Highly Enantioselective Production of (R)-Halohydrins with Whole Cells of Rhodotorula rubra KCh 82 Culture
Abstract
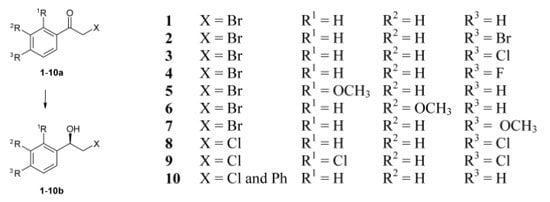
:1. Introduction
2. Results and Discussion
| Reaction | Product | Time (Days) | Conversion (%) a | ee (%) a | Config. |
|---|---|---|---|---|---|
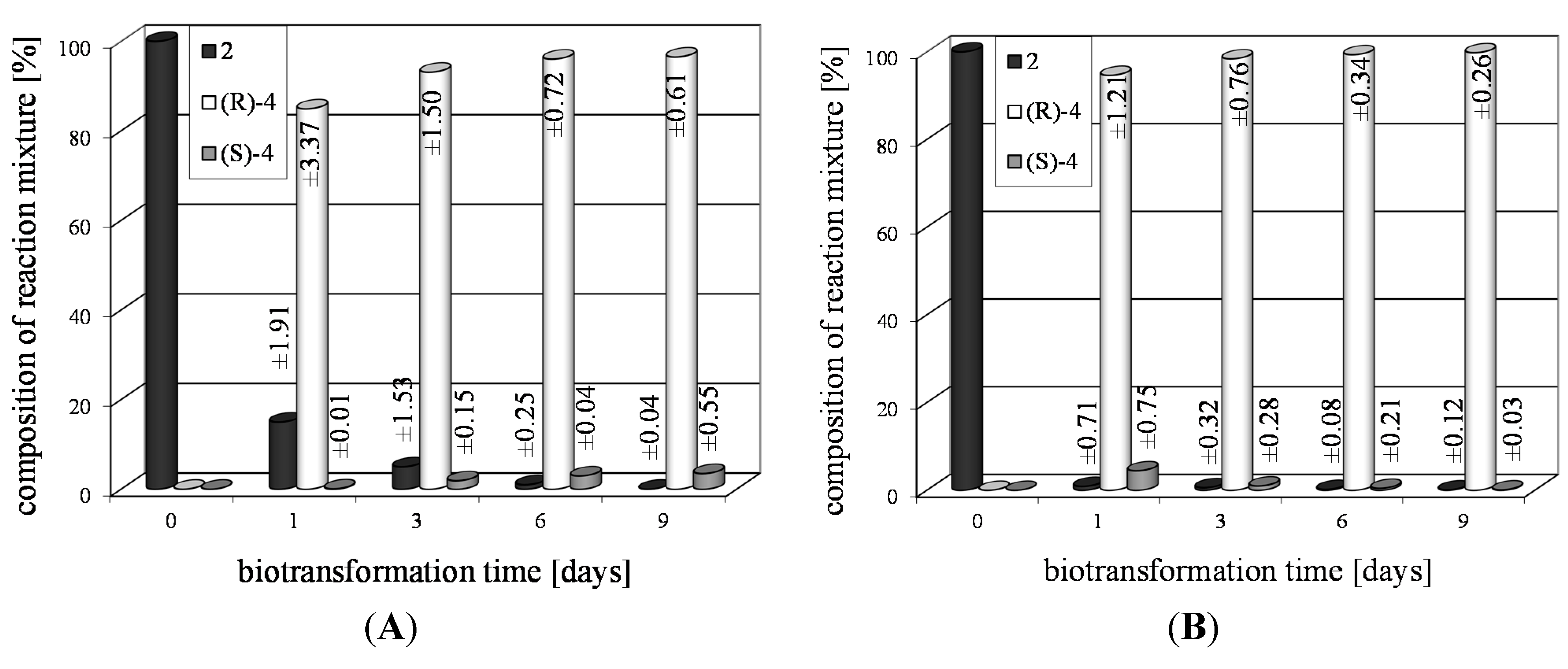
 | 2a | 1 | >99 | 88 | R |
| 3 | >99 | 96 | |||
| 6 | >99 | 97 | |||
| 9 | >99 | 97 | |||
 | 2b | 1 | >99 | 99 | R |
| 3 | >99 | 99 | |||
| 6 | >99 | 99 | |||
| 9 | >99 | 99 | |||
 | 2c | 1 | 85 | >99 | R |
| 3 | 95 | 96 | |||
| 6 | >99 | 94 | |||
| 9 | >99 | 93 | |||
 | 2d | 1 | 97 | 96 | R |
| 3 | 98 | 98 | |||
| 6 | >99 | 99 | |||
| 9 | >99 | >99 | |||
 | 2e | 1 | >99 | 91 | R |
| 3 | >99 | 98 | |||
| 6 | >99 | >99 | |||
| 9 | >99 | >99 | |||
 | 2f | 1 | >99 | 98 | R |
| 3 | >99 | 99 | |||
| 6 | >99 | >99 | |||
| 9 | >99 | >99 | |||
 | 2g | 1 | >99 | 98 | R |
| 3 | >99 | 97 | |||
| 6 | >99 | 97 | |||
| 9 | >99 | 98 | |||
 | 2h | 1 | >99 | 93 | R |
| 3 | >99 | 93 | |||
| 6 | >99 | 93 | |||
| 9 | >99 | 93 | |||
 | 2i | 1 | >99 | 93 | R |
| 3 | >99 | 95 | |||
| 6 | >99 | 96 | |||
| 9 | >99 | 96 |
3. Experimental Section
3.1. Materials
3.2. Analytical Methods
| Compound Number | Starting T (°C) 1 min | Gradient (°C·min−1) | T (°C) 0 min | Gradient (°C·min−1) | Final T (°C) 5 min | Rt of S-Isomer (min) | Rt of R-Isomer (min) |
|---|---|---|---|---|---|---|---|
| 2a | 102 | 1.5 | 126 | 20 | 200 | 19.2 | 19.5 |
| 2b | 150 | 2 | 177 | 20 | 200 | 11.4 | 11.8 |
| 2c | 147 | 2 | 175 | 20 | 200 | 9.3 | 9.8 |
| 2d | 147 | 2 | 165 | 20 | 200 | 8.2 | 8.7 |
| 2e | 150 | 3 | 185 | 20 | 200 | 7.6 | 8.2 |
| 2f | 140 | 2 | 153 | 20 | 200 | 4.1 | 4.5 |
| 2g | 130 | 3 | 155 | 20 | 200 | 10.8 | 11.1 |
| 2h | 120 | 0.1 | 124 | 20 | 200 | 45.3 | 45.8 |
| 2i | 120 | 3 | 155 | 20 | 200 | 14.4 | 14.7 |
3.3. Screening Procedure
3.4. Preparative Biotransformation
3.5. Spectral Data of Isolated Metabolites
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhu, D.; Hyatt, B.A.; Hua, L. Enzymatic hydrogen transfer reduction of α-chloro aromatic ketones catalyzedby a hyperthermophilic alcohol dehydrogenase. J. Mol. Catal. B-Enzym. 2009, 56, 272–276. [Google Scholar]
- Palmqvist, M.; Ibsen, T.; Mellén, A.; Lötvall, J. Comparison of the relative efficacy of formoterol and salmeterol in asthmatic patients. Am. J. Respir. Crit. Care.Med. 1999, 160, 244–249. [Google Scholar]
- Frois, C.; Wu, E.Q.; Ray, S.; Colice, G.L. Inhaled corticosteroids or long-acting β-agonists alone or in fixed-dose combinations in asthma treatment: A systematic review of fluticasone/budesonide and formoterol/salmeterol. Clin. Ther. 2009, 31, 2779–2803. [Google Scholar]
- Cazzola, M.; Materas, M.G.; Santangelo, G.; Vinciguerra, A.; Rossi, F.; D’Amato, G. Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: A dose-response study. Respir. Med. 1995, 89, 357–362. [Google Scholar]
- Lee, D.-M.; Lee, J.-C.; Jeong, N.; Lee, K.-I. Asymmetric transfer hydrogenation of 2-tosyloxy-1-(4-hydroxyphenyl)ethanone derivatives: Synthesis of (R)-tembamide, (R)-aegeline, (R)-octopamine, and (R)-denopamine. Tetrahedron-Asymmetry 2007, 18, 2662–2667. [Google Scholar]
- Yadav, J.S.; Nanda, S.; Ready, P.T.; Rao, A.B. Stereoselective synthesis of (R)-(−)-denopamine, (R)-(−)-tembamide and (R)-(−)-aegeline via asymmetric reduction of azidoketones by Daucus carota in aqueous medium. Tetrahedron-Asymmetry 2001, 12, 3381–3385. [Google Scholar]
- Abrass, I.B.; Davis, J.L.; Scarpace, P.J. Isoproterenol responsiveness and myocardial β-adrenergic receptors in young and old rats. J. Gerontol. 1982, 37, 156–160. [Google Scholar]
- Zhang, G.-X.; Kimura, S.; Nishiyama, A.; Shokoji, T.; Rahman, M.; Yao, L.; Nagai, Y.; Fujisawa, Y.; Miyatake, A.; Abe, Y. Cardiac oxidative stress in acute and chronic isoproterenol-infused rats. Cardiovasc. Res. 2005, 65, 230–238. [Google Scholar]
- Morris, D.J.; Hayes, A.M.; Wills, M. The “Reverse-Tethered” ruthenium (II) catalyst for asymmetric transfer hydrogenation: Further applications. J. Org. Chem. 2006, 71, 7035–7044. [Google Scholar]
- Ohkuma, T.; Tsutsumi, K.; Utsumi, N.; Arai, N.; Noyori, R.; Murata, K. Asymmetric hydrogenation of α-chloro aromatic ketones catalyzed by η6-arene/TsDPEN−ruthenium(II) complexes. Org. Lett. 2007, 9, 255–257. [Google Scholar]
- Wu, X.-F.; Min, C.; Nyamzundui, E.; Zhou, H.-B.; Dong, C. A novel C3-symmetric prolinol-squaramide catalyst for the asymmetric reduction of ketones by borane. Tetrahedron-Asymmetry 2011, 22, 1640–1643. [Google Scholar]
- Aguirre-Pranzoni, C.; Bisogno, F.R.; Orden, A.A.; Kurina-Sanz, M. Lyophilized Rhodotorula yeast as all-in-one redox biocatalyst: Access to enantiopure building blocks by simple chemoenzymatic one-pot procedures. J. Mol. Catal. B-Enzym 2014. [Google Scholar] [CrossRef]
- Fardelone, L.C.; Rodrigues, J.A.R.; Moran, P.J.S. Chiral pharmaceutical intermediaries obtained by reduction of 2-halo-1-(4-substituted phenyl)-ethanones mediated by Geotrichum candidum CCT 1205 and Rhodotorula glutinis CCT 2182. Enzym. Res. 2011, 2011, 976368. [Google Scholar]
- Taketomi, S.; Asano, M.; Higashi, T.; Shoji, M.; Sugai, T. Chemo-enzymatic route for (R)-terbutaline hydrochloride based on microbial asymmetric reduction of a substituted α-chloroacetophenone derivative. J. Mol. Catal. B-Enzym. 2012, 84, 83–88. [Google Scholar]
- Tokoshima, D.; Hanaya, K.; Shoji, M.; Sugai, T. Whole-cell yeast-mediated preparation of (R)-2-chloro-1-(3-nitrophenyl)ethanol as a synthetic precursor for (R)-phenylephrine. J. Mol. Catal. B-Enzym. 2013, 97, 95–99. [Google Scholar]
- Asami, K.; Machida, T.; Jung, S.; Hanaya, K.; Shoji, M.; Sugai, T. Synthesis of (R)-bambuterol based on asymmetric reduction of 1-[3,5-bis(dimethylcarbamoyloxy)phenyl]-2-chloroethanone with incubated whole cells of Williopsis californica JCM 3600. J. Mol. Catal. B-Enzym. 2013, 97, 106–109. [Google Scholar]
- Ren, J.; Dong, W.; Yu, B.; Wu, Q.; Zhu, D. Synthesis of optically active a-bromohydrins via reduction of α-bromoacetophenone analogues catalyzed by an isolated carbonyl reductase. Tetrahedron-Asymmetry 2012, 23, 497–500. [Google Scholar]
- Rowan, A.S.; Moody, T.S.; Howard, R.M.; Underwood, T.J.; Miskelly, I.R.; He, Y.; Wang, B. Preparative access to medicinal chemistry related chiral alcohols using carbonyl reductase technology. Tetrahedron-Asymmetry 2013, 24, 1369–1381. [Google Scholar]
- Xia, S.W.; Lin, H.; Chen, Y.Z. Preparation of (R)-2-chloro-1-(m-chlorophenyl)ethanol by Lipozyme TL IM-catalyzed second resolution. Chin. Chem. Lett. 2012, 23, 289–292. [Google Scholar]
- Rocha, L.C.; Ferreira, H.V.; Pimenta, E.F.; Souza Berlinck, R.G.; Oliveira Rezende, M.O.; Landgraf, M.D.; Regali Seleghim, M.H.; Durães Sette, L.; Meleiro Porto, A.L. Biotransformation of α-bromoacetophenones by the marine fungus Aspergillus sydowii. Mar. Biotechnol. 2010, 12, 552–557. [Google Scholar]
- Utsukihara, T.; Okada, S.; Kato, N.; Horiuchi, C.A. Biotransformation of α-bromo and α,α'-dibromo alkanone to α-hydroxyketone and α-diketone by Spirulina platensis. J. Mol. Catal. B-Enzym. 2007, 45, 68–72. [Google Scholar]
- Bisogno, F.R.; Cuetos, A.; Orden, A.A.; Kurina-Sanz, M.; Lavandera, I.; Gotor, V. Chemo- and stereodivergent preparation of terminal epoxides and bromohydrins through one-pot biocatalysed reactions: Access to enantiopure five- and six-membered N-heterocycles. Adv. Synth. Catal. 2010, 352, 1657–1661. [Google Scholar]
- Gopishetty, B.; Gogoi, S.; Dutta, A.K. An improved asymmetric synthetic route to a novel triple uptake inhibitor antidepressant (2S,4R,5R)-2-benzhydryl-5-((4-methoxybenzyl)amino)tetrahydro-2H-pyran-4-ol (D-142). Tetrahedron-Asymmetry 2011, 22, 1081–1086. [Google Scholar]
- Janeczko, T.; Gładkowski, W.; Kostrzewa-Susłowa, E. Microbial production of dihydrochalcones and its derivatives as the food sweeteners. J. Mol. Catal. B-Enzym. 2013, 98, 55–61. [Google Scholar]
- Janeczko, T.; Kostrzewa-Susłowa, E. Enantioselective reduction of propiophenone formed from 3-chloropropiophenone and stereoinversion of resulting alcohols in selected yeast cultures. Tetrahedron-Asymmetry 2014, 25, 1264–1269. [Google Scholar]
- Janeczko, T.; Dymarska, M.; Siepka, M.; Gniłka, R.; Leśniak, A.; Popłoński, J.; Kostrzewa-Susłow, E. Enantioselective reduction of flavanone and oxidation of cis- and trans-flavan-4-ol by selected yeast cultures. J. Mol. Catal. B-Enzym. 2014, 109, 47–52. [Google Scholar]
- Banzatto, D.; de Freita, L.A.; Mutton, M.J.R. Carotenoid production by Rhodotorula rubra cultivated in sugarcane juice, molasses, and syrup. Ciênc. Tecnol. Aliment. 2013, 33, 14–18. [Google Scholar]
- De Oliveira Lopes, R.; Benzaquem Ribeiro, J.; de Souza Ramos, A.; Miranda, L.S.M.; Ramos Leal, I.C.; Gomes Ferreira Leite, S.; Alves de Souza, R.O.M. Highly enantioselective bioreduction of 4-bromoacetophenone. Tetrahedron-Asymmetry 2011, 22, 1763–1766. [Google Scholar]
- Roy, S.; Alexandre, V.; Neuwels, M.; le Texier, L. Asymmetric bioreduction of a bulky ketone: 1-Phenyl-1-(2-phenylthiazol-5-yl)-methanone. Adv. Synth. Catal. 2001, 343, 738–743. [Google Scholar]
- Lorraine, K.; King, S.; Greasham, R.; Chartrain, M. Asymmetric bioreduction of a ketosulfone to the corresponding trans-hydroxysulfone by the yeast Rhodotorula rubra MY 2169. Enzym. Microb. Technol. 1996, 19, 250–255. [Google Scholar]
- Brzezińska-Rodak, M.; Żymańczyk-Duda, E.; Kafarski, P.; Lejczak, B. Application of fungi as biocatalysts for the reduction of diethyl 1-oxoalkylphosphonates in anhydrous hexane. Biotechnol. Prog. 2002, 18, 1287–1291. [Google Scholar]
- Żymańczyk-Duda, E.; Klimek-Ochab, M.; Kafarski, P.; Lejczak, B. Stereochemical control of biocatalytic asymmetric reduction of diethyl 2-oxopropylphosphonate employing yeasts. J. Organomet. Chem. 2005, 690, 2593–2596. [Google Scholar]
- Olejniczak, T.; Grabarczyk, M.; Wawrzeńczyk, C. Lactones 7: Enantioselective lactonization of racemic ethyl (5,5-dimethyl-2,3-epoxycyclohex-1-yl)acetate. J. Mol. Catal. B-Enzym. 2001, 11, 243–247. [Google Scholar]
- Goswami, A.; Bezbaruah, R.L.; Goswami, J.; Borthakur, N.; Dey, D.; Hazarika, A.K. Microbial reduction of ω-bromoacetophenones in the presence of surfactants. Tetrahedron-Asymmetry 2000, 11, 3701–3709. [Google Scholar]
- Hiratake, J.; Inagaki, M.; Nishioka, T.; Oda, J. Irreversible and highly enantioselective acylation of 2-halo-1-arylethanols in organic solvents catalyzed by a lipase from Pseudomonas fluorescens. J. Org. Chem. 1988, 53, 6130–6133. [Google Scholar]
- Basavaiah, D.; Jayapal Reddy, G.; Chandrashekar, V. A novel and effective chiral phosphoramide catalyst for the borane-mediated asymmetric reduction of prochiral a-halo ketones. Tetrahedron-Asymmetry 2001, 12, 685–689. [Google Scholar]
- Olivares-Romero, J.L.; Juaristi, E. Synthesis of three novel chiral diamines derived from (S)-proline and their evaluation as precursors of diazaborolidines for the catalytic borane-mediated enantioselective reduction of prochiral ketones. Tetrahedron 2008, 64, 9992–9998. [Google Scholar]
- Janeczko, T.; Bakowski, W.; Walczak, E.; Robak, M.; Dmochowska-Gładysz, J.; Kostrzewa-Susłow, E. Biotransformation of acetophenone and its halogen derivatives by Yarrowia lipolytica strains. Ann. Microbiol. 2014. [Google Scholar] [CrossRef]
- Bandini, M.; Cozzi, P.G.; Negro, L.; Umani-Ronchi, A. Enantioselective reduction of ketones with triethoxysilane catalyzed by chiral bis-oxazoline titanium complexes. Chem. Commun. 1999, 39. [Google Scholar]
- Kizaki, N.; Sawa, I.; Yano, M.; Yasohara, Y.; Hasegawa, J. Purification and characterization of a yeast carbonyl reductase for synthesis of optically active (R)-styrene oxide derivatives. Biosci. Biotechnol. Biochem. 2005, 69, 79–86. [Google Scholar]
- Zhu, D.; Mukherjee, C.; Hua, L. “Green” synthesis of important pharmaceutical building blocks: Enzymatic access to enantiomerically pure α-chloroalcohols. Tetrahedron-Asymmetry 2005, 16, 3275–3278. [Google Scholar]
- Wei, Z.-L.; Li, Z.-Y.; Lin, G.-Q. Anti-Prelog microbial reduction of aryl α-halomethyl or α-hydroxymethyl ketones with Geotrichum sp. 38. Tetrahedron 1998, 54, 13059–13072. [Google Scholar]
- Huanga, X.; Ying, J.Y. Asymmetric transfer hydrogenation over Ru–TsDPEN catalysts supported on siliceous mesocellular foam. Chem. Commun. 2007, 18, 1825–1827. [Google Scholar]
- Yang, Y.; Zhu, D.; Piegat, T.J.; Hua, L. Enzymatic ketone reduction: mapping the substrate profile of a short-chain alcohol dehydrogenase (YMR226c) from Saccharomyces cerevisiae. Tetrahedron-Asymmetry 2007, 18, 1799–1803. [Google Scholar]
- Pàmies, O.; Bäckvall, J.-E. Chemoenzymatic dynamic kinetic resolution of β-halo alcohols. An efficient route to chiral epoxides. J. Org. Chem. 2002, 67, 9006–9010. [Google Scholar]
- Cordes, D.B.; Kwong, T.J.; Morgan, K.A.; Singaram, B. Chiral styrene oxides from α-haloacetophenones using NaBH4 and TarB-NO2, a chiral Lewis acid. Tetrahedron Lett. 2006, 47, 349–351. [Google Scholar]
- Perrone, R.; Berardi, F.; Leopoldo, M.; Tortorella, V. Oxygen isosteric derivatives of 3-(3-hydroxypheny1)-N-n-propylpiperidine. J. Med. Chem. 1992, 35, 3045–3049. [Google Scholar]
- Wang, F.; Liu, H.; Cun, L.; Zhu, J.; Deng, J.; Jiang, Y. Asymmetric transfer hydrogenation of ketones catalyzed by hydrophobic metal-amido complexes in aqueous micelles and vesicles. J. Org. Chem. 2005, 70, 9424–9429. [Google Scholar]
- Smith, H.E.; Fontana, L.P. Optically active amines A sector rule for the circular dichroism of the benzene chromophore. J. Org. Chem. 1991, 56, 432–435. [Google Scholar]
- Basavaiah, D.; Reddy, G.J.; Chandrashekar, V. A new chiral catalytic source with an N–P2O structural framework containing a proximal hydroxyl group for the borane-mediated asymmetric reduction of prochiral ketones. Tetrahedron-Asymmetry 2004, 15, 47–52. [Google Scholar]
- Lin, H.; Chen, Y.; Xu, X.; Xia, S.; Wang, L. Preparation of key intermediates of adrenergic receptor agonists: Highly enantioselective production of (R)-α-halohydrins with Saccharomyces cerevisiae CGMCC 2.396. J. Mol. Catal. B-Enzym. 2009, 57, 1–5. [Google Scholar]
- Yang, S.-D.; Shi, Y.; Sun, Z.-H.; Zhaoa, Y.-B.; Liang, Y.-M. Asymmetric borane reduction of prochiral ketones using imidazolium-tagged sulfonamide catalyst. Tetrahedron-Asymmetry 2006, 17, 1895–1900. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janeczko, T.; Dymarska, M.; Kostrzewa-Susłow, E. Highly Enantioselective Production of (R)-Halohydrins with Whole Cells of Rhodotorula rubra KCh 82 Culture. Int. J. Mol. Sci. 2014, 15, 22392-22404. https://doi.org/10.3390/ijms151222392
Janeczko T, Dymarska M, Kostrzewa-Susłow E. Highly Enantioselective Production of (R)-Halohydrins with Whole Cells of Rhodotorula rubra KCh 82 Culture. International Journal of Molecular Sciences. 2014; 15(12):22392-22404. https://doi.org/10.3390/ijms151222392
Chicago/Turabian StyleJaneczko, Tomasz, Monika Dymarska, and Edyta Kostrzewa-Susłow. 2014. "Highly Enantioselective Production of (R)-Halohydrins with Whole Cells of Rhodotorula rubra KCh 82 Culture" International Journal of Molecular Sciences 15, no. 12: 22392-22404. https://doi.org/10.3390/ijms151222392