Pheophytin a Inhibits Inflammation via Suppression of LPS-Induced Nitric Oxide Synthase-2, Prostaglandin E2, and Interleukin-1β of Macrophages
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cytotoxicity of Pheophytin a on Lipopolysaccharides (LPS)-Stimulated Macrophages
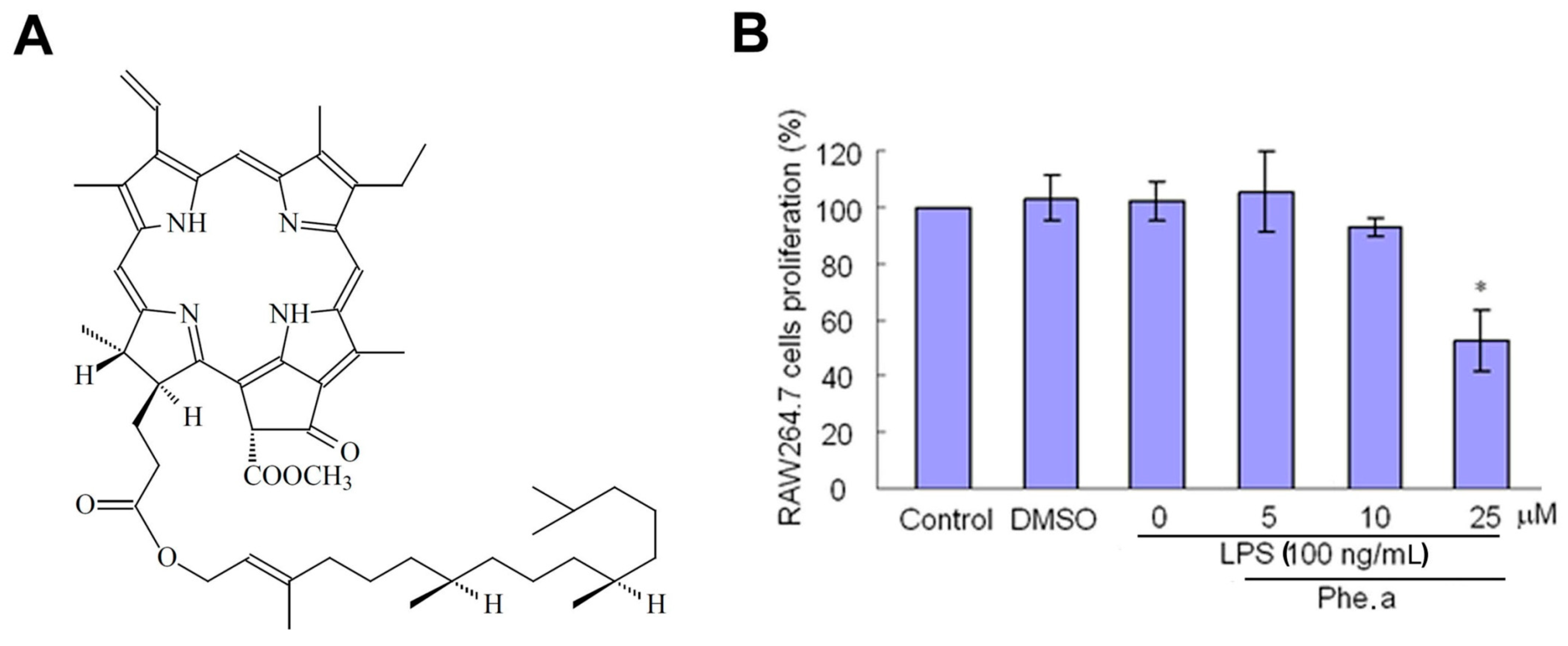

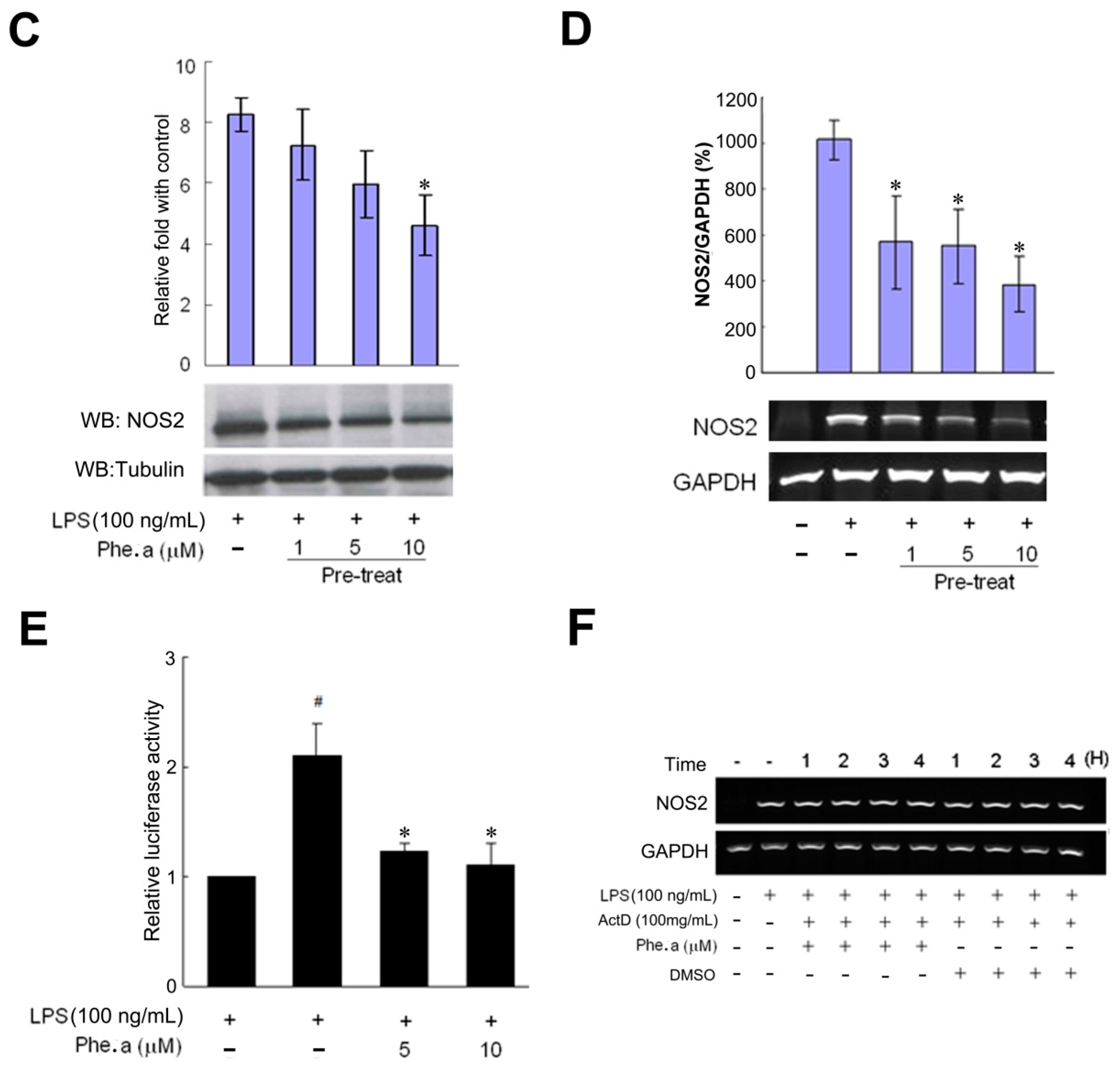
2.2. Effects of Pheophytin a on Nitric Oxide (NO) Production in LPS-Stimulated Macrophages
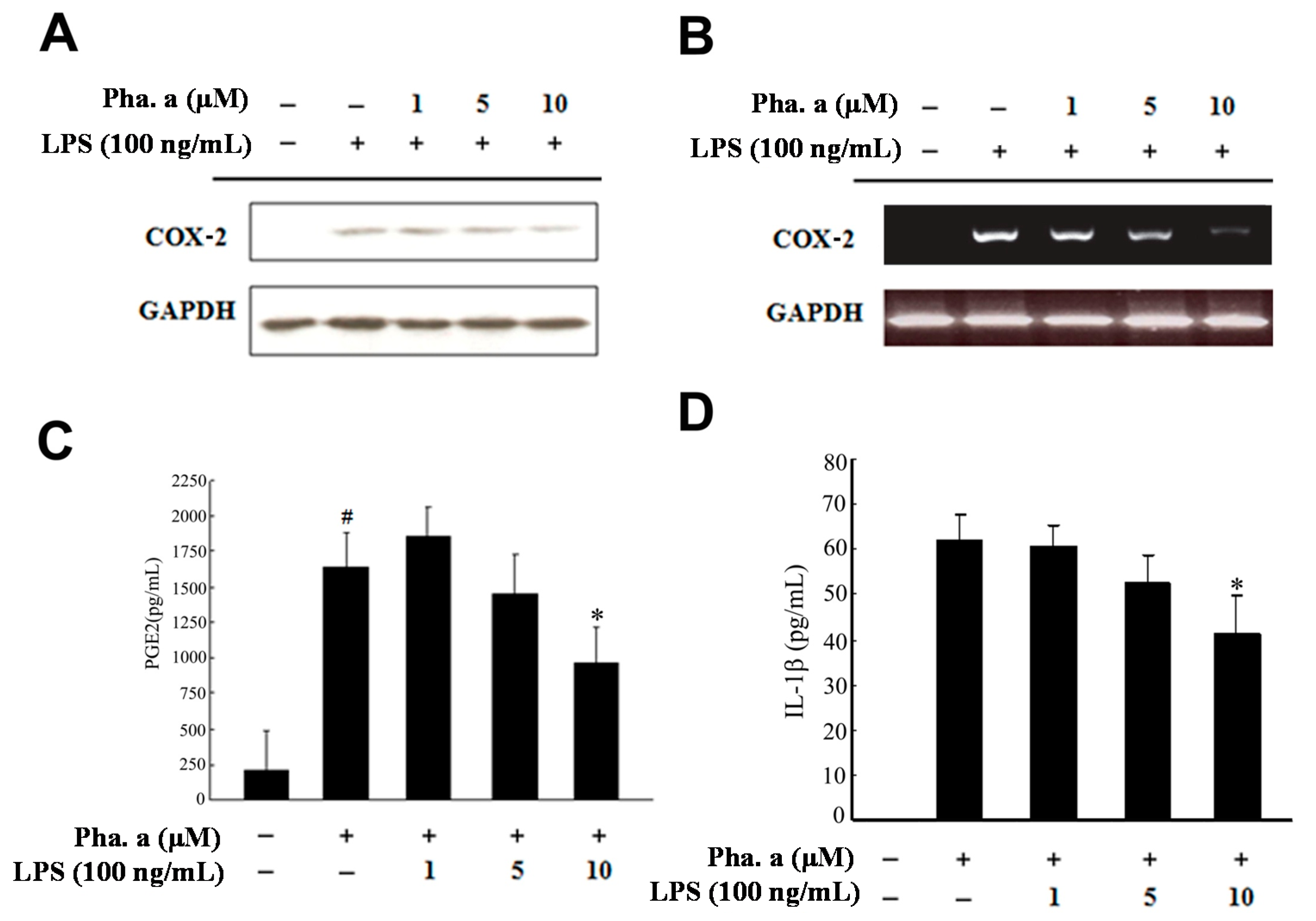
2.3. Effects of Pheophytin a on Prostaglandin E2 (PGE2), Cyclooxygenase-2 (COX-2) and Interleukin-1β (IL-1β) Production in LPS-Stimulated Macrophages
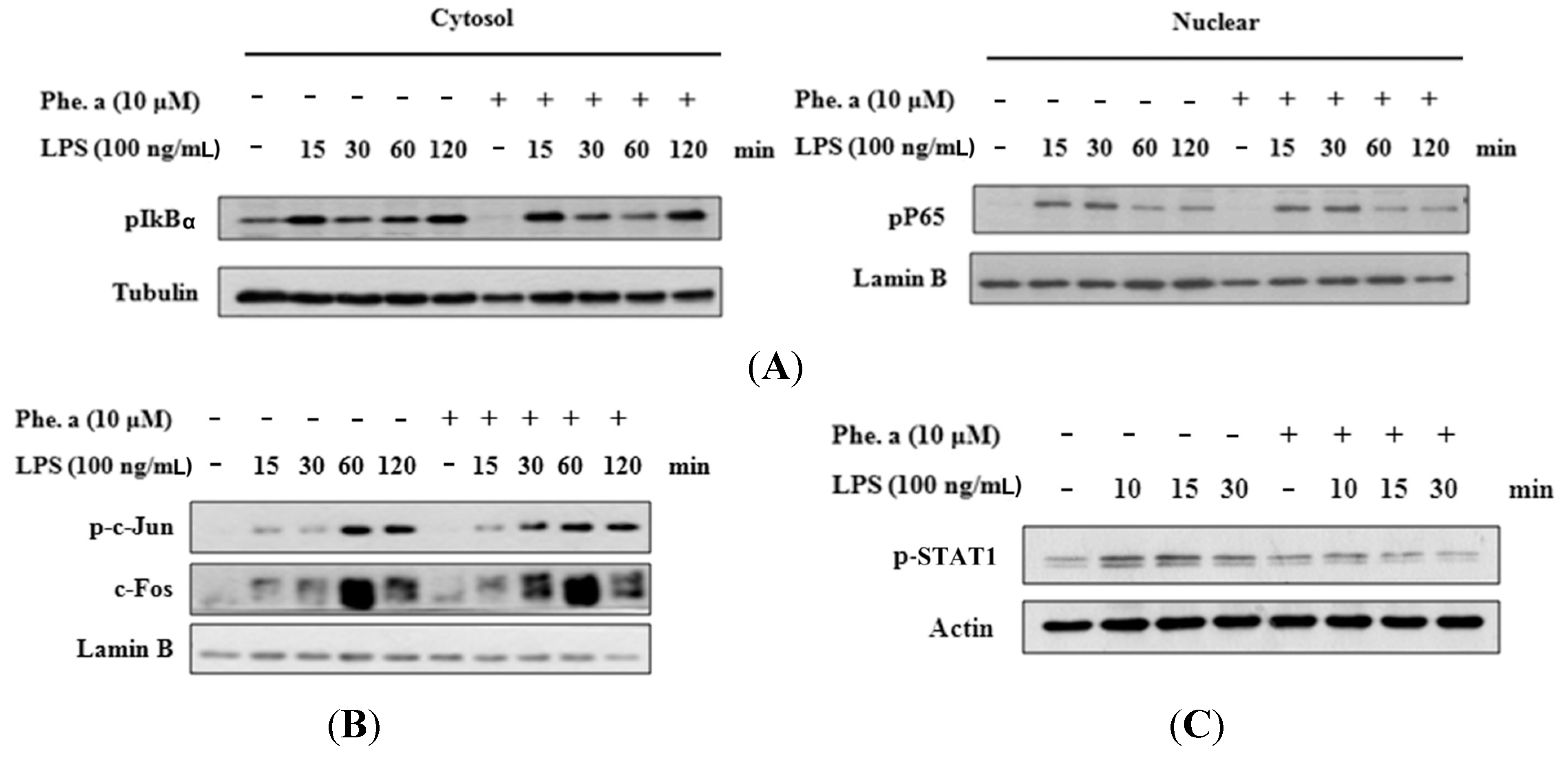
2.4. Effects of Pheophytin a on Signal Transduction Pathways in LPS-Stimulated Macrophages
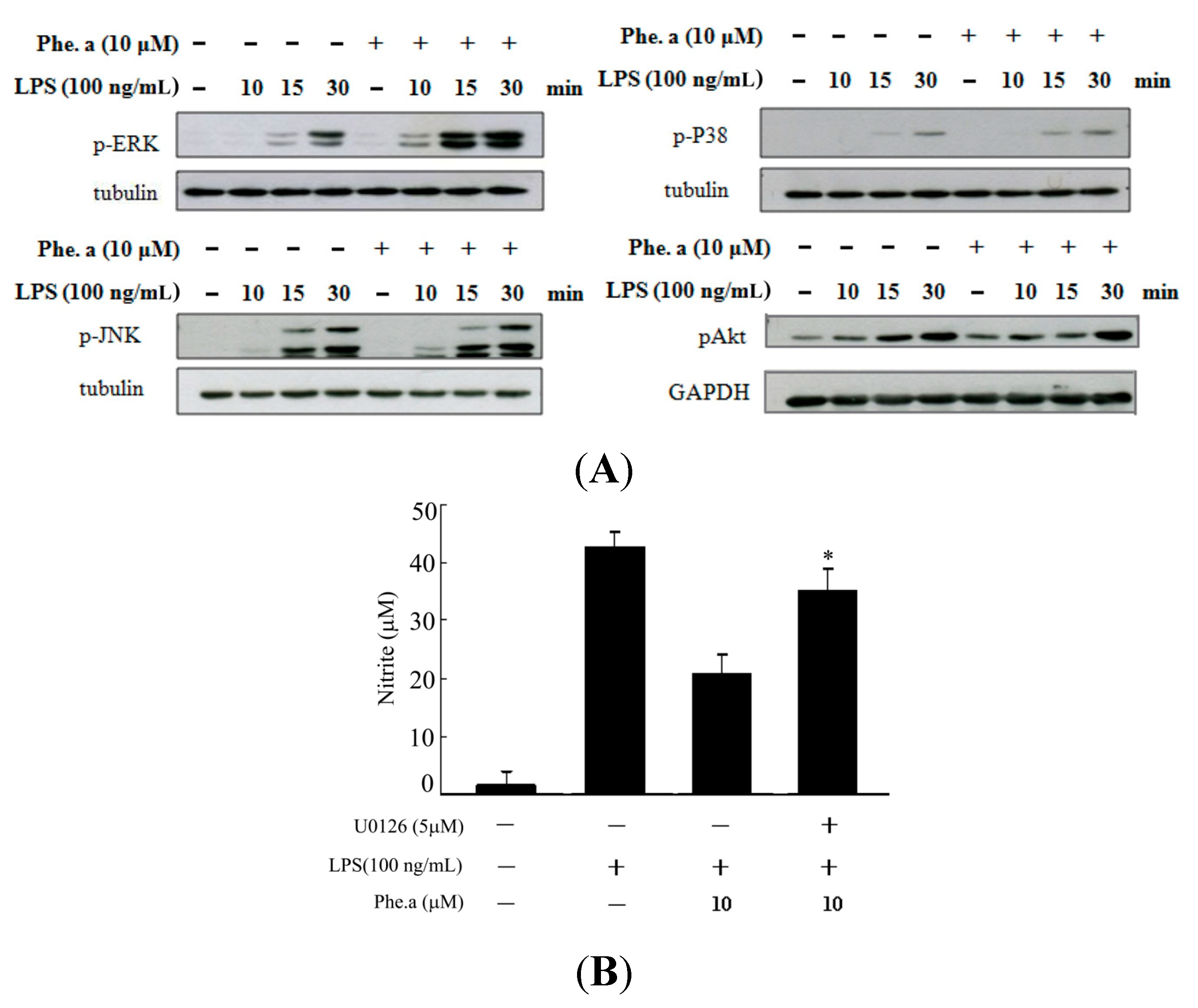
2.5. Effects of Pheophytin a on the Activation of NF-κB, AP-1 and STAT-1 in LPS-Stimulated Macrophages
3. Experimental Section
3.1. The Purification of Pheophytin a
3.2. The Chemical Structure and Purity of Pheophytin a
3.3. Reagents and Antibodies
3.4. Cell Culture
3.5. Cytotoxicity of Pheophytin a
3.6. Pheophytin a Treatment and LPS Stimulation of RAW 264.7 Cells
3.7. Measurement of Nitrite Release
3.8. Western Blot Measurement of the Protein Levels of NOS2 and COX-2
3.9. Real-Time Quantitative PCR
3.10. NOS2 Promoter Activity Assay
3.11. NF-κB Translocation Assay
3.12. Enzyme-Linked Immunosorbent Assay (ELISA)
3.13. Signal Transduction Pathway Assay
3.14. Statistical Analysis
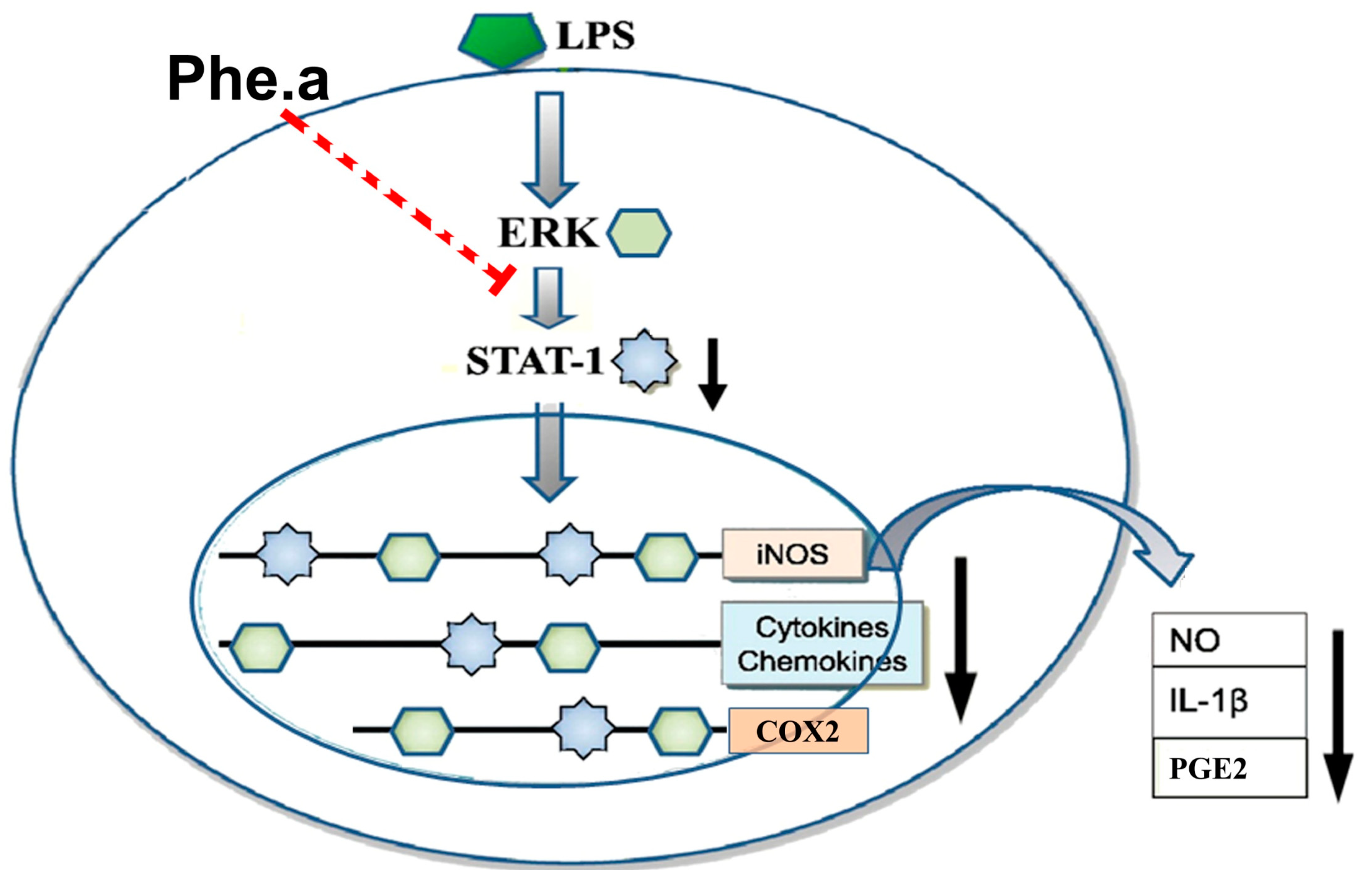
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [PubMed]
- Angus, D.C.; van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. The immunopathogenesis of sepsis. Nature 2002, 420, 8858–8891. [Google Scholar]
- Sands, K.E.; Bates, D.W.; Lanken, P.N.; Graman, P.S.; Hibberd, P.L.; Kahn, K.L.; Parsonnet, J.; Panzer, R.; Orav, E.J.; Snydman, D.R.; et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA 1997, 278, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.L.; Li, P.; Ho, B. The Sushi peptides: Structural characterization and mode of action against Gram-negative bacteria. Cell. Mol. Life Sci. 2008, 65, 1202–1219. [Google Scholar] [CrossRef]
- Hardaway, R.M. A review of septic shock. Am. Surg. 2000, 66, 22–29. [Google Scholar] [PubMed]
- Joo, S.Y.; Song, Y.A.; Park, Y.L.; Myung, E.; Chung, C.Y.; Park, K.J.; Cho, S.B.; Lee, W.S.; Kim, H.S.; Rew, J.S.; et al. Epigallocatechin-3-gallate inhibits LPS-induced NF-κB and MAPK signaling pathways in bone marrow-derived macrophages. Gut Liver 2012, 6, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lafuente, A.; Moro, C.; Manchon, N.; Gonzalo-Ruiz, A.; Villares, A.; Guillamon, E.; Rostagno, M.; Mateo-Vivaracho, L. In vitro anti-inflammatory activity of phenolic rich extracts from white and red common beans. Food Chem. 2014, 161, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M. Regulation of tissue homeostasis by NF-κB signalling: Implications for inflammatory diseases. Nat. Rev. Immunol. 2009, 9, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.K.; Lin, C.K.; Chang, H.W.; Wu, Y.H.; Yen, F.L.; Chang, F.R.; Chen, W.C.; Yeh, C.C.; Lee, J.C. Aqueous extract of Gracilaria tenuistipitata suppresses LPS-induced NF-κB and MAPK activation in RAW 264.7 and rat peritoneal macrophages and exerts hepatoprotective effects on carbon tetrachloride-treated rat. PLoS One 2014, 9, e86557. [Google Scholar] [PubMed]
- Guijarro-Munoz, I.; Compte, M.; Alvarez-Cienfuegos, A.; Alvarez-Vallina, L.; Sanz, L. Lipopolysaccharide activates toll-like receptor 4 (TLR4)-mediated NF-κB signaling pathway and proinflammatory response in human pericytes. J. Biol. Chem. 2014, 289, 2457–2468. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, J.J.; Park, B.K.; Yoon, S.J.; Choi, J.E.; Jin, M. Pheophytin a and chlorophyll a suppress neuroinflammatory responses in lipopolysaccharide and interferon-γ-stimulated BV2 microglia. Life Sci. 2014, 103, 59–67. [Google Scholar] [PubMed]
- Zhang, W.J.; Wei, H.; Hagen, T.; Frei, B. Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc. Natl. Acad. Sci. USA 2007, 104, 4077–4082. [Google Scholar] [CrossRef]
- Bak, M.J.; Truong, V.L.; Kang, H.S.; Jun, M.; Jeong, W.S. Anti-inflammatory effect of procyanidins from wild grape (Vitis amurensis) seeds in LPS-induced RAW 264.7 cells. Oxid. Med. Cell Longev. 2013, 2013, 409321. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Yin, F.; Lu, L.; Shen, L.; Qi, S.; Lan, L.; Luo, L.; Yin, Z. Baicalein reduces lipopolysaccharide-induced inflammation via suppressing JAK/STATs activation and ROS production. Inflamm. Res. 2013, 62, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Ina, A.; Hayashi, K.; Nozaki, H.; Kamei, Y. Pheophytin a, a low molecular weight compound found in the marine brown alga Sargassum fulvellum, promotes the differentiation of PC12 cells. Int. J. Dev. Neurosci. 2007, 25, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Okai, Y.; Higashi-Okai, K. Potent anti-inflammatory activity of pheophytin a derived from edible green alga, Enteromorpha prolifera (Sujiao-nori). Int. J. Immunopharmacol. 1997, 19, 355–358. [Google Scholar] [CrossRef]
- Lin, C.Y.; Wei, P.L.; Chang, W.J.; Huang, Y.K.; Feng, S.W.; Lin, C.T.; Lee, S.Y.; Huang, H.M. Slow freezing coupled static magnetic field exposure enhances cryopreservative efficiency—A study on human erythrocytes. PLoS One 2013, 8, e58988. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, M.; Sakaeda, Y.; Yamaguchi, H.; Ohmori, Y. Anti-inflammatory cytokine interleukin-4 inhibits inducible nitric oxide synthase gene expression in the mouse macrophage cell line RAW264.7 through the repression of octamer-dependent transcription. Mediat. Inflamm. 2013, 2013, 369693. [Google Scholar]
- Lupp, C.; Baasner, S.; Ince, C.; Nocken, F.; Stover, J.F.; Westphal, M. Differentiated control of deranged nitric oxide metabolism: A therapeutic option in sepsis? Crit. Care 2013, 17, 311. [Google Scholar] [CrossRef]
- Zhong, Y.; Chiou, Y.S.; Pan, M.H.; Shahidi, F. Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages. Food Chem. 2012, 134, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Huang, W.C.; Liou, C.J. Evaluation of the anti-inflammatory effects of phloretin and phlorizin in lipopolysaccharide-stimulated mouse macrophages. Food Chem. 2012, 134, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Ruland, J. Return to homeostasis: Down-regulation of NF-κB responses. Nat. Immunol. 2011, 12, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef]
- Frazier, W.J.; Wang, X.; Wancket, L.M.; Li, X.A.; Meng, X.; Nelin, L.D.; Cato, A.C.; Liu, Y. Increased inflammation, impaired bacterial clearance, and metabolic disruption after gram-negative sepsis in MKP-1-deficient mice. J. Immunol. 2009, 183, 7411–7419. [Google Scholar] [CrossRef]
- Zong, Y.; Sun, L.; Liu, B.; Deng, Y.S.; Zhan, D.; Chen, Y.L.; He, Y.; Liu, J.; Zhang, Z.J.; Sun, J.; et al. Resveratrol inhibits LPS-induced MAPKs activation via activation of the phosphatidylinositol 3-kinase pathway in murine RAW 264.7 macrophage cells. PLoS One 2012, 7, e44107. [Google Scholar] [PubMed]
- Levy, D.E.; Darnell, J.E., Jr. STATs: Transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002, 3, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Ishita, I.J.; Jin, S.E.; Choi, R.J.; Lee, C.M.; Kim, Y.S.; Jung, H.A.; Choi, J.S. Anti-inflammatory activity of edible brown alga Saccharina japonica and its constituents pheophorbide a and pheophytin a in LPS-stimulated RAW 264.7 macrophage cells. Food Chem. Toxicol. 2013, 55, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Buapool, D.; Mongkol, N.; Chantimal, J.; Roytrakul, S.; Srisook, E.; Srisook, K. Molecular mechanism of anti-inflammatory activity of Pluchea indica leaves in macrophages RAW 264.7 and its action in animal models of inflammation. J. Ethnopharmacol. 2013, 146, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Layne, M.D.; Chung, S.W.; Ejima, K.; Baron, R.M.; Yet, S.F.; Perrella, M.A. Elk-3 is a transcriptional repressor of nitric-oxide synthase 2. J. Biol. Chem. 2003, 278, 39572–39577. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Z.; Tang, Y.Z.; Liu, Y.H. Wogonoside displays anti-inflammatory effects through modulating inflammatory mediator expression using RAW264.7 cells. J. Ethnopharmacol. 2013, 148, 271–276. [Google Scholar]
- Kato, T.; Fujino, H.; Oyama, S.; Kawashima, T.; Murayama, T. Indomethacin induces cellular morphological change and migration via epithelial-mesenchymal transition in A549 human lung cancer cells: A novel cyclooxygenase-inhibition-independent effect. Biochem. Pharmacol. 2011, 82, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Qi, S.; Dai, W.; Luo, L.; Yin, Z. Arctigenin inhibits lipopolysaccharide-induced iNOS expression in RAW264.7 cells through suppressing JAK-STAT signal pathway. Int. Immunopharmacol. 2011, 11, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Zhang, J.; Chen, K.; Liu, Y.; Schopfer, F.J.; Baker, P.R.; Freeman, B.A.; Chen, Y.E.; Cui, T. Nitroalkenes suppress lipopolysaccharide-induced signal transducer and activator of transcription signaling in macrophages: A critical role of mitogen-activated protein kinase phosphatase 1. Endocrinology 2008, 149, 4086–4094. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Li, J.; Nagarkatti, P.; Nagarkatti, M.; Hofseth, L.J.; Windust, A.; Cui, T. American ginseng preferentially suppresses STAT/iNOS signaling in activated macrophages. J. Ethnopharmacol. 2009, 125, 145–150. [Google Scholar] [CrossRef]
- Zhu, Z.G.; Jin, H.; Yu, P.J.; Tian, Y.X.; Zhang, J.J.; Wu, S.G. Mollugin inhibits the inflammatory response in lipopolysaccharide-stimulated RAW264.7 macrophages by blocking the Janus kinase-signal transducers and activators of transcription signaling pathway. Biol. Pharm. Bull. 2013, 36, 399–406. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Lee, C.-H.; Chang, Y.-W.; Wang, H.-M.; Chen, C.-Y.; Chen, Y.-H. Pheophytin a Inhibits Inflammation via Suppression of LPS-Induced Nitric Oxide Synthase-2, Prostaglandin E2, and Interleukin-1β of Macrophages. Int. J. Mol. Sci. 2014, 15, 22819-22834. https://doi.org/10.3390/ijms151222819
Lin C-Y, Lee C-H, Chang Y-W, Wang H-M, Chen C-Y, Chen Y-H. Pheophytin a Inhibits Inflammation via Suppression of LPS-Induced Nitric Oxide Synthase-2, Prostaglandin E2, and Interleukin-1β of Macrophages. International Journal of Molecular Sciences. 2014; 15(12):22819-22834. https://doi.org/10.3390/ijms151222819
Chicago/Turabian StyleLin, Chun-Yu, Chien-Hsing Lee, Yu-Wei Chang, Hui-Min Wang, Chung-Yi Chen, and Yen-Hsu Chen. 2014. "Pheophytin a Inhibits Inflammation via Suppression of LPS-Induced Nitric Oxide Synthase-2, Prostaglandin E2, and Interleukin-1β of Macrophages" International Journal of Molecular Sciences 15, no. 12: 22819-22834. https://doi.org/10.3390/ijms151222819




