The Study of NADPH-Dependent Flavoenzyme-Catalyzed Reduction of Benzo[1,2-c]1,2,5-oxadiazole N-Oxides (Benzofuroxans)
Abstract
:1. Introduction
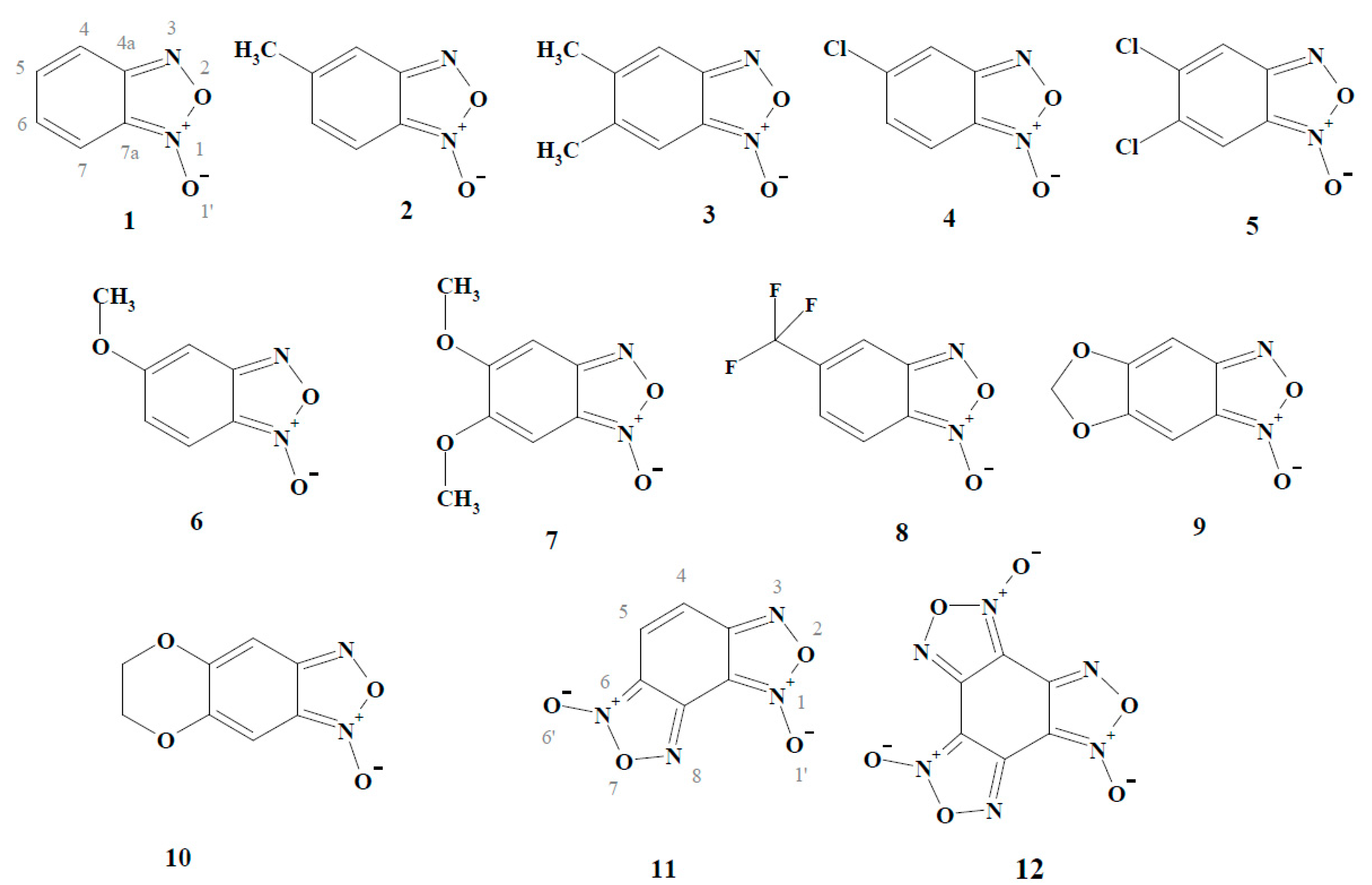
2. Results and Discussion
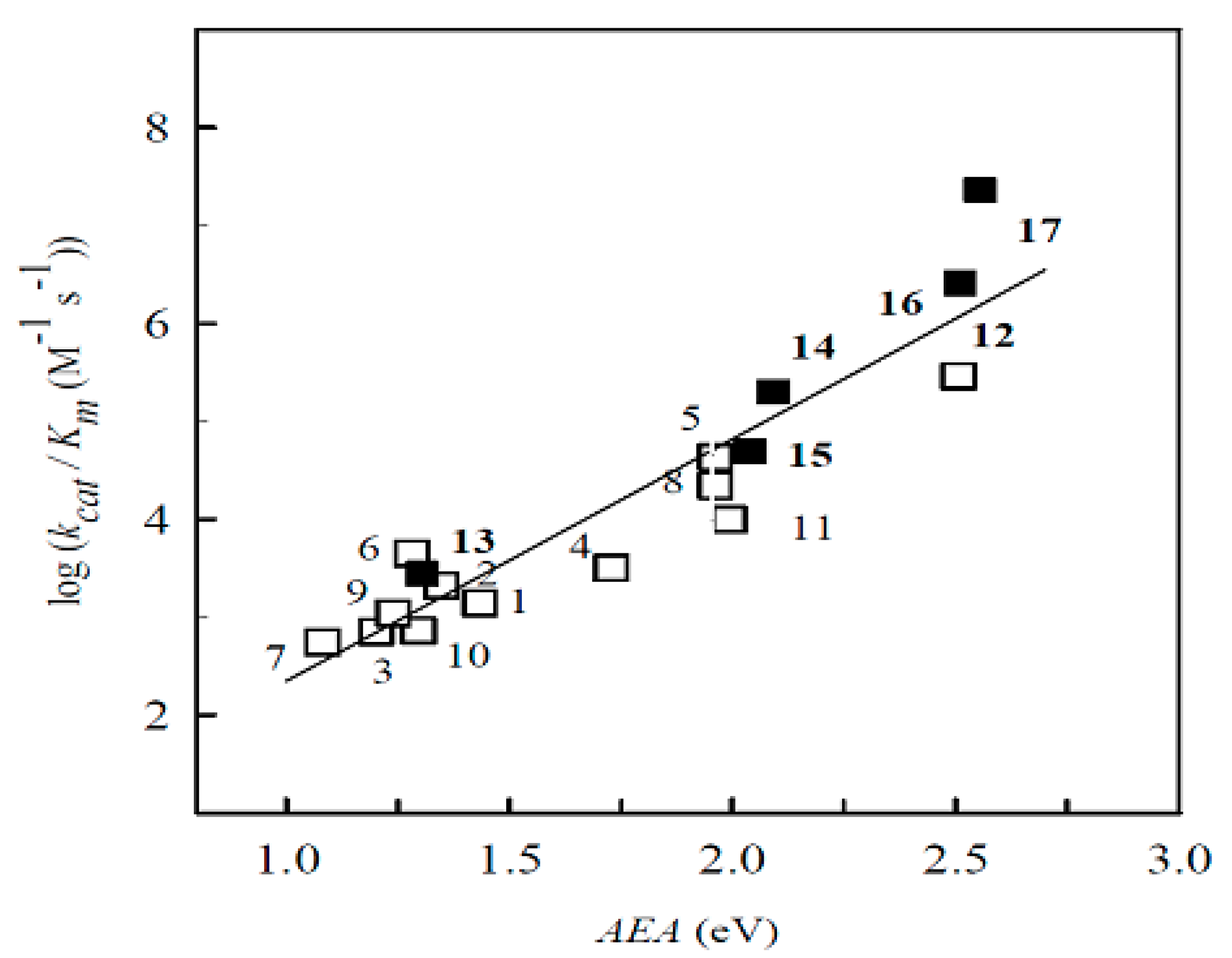
2.1. The Computational Study of the Electron-Accepting Properties of BFXs
| No. | Compound | E17 (V) | VEA (eV) | AEA (eV) | S (eV−1) | Χ (eV) | ϖ (eV) |
|---|---|---|---|---|---|---|---|
| Benzofuroxans | |||||||
| 1. | Benzofuroxan | 1.241 | 1.433 | 0.142 | 4.799 | 3.262 | |
| 2. | 5-Methylbenzofuroxan | 1.146 | 1.348 | 0.140 | 4.668 | 3.040 | |
| 3. | 5,6-Dimethylbenzofuroxan | 1.093 | 1.202 | 0.141 | 4.527 | 2.885 | |
| 4. | 5-Chlorobenzofuroxan | 1.524 | 1.729 | 0.142 | 5.029 | 3.580 | |
| 5. | 5,6-Dichlorobenzofuroxan | 1.760 | 1.962 | 0.146 | 5.150 | 3.877 | |
| 6. | 5-Methoxybenzofuroxan | 1.051 | 1.296 | 0.136 | 4.595 | 2.897 | |
| 7. | 5,6-Dimethoxybenzofuroxan | 0.815 | 1.082 | 0.137 | 4.274 | 2.499 | |
| 8. | 5-Trifluorobenzofuroxan | 1.734 | 1.962 | 0.143 | 5.253 | 3.928 | |
| 9. | 5,6-Methylenedioxybenzofuroxan | 0.990 | 1.240 | 0.137 | 4.514 | 2.795 | |
| 10. | 5,6-Ethylenedioxybenzofuroxan | 1.035 | 1.282 | 0.146 | 4.419 | 2.852 | |
| 11. | Benzodifuroxan | 1.710 | 1.992 | 0.125 | 5.382 | 3.621 | |
| 12. | Benzotrifuroxan | 2.384 | 2.507 | 0.121 | 5.947 | 4.278 | |
| Nitrobenzenes | |||||||
| 13. | Nitrobenzene | −0.485 | 0.991 | 1.304 | 0.099 | 5.435 | 2.933 |
| 14. | 1,2-Dinitrobenzene | −0.287 | 1.817 | 2.093 | 0.097 | 6.192 | 3.548 |
| 15. | 1,3-Dinitrobenzene | −0.345 | 1.703 | 2.040 | 0.102 | 5.902 | 3.694 |
| 16. | 1,4-Dinitrobenzene | −0.257 | 2.212 | 2.510 | 0.105 | 6.332 | 4.196 |
| 17. | 2,4,6-Trinitrotoluene | −0.253 | 2.370 | 2.555 | 0.102 | 6.378 | 4.130 |
2.2. The Study of Enzymatic Reactivity of BFXs
2.2.1. P-450R-Catalyzed Reduction of BFXs
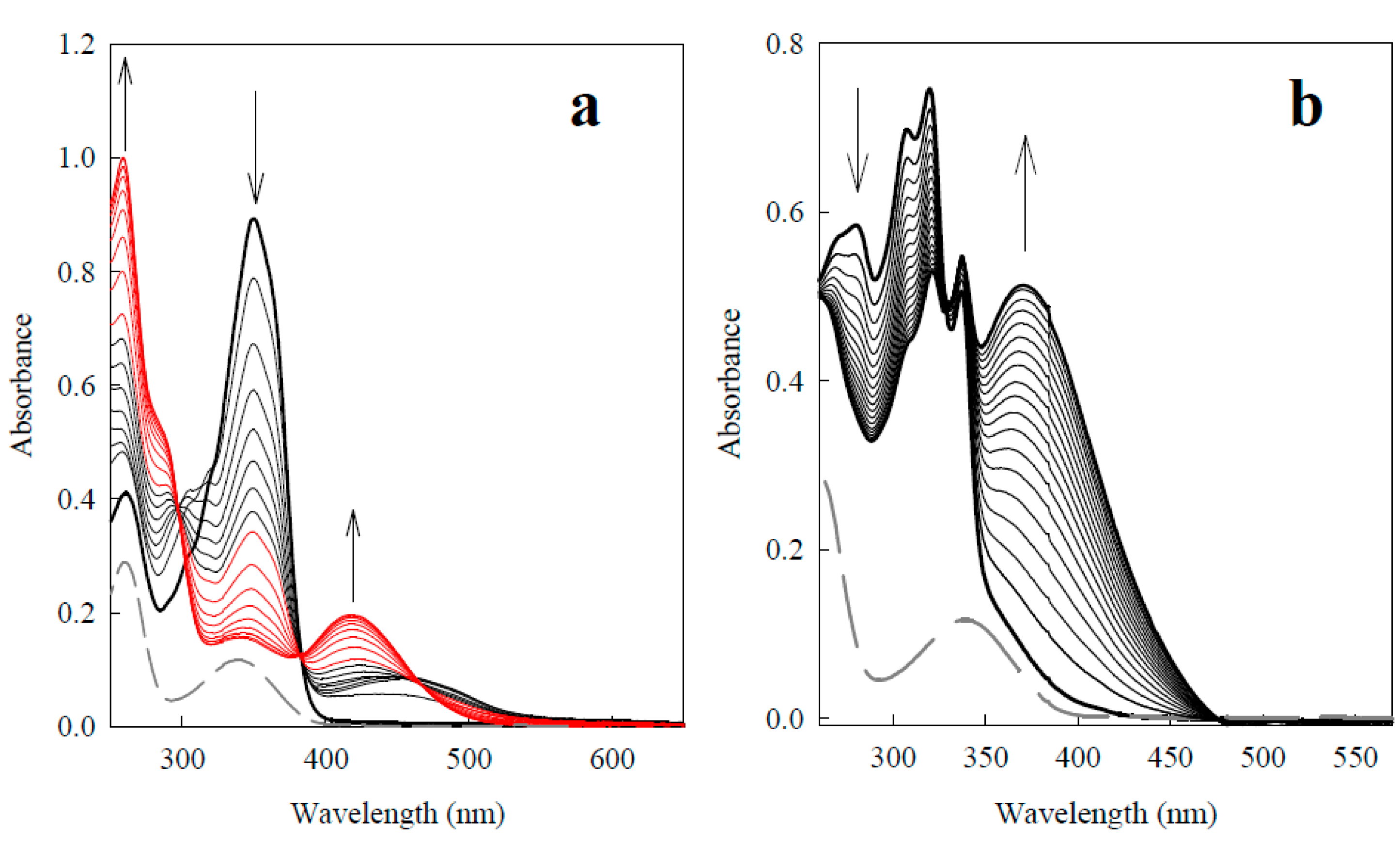
| No. | Compound | kcat/Km (M−1·s−1) |
|---|---|---|
| Benzofuroxans | ||
| 1. | Benzofuroxan | 1.4 ± 0.2 × 103 |
| 2. | 5-Methylbenzofuroxan | 2.1 ± 0.1 × 103 |
| 3. | 5-Dimethylbenzofuroxan | 7.1 ± 0.8 × 102 |
| 4. | 5-Chlorobenzofuroxan | 3.2 ± 0.2 × 103 |
| 5. | 5,6-Dichlorobenzofuroxan | 4.3 ± 0.3 × 104 |
| 6. | 5-Methoxybenzofuroxan | 7.3 ± 0.3 × 102 |
| 7. | 5,6-Dimethoxybenzofuroxan | 5.6 ± 0.5 × 102 |
| 8. | 5-Trifluorobenzofuroxan | 1.1 ± 0.1 × 104 |
| 9. | 5,6-Methylendioxybenzofuroxan | 1.1 ± 0.1 × 103 |
| 10. | 5,6-Ethylendioxybenzofuroxan | 4.5 ± 0.5 × 103 |
| 11. | Benzodifuroxan | 1.0 ± 0.1 × 104 |
| 12. | Benzotrifuroxan | 2.9 ± 0.2 × 105 |
| Nitrobenzenes | ||
| 13. | Nitrobenzene | 2.8 ± 0.3 × 103 |
| 14. | 1,2-Dinitrobenzene | 2.0 ± 0.2 × 105 |
| 15. | 1,3-Dinitrobenzene | 4.9 ± 0.4 × 104 |
| 16. | 1,4-Dinitrobenzene | 2.6 ± 0.3 × 106 |
| 17. | 2,4,6-Trinitrotoluene | 2.3 ± 0.2 × 107 |
2.2.2. NQO1-Catalyzed Reduction of BFXs
| No. | Compound | kcat (s−1) | kcat/Km (M−1·s−1) |
|---|---|---|---|
| Benzofuroxans | |||
| 1. | Benzofuroxan | 11.0 ± 1.5 | 8.4 ± 0.3 × 104 |
| 2. | 5-Methylbenzofuroxan | 2.4 ± 0.4 | 1.4 ± 0.1 × 104 |
| 3. | 5-Dimethylbenzofuroxan | 1.8 ± 0.2 | 4.7 ± 1.1 × 103 |
| 4. | 5-Chlorobenzofuroxan | 5.6 ± 0.6 | 6.2 ± 0.5 × 104 |
| 5. | 5,6-Dichlorobenzofuroxan | 6.9 ± 0.1 | 1.3 ± 0.1 × 105 |
| 6. | 5-Methoxybenzofuroxan | 4.4 ± 0.1 | 2.5 ± 0.4 × 104 |
| 7. | 5,6-Dimethoxybenzofuroxan | 1.1 ± 0.1 | 1.4 ± 0.3 × 104 |
| 8. | 5-Trifluorobenzofuroxan | 7.6 ± 0.9 | 2.5 ± 0.4 × 105 |
| 9. | 5,6-Methylendioxybenzofuroxan | 1.2 ± 0.4 | 4.6 ± 0.9 × 103 |
| 10. | 5,6-Ethylendioxybenzofuroxan | 0.7 ± 0.1 | 8.1 ± 1.7 × 103 |
| 11. | Benzodifuroxan | 2.3 ± 0.4 | 1.6 ± 0.3 × 104 |
| 12. | Benzotrifuroxan | 2.6 ± 0.3 | 3.8 ± 0.4 × 104 |
| Nitrobenzenes | |||
| 13. | Nitrobenzene | <0.1 | 2.4 ± 0.2 × 101 |
| 14. | 1,2-Dinitrobenzene | <0.1 | 6.6 ± 0.7 × 102 |
| 15. | 1,3-Dinitrobenzene | 0.3 | 7.4 ± 0.7 × 102 |
| 16. | 1,4-Dinitrobenzene | 0.6 ± 0.1 | 2.4 ± 0.1 × 103 |
| 17. | 2,4,6-Trinitrotoluene | 1.0 | 6.7 ± 0.7 × 102 |

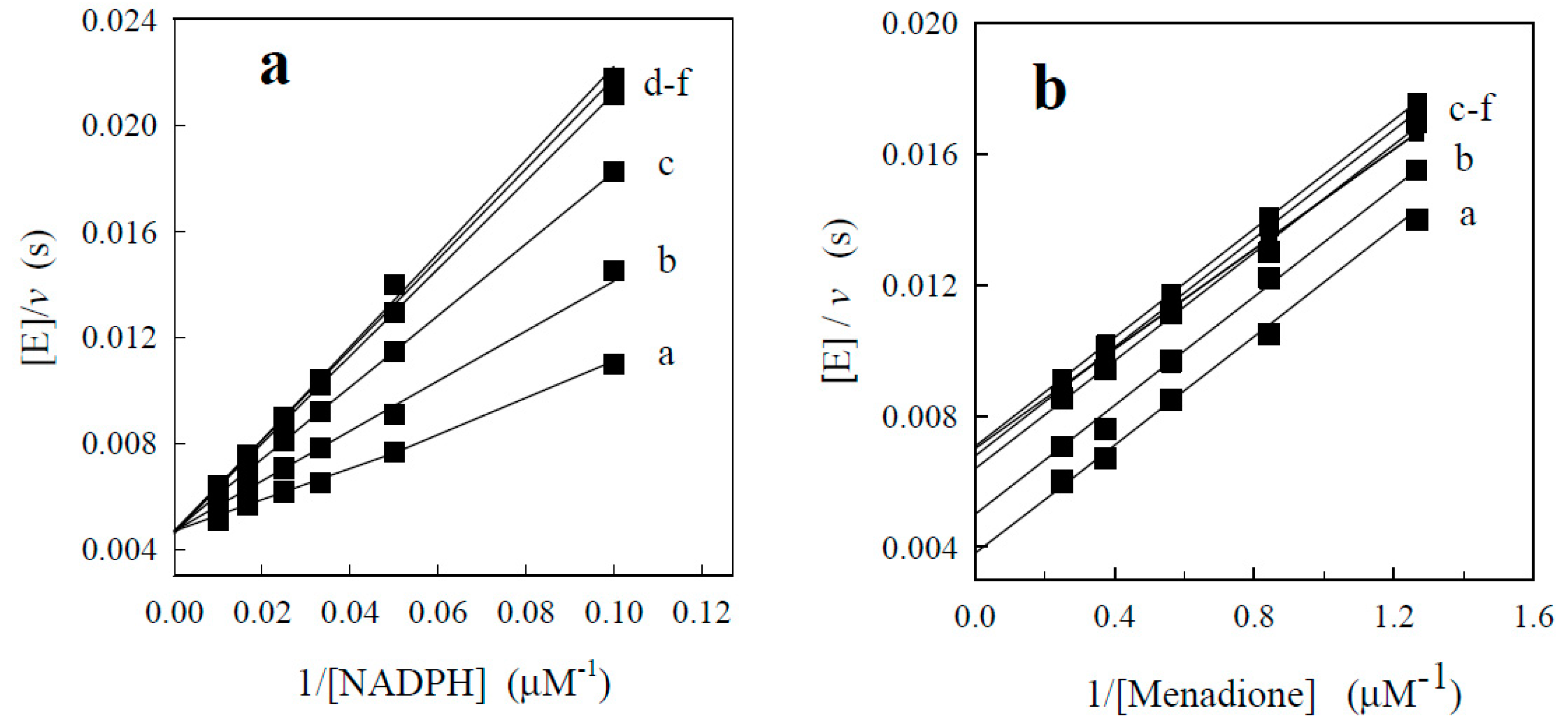

| No. | Compound | Ki (µM) | εmax (%) |
|---|---|---|---|
| Benzofuroxans | |||
| 1. | Benzofuroxan | 20.3 ± 3.9 | 72.9 ± 3.0 |
| 2. | 5-Methylbenzofuroxan | 25.7 ± 4.2 | 73.3 ± 2.5 |
| 3. | 5-Dimethylbenzofuroxan | 29.3 ± 2.5 | 87.0 ± 2.6 |
| 4. | 5-Chlorobenzofuroxan | 33.8 ± 4.2 | 82.0 ± 2.3 |
| 5. | 5,6-Dichlorobenzofuroxan | 22.1 ± 5.7 | 84.7 ± 4.2 |
| 6. | 5-Methoxybenzofuroxan | 32.9 ± 3.4 | 89.4 ± 1.2 |
| 7. | 5,6-Dimethoxybenzofuroxan | 55.3 ± 3.4 | 89.4 ± 1.2 |
| 8. | 5-Trifluorobenzofuroxan | 49.0 ± 2.8 | 77.0 ± 1.6 |
| 9. | 5,6-Methylendioxybenzofuroxan | 110 ± 19 | 75.9 ± 4.0 |
| 10. | 5,6-Ethylendioxybenzofuroxan | 112 ± 12 | 81.3 ± 2.8 |
| 11. | Benzodifuroxan | 41.5 ± 5.0 | 100 |
| 12. | Benzotrifuroxan | 46.0 ± 5.6 | 100 |
| Nitrobenzenes | |||
| 13. | Nitrobenzene | 900 | 100 |
| 14. | 1,2-Dinitrobenzene | ||
| 15. | 1,3-Dinitrobenzene | ||
| 16. | 1,4-Dinitrobenzene | 200 | 100 |
| 17. | 2,4,6-Trinitrotoluene | 80 | 100 |
| Compound | kcat (s−1) | kcat/Km (M−1·s−1) | ∆H≠ (kJ·mol−1) | ∆S≠ (eu) |
|---|---|---|---|---|
| Tetramethyl-1,4-benzoquinone a | 1000 ± 90 | 6.7 ± 0.8 × 107 | 4.44 ± 1.05 | −76.09 ± 3.44 |
| 2-Hydroxy-1,4-nathphoquinone a | 232 ± 25 | 5.9 ± 0.5 × 106 | 10.10 ± 3.35 | −84.43 ± 8.30 |
| Benzofuroxan | 11.0 ± 1.5 | 8.4 ± 0.3 × 104 | 30.45 ± 6.46 | −42.52 ± 8.30 |
| 5,6-Dichlorobenzofuroxan | 6.9 ± 0.1 | 1.3 ± 0.1 × 105 | 30.87 ± 1.70 | −46.06 ± 5.72 |
| 1,4-Dinitrobenzene b | 0.6 ± 0.1 | 2.3 ± 0.1 × 103 | 50.58 ± 10.31 | −8.81 ± 2.25 |
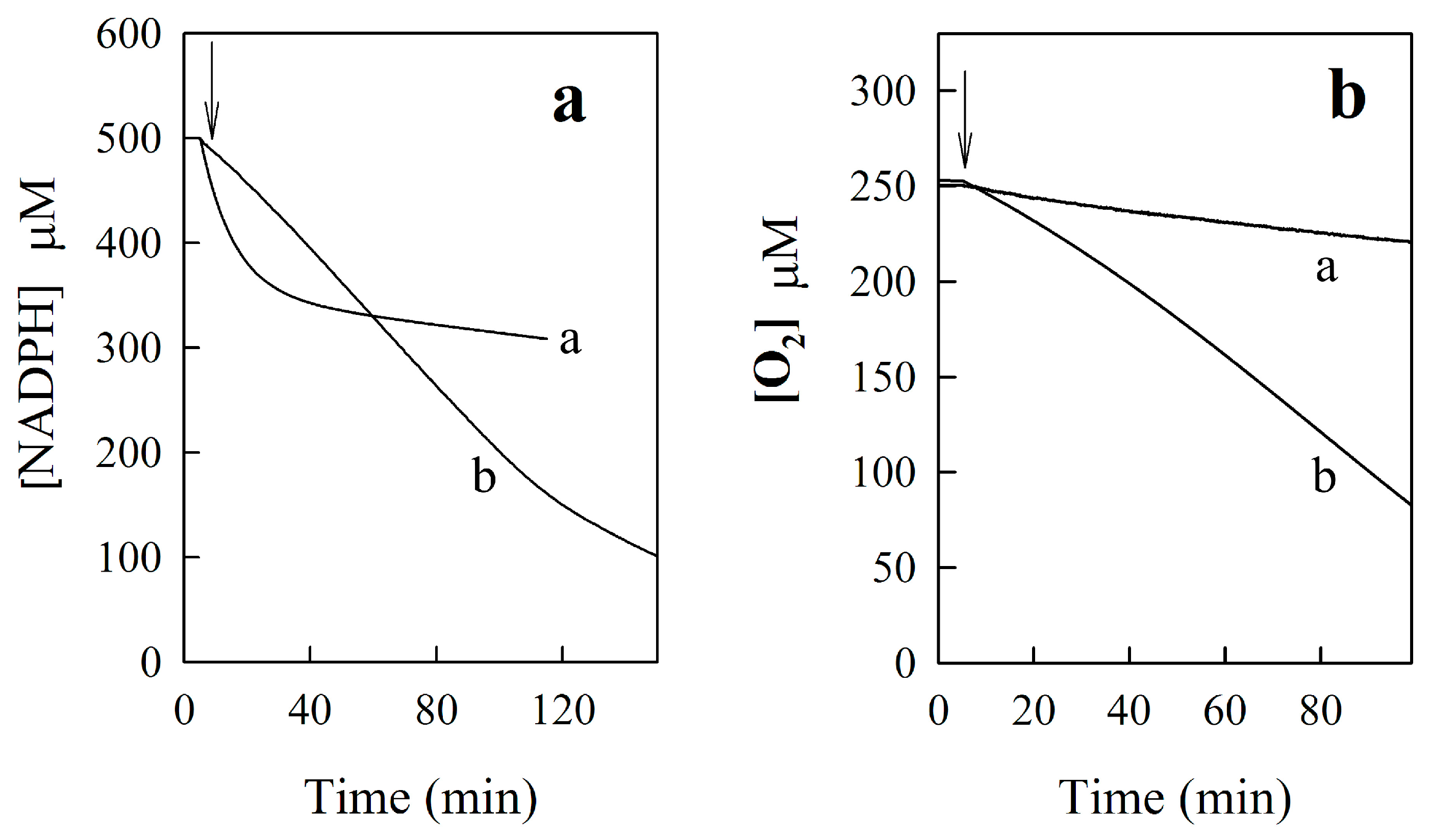
2.2.3. o-Benzoquinone Dioxime and 2,3-Diaminophenazine as the P-450R- and NQO1-Catalyzed Prime Reductive Intermediate and the Final Product of Benzofuroxan, Respectively
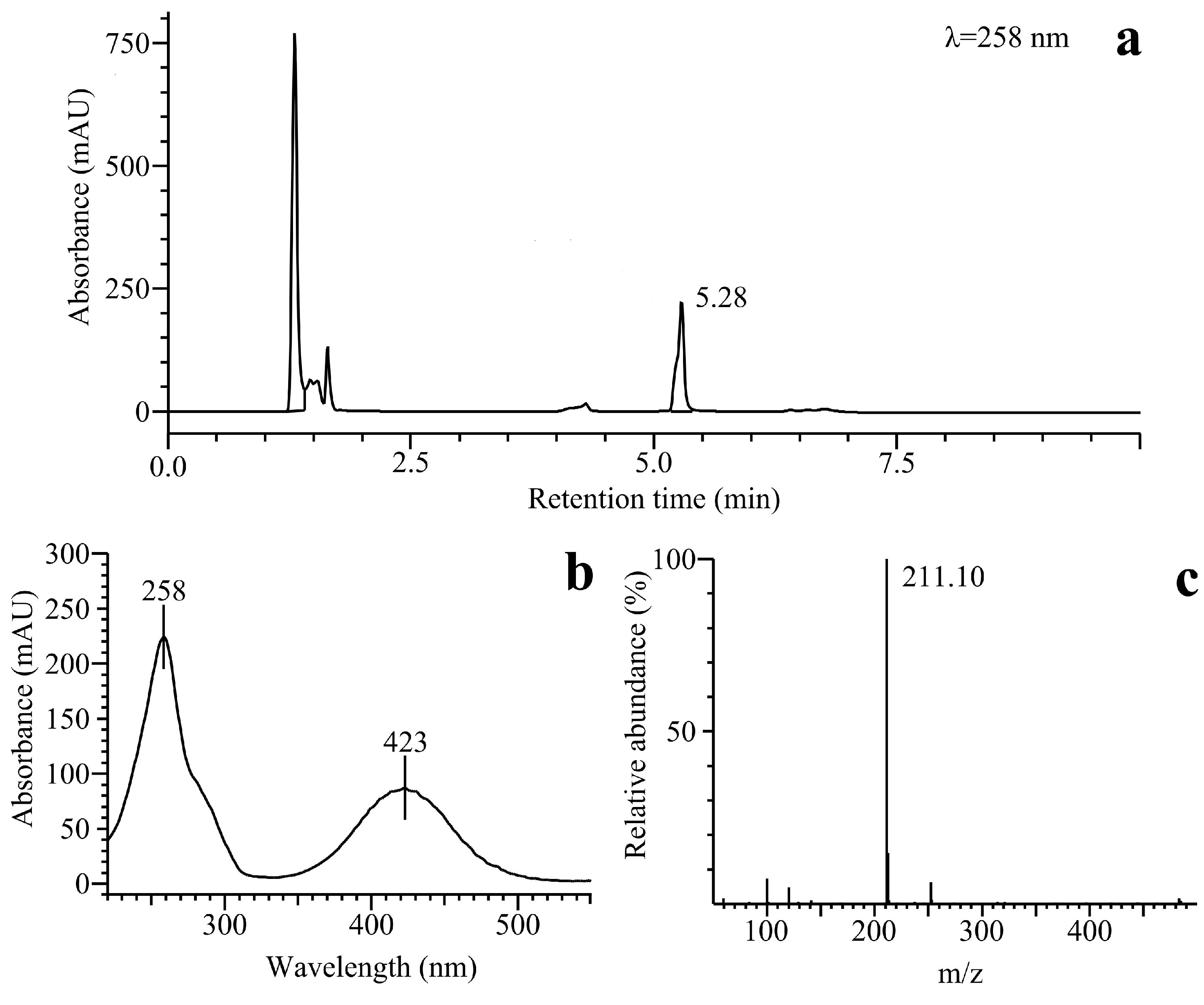

3. Experimental Section
3.1. The Computational Details
3.2. Chemicals and Enzymes
3.3. Enzymatic Assay and Estimation of Kinetic Data

3.4. Liquid Chromatography and Mass Spectrometry (LC-MS)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cerecetto, H.; Gonzalez, M. Benzofuroxan and furoxan. Chemistry and biology. Top Heterocycl. Chem. J. 2007, 10, 265–308. [Google Scholar]
- Cerecetto, H.; Porcal, H. Pharmacological properties of furoxans and benzofuroxans: Recent development. Mini Rev. Med. Chem. 2005, 5, 57–71. [Google Scholar]
- Aguirre, G.; Boiani, L.; Cerecetto, H.; di Maio, R.; Gonzalez, M.; Porcal, M.; Denicola, A.; Moller, M.; Thomson, L.; Tortora, V. Benzo[1,2-c]1,2,5-oxadiazole N-oxide derivatives as potential antitrypanosomal drugs. Part 3: Substituents-clustering methodology in the search for new active compounds. Bioorg. Med. Chem. 2005, 13, 6324–6335. [Google Scholar]
- Porcal, W.; Hernandez, P.; Boiani, L.; Boiani, M.; Ferreira, A.; Chidichimo, A.; Cazzulo, J.J.; Olea-Azar, C.; Gonzalez, M.; Cerecetto, H. New trypanocidal hybrid compounds from the association of hydrazone moieties and benzofuroxan heterocycle. Bioorg. Med. Chem. 2008, 16, 6995–7004. [Google Scholar]
- Wang, L.; Cong, L.; Zhang, Y.; Qiao, C.; Ye, Y. Synthesis and biological evaluation of benzofuroxan derivatives as fungicides against phytopatogenic fungi. J. Agric. Food Chem. 2013, 61, 8632–8640. [Google Scholar]
- Jorge, S.D.; Masunari, A.; Rangel-Yagui, O.C.; Pasqualoto, K.F.M.; Tavares, K.L. Design, synthesis, antimicrobial activity and molecular modeling studies of novel benzofuroxan derivatives against Staphylococcus aureus. Bioorg. Med. Chem. 2009, 17, 3028–3036. [Google Scholar]
- Šarlauskas, J.; Anusevičius, Ž.; Misiūnas, A. Benzofuroxan (Benzo[1,2-c] 1,2,5-oxadiazole N-oxide) derivatives as potential energetic materials: Studies of their synthesis and properties. Cent. Eur. J. Energy Mater. 2012, 9, 365–386. [Google Scholar]
- Severina, I.S.; Axenova, L.N.; Veselevsky, A.V.; Pyatakova, N.V.; Buneeva, O.A.; Ivanov, A.S.; Medvedev, A.E. Nonselective inhibition of monomine oxidases A and B by activators of soluble guanylate cyclase. Biochemistry (Moscow) 2003, 68, 1048–1054. [Google Scholar]
- Medana, C.; di Stilo, A.; Visentin, S.; Fruttero, R.; Gasco, A.; Ghigo, D.; Bosia, A. NO donor and biological properties of different benzofuroxans. Pharm. Res. 1999, 16, 956–960. [Google Scholar]
- Shipton, M.; Stuchbury, T.; Brocklehurst, K.; Herbert, L.; Suschitzky, H. Evaluation of benzofuroxan as a chemiphoric oxidizing agent for thiol groups by using its reactions with papain, ficin, bromelain and low-molecular-weight thiols. Biochem. J. 1977, 161, 627–637. [Google Scholar]
- Shipton, M.; Brocklehurst, K. Benzofuroxan as a thiol-specific reactivity probe. Kinetics of its reactions with papain, ficin, bromelanin and low-molecular-weight thiols. Biochem. J. 1977, 167, 799–810. [Google Scholar]
- Witanowski, M.; Stefaniak, L.; Webb, G. Annual Reports on NMR Spectroscopy; Academic Press Inc.: London, UK, 1981; Volume 11B, p. 502. [Google Scholar]
- Cmoch, P.; Wiench, J.W.; Stefaniak, L.; Webb, G.A. An NMR study and ab initio molecular orbital calculation of substituted benzofuroxans and the salt of 4,6-dinitrobenzofuroxan. Spectrochim. Acta Part A 1999, 55, 2207–2214. [Google Scholar]
- Orna, M.V.; Mason, R.P. Correlation of kinetic parameters of nitroreductase enzymes with redox properties of nitroaromatic compounds. J. Biol. Chem. 1989, 264, 12379–12384. [Google Scholar]
- Čėnas, N.; Anusevičius, Ž.; Bironaitė, D.; Bachmanova, G.I.; Archakov, A.I.; Ollinger, K. The electron transfer reactions of NADPH:cytochrome P-450 reductase with non-physiological oxidants. Arch. Biochem. Biophys. 1994, 315, 400–406. [Google Scholar]
- Riefler, R.G.; Smets, B.F. Enzymatic reduction of 2,4,6-trinitrotoluene and related nitroarenes: Kinetics linked to one-electron redox potentials. Environ. Sci. Technol. 2000, 34, 3900–2906. [Google Scholar]
- Čėnas, N.; Nemeikaitė-Čėnienė, A.; Sergedienė, E.; Nivinskas, H.; Anusevičius, Ž.; Šarlauskas, J. Quantitative-structure-activity relationships in enzymatic single-electron reduction of nitroaromatic explosives: Implications for their cytotoxicity. Biochim. Biophys. Acta 2001, 1528, 31–38. [Google Scholar]
- Šarlauskas, J.; Nemeikaitė-Čėnienė, A.; Anusevičius, Ž.; Misevičienė, L.; Martinez-Julvez, M.; Medina, M.; Gomez-Moreno, C.; Čėnas, N. Flavoenzyme-catalyzed redox-cycling of hydroxylamino- and amino metabilites of 2,4,6-trinitrotoluene: Implications for their cytotoxicity. Arch. Biochem. Biophys 2004, 425, 184–192. [Google Scholar]
- Knox, R.J.; Friedlos, F.; Boland, M. The bioactivation of CB 1954 and its use as a prodrug in antibody directed enzyme pro-drug therapy (ADEPT). Cancer Metastasis Rev. 1993, 12, 195–212. [Google Scholar]
- Medana, C.; Visentin, S.; Grosa, G.; Fruttero, R.; Gasco, A. Nitroanilines are the reducton products of benzofuroxan system by oxyhemoglobin (HbO22+). Il Farmaco 2001, 56, 799–802. [Google Scholar]
- Rolando, B.; Fruttero, R.; Gervasio, G.; Gasco, A. Identification of diaminophenazine and of benzoquinone dioxime as major in vitro metabolites of benzofuroxan. Xenobiotica 2004, 34, 345–352. [Google Scholar]
- Marcus, R.A.; Sutin, N. Electron transfer in chemistry and biology. Biochim. Biophys. Acta 1985, 811, 265–322. [Google Scholar]
- Misevičienė, L.; Anusevičius, Ž.; Šarlauskas, J.; Čėnas, N. Reduction of nitroaromatic compounds by NAD(P)H:quinone oxidoreductase (NQO1): The role of electron-accepting potency and structural parameters in the susbstrate specificity. Acta Biochim. Pol. 2006, 53, 569–576. [Google Scholar]
- Wardman, P. Reduction potentials of one-electron couples involving free radicals in aqueous solution. J. Phys. Chem. Ref. Data 1984, 18, 1637–1755. [Google Scholar]
- Janak, J.F. Proof that dE/dni = Ei in density functional theory. Phys. Rev. B 1978, 18, 7165–7168. [Google Scholar]
- Pearson, R.G. Absolute electronegativity and hardness correlated with molecular orbital theory. Proc. Natl. Acad. Sci. USA 1986, 83, 8440–8441. [Google Scholar]
- Pearson, R.G. Chemical hardness and density functional theory. J. Chem. Sci. 2005, 117, 369–377. [Google Scholar]
- Parr, R.G.; Szentpaly, L.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar]
- Geerlings, P.; de Proft, F.; Langenaeker, W. Conceptual density functional theory. Chem. Rev. 2003, 103, 1793–1873. [Google Scholar]
- De Proft, F.; Geeling, P. Computational DFT in the study of aromaticity. Chem. Rev. 2001, 101, 1451–1464. [Google Scholar]
- Campodonico, P.R.; Aizman, A.; Contreras, R. Electrophilicity of quinones and its relationship with hydride affinity. Chem. Phys. Lett. 2009, 471, 168–173. [Google Scholar]
- Beheshti, A.; Norouzi, P.; Ganjali, M.R. A simple and robust model for predictivity of the reduction potential of quinones family; electrophilicity index effect. Int. J. Electrochem. Sci. 2012, 7, 4811–4821. [Google Scholar]
- Kuhn, A.; Eschwege, K.G.; Conradie, J. The reduction potentials of para-substituted nitrobenzenes- an infrared, nuclear, magnetic resonance, and density functional theory study. J. Phys. Org. Chem. 2012, 25, 58–68. [Google Scholar]
- About, H.I. Density functional theory calculations for nitrobenzene molecules group. Br. J. Sci. 2012, 6, 51–60. [Google Scholar]
- Hosoda, S.; Nakamura, W.; Hayashi, K. Properties and reduction mechanism of DT-diaphorase from rat liver. J. Biol. Chem. 1974, 219, 6416–6423. [Google Scholar]
- Tedeschi, G.; Chen, S.; Massey, V. DT-diaphorase. Redox potential, steady-state, and rapid steady-state studies. J. Biol. Chem. 1995, 270, 1198–1204. [Google Scholar]
- Anusevičius, Ž.; Šarlauskas, J.; Čėnas, N. Two-electron reduction of quinones by rat liver NAD(P)H:quinone oxidoreductase: Quantitative structure-activity relationships. Arch. Biochem. Biophys. 2002, 404, 254–262. [Google Scholar]
- Thompson, C.D.; Foley, R.T. Electrochemical reduction of benzofuroxan: I. Aqueous solutions. J. Electrochem. Soc. 1972, 119, 177–183. [Google Scholar]
- Contreras, R.R.; Fuentealba, P.; Galvan, M.G.; Perez, P. A direct evaluation of regional Fukui functions in molecules. Chem. Phys. Lett. 1999, 304, 405–413. [Google Scholar]
- Britton, D.; Noland, W. Benzofuroxans. The crystal and molecular structure of 5-chlorobenzfurazan 1-oxide and 5-bromobenzfurazan 1-oxide. J. Org. Chem. 1962, 27, 3218–3223. [Google Scholar]
- Gaughran, R.J.; Picard, J.P.; Kaufman, J.V.R. Contribution to the chemistry of benzofuroxan and benzofurozan derivatives. J. Am. Chem. Soc. 1954, 76, 2233–2236. [Google Scholar]
- Cillo, C.M.; Lash, T.D.J. Benzo[1,2-c:3,4-c']bis[1,2,5]selenadiazole, [1,2,5]selenadiazolo[3,4-e]-2,1,3-benzothiadiazole, furazanobenzo-2,1,3-thiadiazole, furazanobenzo-2,1,3-selenadiazole and related heterocyclic systems. J. Heterocycl. Chem. 2004, 41, 955–962. [Google Scholar]
- Chugunova, E.A.; Timasheva, R.E.; Gibadullina, E.M.; Burilov, A.R.; Goumont, G.R. First synthesis of benzotrifuroxan at low temperature: Unexpected behavior of 5,7-dichloro-4,6-dinitrobenzo-furoxan with sodium azide. Propellants Explos. Pyrotech. 2012, 37, 390–392. [Google Scholar]
- Cabrera, M.; Lavaggi, M.L.; Hernández, P.; Merlino, A.; Gerpe, A.; Porcal, W.; Boiani, M.; Ferreira, A.; Monge, A.; López de Cerain, A.; et al. Cytotoxic, mutagenic and genotoxic effects of new anti-T. cruzi 5-phenylethenylbenzofuroxans. Contribution of phase I metabolites on the mutagenicity induction. Toxicol. Lett. 2009, 190, 140–149. [Google Scholar]
- Ughetto, G.; Wang, A.H.; Quigley, G.J.; van der Marel, G.A.; van Boom, H.J.; Rich, A. A comparison of the structure of echinomycin and triostin A complexed to a DNA fragment. Nucleic Acids Res. 1985, 13, 2305–2323. [Google Scholar]
- Pechurskaya, T.A.; Harnastai, I.N.; Grabovec, I.P.; Gilep, A.A.; Usanov, S.A. Adrenodoxin supports reactions catalyzed by microsomal steroidgenetic cytochrome P450s. Biochem. Biophys. Res. Commun. 2007, 353, 598–604. [Google Scholar]
- Prochaska, H.J. Purification and crystallization of rat liver NAD(P)H:quinone-acceptor) oxidoreductase by cibacron blue affinity chromatography: Identification of a new and potent inhibitor. Arch. Biochem. Biophys. 1988, 267, 529–538. [Google Scholar]
- Kolm, R.H.; Danielson, U.H.; Zhang, Y.; Talalay, P.; Mannervik, B. Isothiocyanates as substrates for human glutathione transferases: Structure-activity studies. Biochem. J. 1995, 311, 453–459. [Google Scholar]
- Moetner, M.; Neta, P. Kinetics of electron transfer from nitroaromatic radical-anions in aqueous solution. Effects of temperature and steric configuration. J. Phys. Chem. 1986, 90, 4648–4650. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šarlauskas, J.; Misevičienė, L.; Marozienė, A.; Karvelis, L.; Stankevičiūtė, J.; Krikštopaitis, K.; Čėnas, N.; Yantsevich, A.; Laurynėnas, A.; Anusevičius, Ž. The Study of NADPH-Dependent Flavoenzyme-Catalyzed Reduction of Benzo[1,2-c]1,2,5-oxadiazole N-Oxides (Benzofuroxans). Int. J. Mol. Sci. 2014, 15, 23307-23331. https://doi.org/10.3390/ijms151223307
Šarlauskas J, Misevičienė L, Marozienė A, Karvelis L, Stankevičiūtė J, Krikštopaitis K, Čėnas N, Yantsevich A, Laurynėnas A, Anusevičius Ž. The Study of NADPH-Dependent Flavoenzyme-Catalyzed Reduction of Benzo[1,2-c]1,2,5-oxadiazole N-Oxides (Benzofuroxans). International Journal of Molecular Sciences. 2014; 15(12):23307-23331. https://doi.org/10.3390/ijms151223307
Chicago/Turabian StyleŠarlauskas, Jonas, Lina Misevičienė, Audronė Marozienė, Laimonas Karvelis, Jonita Stankevičiūtė, Kastis Krikštopaitis, Narimantas Čėnas, Aleksey Yantsevich, Audrius Laurynėnas, and Žilvinas Anusevičius. 2014. "The Study of NADPH-Dependent Flavoenzyme-Catalyzed Reduction of Benzo[1,2-c]1,2,5-oxadiazole N-Oxides (Benzofuroxans)" International Journal of Molecular Sciences 15, no. 12: 23307-23331. https://doi.org/10.3390/ijms151223307
APA StyleŠarlauskas, J., Misevičienė, L., Marozienė, A., Karvelis, L., Stankevičiūtė, J., Krikštopaitis, K., Čėnas, N., Yantsevich, A., Laurynėnas, A., & Anusevičius, Ž. (2014). The Study of NADPH-Dependent Flavoenzyme-Catalyzed Reduction of Benzo[1,2-c]1,2,5-oxadiazole N-Oxides (Benzofuroxans). International Journal of Molecular Sciences, 15(12), 23307-23331. https://doi.org/10.3390/ijms151223307





