Suppressed the UVA-Induced Malondialdehyde Accumulation in Human Dermal Fibroblasts
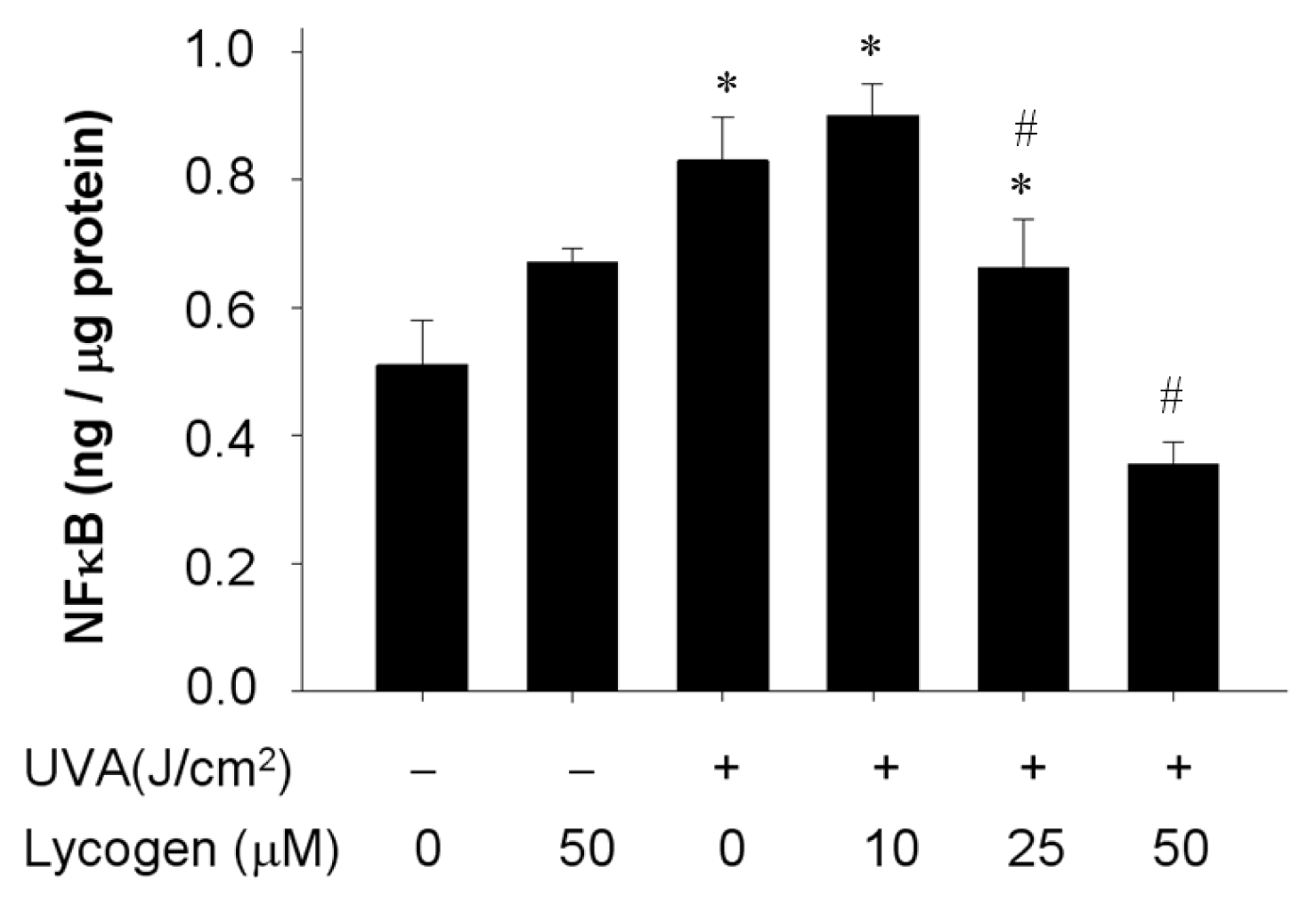
2.7. Chronic Exposure to Lycogen™ Inhibited the UVA-Induced Upregulation of NFκB Levels in Human Dermal Fibroblasts
2.8. Discussion
3. Materials and methods
3.1. Reagents
3.2. Lycogen™, Cell Culture and UVA Irradiation
3.3. Measurement of Cell Viability
3.4. Measurement of Lipid Peroxidation
3.5. Western Blot Assay
3.6. Measurement of NFκB
3.7. Data Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kohl, E.; Steinbauer, J.; Landthaler, M.; Szeimies, R.M. Skin ageing. J. Eur. Acad. Dermatol. Venereol 2011, 25, 873–884. [Google Scholar]
- Biesalski, H.K.; Berneburg, M.; Grune, T.; Kerscher, M.; Krutmann, J.; Raab, W.; Reimann, J.; Reuther, T.; Robert, L.; Schwarz, T. Hohenheimer Consensus Talk. Oxidative and premature skin ageing. Exp. Dermatol 2003, 12, 3–15. [Google Scholar]
- Kim, J.A.; Ahn, B.N.; Kong, C.S.; Kim, S.K. The chromene sargachromanol E inhibits ultraviolet A-induced ageing of skin in human dermal fibroblasts. Br. J. Dermatol 2013, 168, 968–976. [Google Scholar]
- Scharffetter, K.; Wlaschek, M.; Hogg, A.; Bolsen, K.; Schothorst, A.; Goerz, G.; Krieg, T.; Plewig, G. UVA irradiation induces collagenase in human dermal fibroblasts in vitro and in vivo. Arch. Dermatol. Res. 1991, 283, 506–511. [Google Scholar]
- Krutmann, J. The role of UVA rays in skin aging. Eur. J. Dermatol 2001, 11, 170–171. [Google Scholar]
- Wlaschek, M.; Tantcheva-Poor, I.; Naderi, L.; Ma, W.; Schneider, L.A.; Razi-Wolf, Z.; Schuller, J.; Scharffetter-Kochanek, K. Solar UV irradiation and dermal photoaging. J. Photochem. Photobiol. B 2001, 63, 41–51. [Google Scholar]
- Greinert, R.; Volkmer, B.; Henning, S.; Breitbart, E.W.; Greulich, K.O.; Cardoso, M.C.; Rapp, A. UVA-induced DNA double-strand breaks result from the repair of clustered oxidative DNA damages. Nucleic Acids Res 2012, 40, 10263–10273. [Google Scholar]
- Evans, J.A.; Johnson, E.J. The role of phytonutrients in skin health. Nutrients 2010, 2, 903–928. [Google Scholar]
- Binic, I.; Lazarevic, V.; Ljubenovic, M.; Mojsa, J.; Sokolovic, D. Skin ageing: Natural weapons and strategies. Evid. Based Complement Alternat. Med 2013, 2013, 827248. [Google Scholar]
- Darvin, M.; Patzelt, A.; Gehse, S.; Schanzer, S.; Benderoth, C.; Sterry, W.; Lademann, J. Cutaneous concentration of lycopene correlates significantly with the roughness of the skin. Eur. J. Pharm. Biopharm 2008, 69, 943–947. [Google Scholar]
- Lopes, L.B.; Reed, R. A simple and rapid method to assess lycopene in multiple layers of skin samples. Biomed. Chromatogr 2010, 24, 154–159. [Google Scholar]
- Marcotorchino, J.; Romier, B.; Gouranton, E.; Riollet, C.; Gleize, B.; Malezet-Desmoulins, C.; Landrier, J.F. Lycopene attenuates LPS-induced TNF-alpha secretion in macrophages and inflammatory markers in adipocytes exposed to macrophage-conditioned media. Mol. Nutr. Food Res 2012, 56, 725–732. [Google Scholar]
- Chen, M.L.; Lin, Y.H.; Yang, C.M.; Hu, M.L. Lycopene inhibits angiogenesis both in vitro and in vivo by inhibiting MMP-2/uPA system through VEGFR2-mediated PI3K-Akt and ERK/p38 signaling pathways. Mol. Nutr. Food Res 2012, 56, 889–899. [Google Scholar]
- Holzapfel, N.P.; Holzapfel, B.M.; Champ, S.; Feldthusen, J.; Clements, J.; Hutmacher, D.W. The potential role of lycopene for the prevention and therapy of prostate cancer: From molecular mechanisms to clinical evidence. Int. J. Mol. Sci 2013, 14, 14620–14646. [Google Scholar]
- Lorenz, M.; Fechner, M.; Kalkowski, J.; Frohlich, K.; Trautmann, A.; Bohm, V.; Liebisch, G.; Lehneis, S.; Schmitz, G.; Ludwig, A. Effects of lycopene on the initial state of atherosclerosis in New Zealand White (NZW) rabbits. PLoS One 2012, 7, e30808. [Google Scholar]
- Topal, U.; Sasaki, M.; Goto, M.; Hayakawa, K. Extraction of lycopene from tomato skin with supercritical carbon dioxide: Effect of operating conditions and solubility analysis. J. Agric. Food Chem 2006, 54, 5604–5610. [Google Scholar]
- Liu, W.S.; Chen, M.C.; Chiu, K.H.; Wen, Z.H.; Lee, C.H. Amelioration of dextran sodium sulfate-induced colitis in mice by Rhodobacter sphaeroides extract. Molecules 2012, 17, 13622–13630. [Google Scholar]
- Liu, W.S.; Kuan, Y.D.; Chiu, K.H.; Wang, W.K.; Chang, F.H.; Liu, C.H.; Lee, C.H. The extract of Rhodobacter sphaeroides inhibits melanogenesis through the MEK/ERK signaling pathway. Mar. Drugs 2013, 11, 1899–1908. [Google Scholar]
- Fulia, F.; Gitto, E.; Cuzzocrea, S.; Reiter, R.J.; Dugo, L.; Gitto, P.; Barberi, S.; Cordaro, S.; Barberi, I. Increased levels of malondialdehyde and nitrite/nitrate in the blood of asphyxiated newborns: Reduction by melatonin. J. Pineal. Res 2001, 31, 343–349. [Google Scholar]
- Thomas, D.R. Vitamins in aging, health, and longevity. Clin. Interv. Aging 2006, 1, 81–91. [Google Scholar]
- Mangels, A.R.; Holden, J.M.; Beecher, G.R.; Forman, M.R.; Lanza, E. Carotenoid content of fruits and vegetables: An evaluation of analytic data. J. Am. Diet Assoc 1993, 93, 284–296. [Google Scholar]
- Johnson, E.J. The role of carotenoids in human health. Nutr. Clin. Care 2002, 5, 56–65. [Google Scholar]
- Agarwal, S.; Rao, A.V. Carotenoids and chronic diseases. Drug Metabol. Drug Interact 2000, 17, 189–210. [Google Scholar]
- Gerster, H. The potential role of lycopene for human health. J. Am. Coll. Nutr 1997, 16, 109–126. [Google Scholar]
- Stahl, W.; Sies, H. Photoprotection by dietary carotenoids: Concept, mechanisms, evidence and future development. Mol. Nutr. Food Res 2012, 56, 287–295. [Google Scholar]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.K. Pharmaceutically active secondary metabolites of marine actinobacteria. Microbiol Res 2013. [Google Scholar] [CrossRef]
- Bhosale, P. Environmental and cultural stimulants in the production of carotenoids from microorganisms. Appl. Microbiol. Biotechnol 2004, 63, 351–361. [Google Scholar]
- Verwaal, R.; Wang, J.; Meijnen, J.P.; Visser, H.; Sandmann, G.; van den Berg, J.A.; van Ooyen, A.J. High-level production of beta-carotene in Saccharomyces cerevisiae by successive transformation with carotenogenic genes from Xanthophyllomyces dendrorhous. Appl. Environ. Microbiol 2007, 73, 4342–4350. [Google Scholar]
- Takaichi, S.; Sandmann, G.; Schnurr, G.; Satomi, Y.; Suzuki, A.; Misawa, N. The carotenoid 7,8-dihydro-psi end group can be cyclized by the lycopene cyclases from the bacterium Erwinia uredovora and the higher plant Capsicum annuum. Eur. J. Biochem 1996, 241, 291–296. [Google Scholar]
- Bae, J.T.; Ko, H.J.; Kim, G.B.; Pyo, H.B.; Lee, G.S. Protective effects of fermented Citrus unshiu peel extract against ultraviolet-A-induced photoageing in human dermal fibrobolasts. Phytother. Res 2012, 26, 1851–1856. [Google Scholar]
- He, D.; Sun, J.; Zhu, X.; Nian, S.; Liu, J. Compound K increases type I procollagen level and decreases matrix metalloproteinase-1 activity and level in ultraviolet-A-irradiated fibroblasts. J. Formos. Med. Assoc 2011, 110, 153–160. [Google Scholar]
- Kato, S.; Saitoh, Y.; Iwai, K.; Miwa, N. Hydrogen-rich electrolyzed warm water represses wrinkle formation against UVA ray together with type-I collagen production and oxidative-stress diminishment in fibroblasts and cell-injury prevention in keratinocytes. J. Photochem. Photobiol. B 2012, 106, 24–33. [Google Scholar]
- Pluemsamran, T.; Onkoksoong, T.; Panich, U. Caffeic acid and ferulic acid inhibit UVA-induced matrix metalloproteinase-1 through regulation of antioxidant defense system in keratinocyte HaCaT cells. Photochem. Photobiol 2012, 88, 961–968. [Google Scholar]
- Lee, Y.K.; Cha, H.J.; Hong, M.; Yoon, Y.; Lee, H.; An, S. Role of NF-kappaB-p53 crosstalk in ultraviolet A-induced cell death and G1 arrest in human dermal fibroblasts. Arch. Dermatol. Res 2012, 304, 73–79. [Google Scholar]
- Loveland, B.E.; Johns, T.G.; Mackay, I.R.; Vaillant, F.; Wang, Z.X.; Hertzog, P.J. Validation of the MTT dye assay for enumeration of cells in proliferative and antiproliferative assays. Biochem. Int 1992, 27, 501–510. [Google Scholar]
- Sano, M.; Motchnik, P.A.; Tapple, A.L. Halogenated hydrocarbonand hydroperoxide-induced peroxidation in rat tissue slices. Free Radic. Biol. Med 1986, 2, 41–48. [Google Scholar]





© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yang, T.-H.; Lai, Y.-H.; Lin, T.-P.; Liu, W.-S.; Kuan, L.-C.; Liu, C.-C. Chronic Exposure to Rhodobacter Sphaeroides Extract Lycogen™ Prevents UVA-Induced Malondialdehyde Accumulation and Procollagen I Down-Regulation in Human Dermal Fibroblasts. Int. J. Mol. Sci. 2014, 15, 1686-1699. https://doi.org/10.3390/ijms15021686
Yang T-H, Lai Y-H, Lin T-P, Liu W-S, Kuan L-C, Liu C-C. Chronic Exposure to Rhodobacter Sphaeroides Extract Lycogen™ Prevents UVA-Induced Malondialdehyde Accumulation and Procollagen I Down-Regulation in Human Dermal Fibroblasts. International Journal of Molecular Sciences. 2014; 15(2):1686-1699. https://doi.org/10.3390/ijms15021686
Chicago/Turabian StyleYang, Tsai-Hsiu, Ying-Hsiu Lai, Tsuey-Pin Lin, Wen-Sheng Liu, Li-Chun Kuan, and Chia-Chyuan Liu. 2014. "Chronic Exposure to Rhodobacter Sphaeroides Extract Lycogen™ Prevents UVA-Induced Malondialdehyde Accumulation and Procollagen I Down-Regulation in Human Dermal Fibroblasts" International Journal of Molecular Sciences 15, no. 2: 1686-1699. https://doi.org/10.3390/ijms15021686



