Physiological and Proteomic Analysis in Chloroplasts of Solanum lycopersicum L. under Silicon Efficiency and Salinity Stress
Abstract
:1. Introduction

2. Results
2.1. Shoot Biomass Analysis

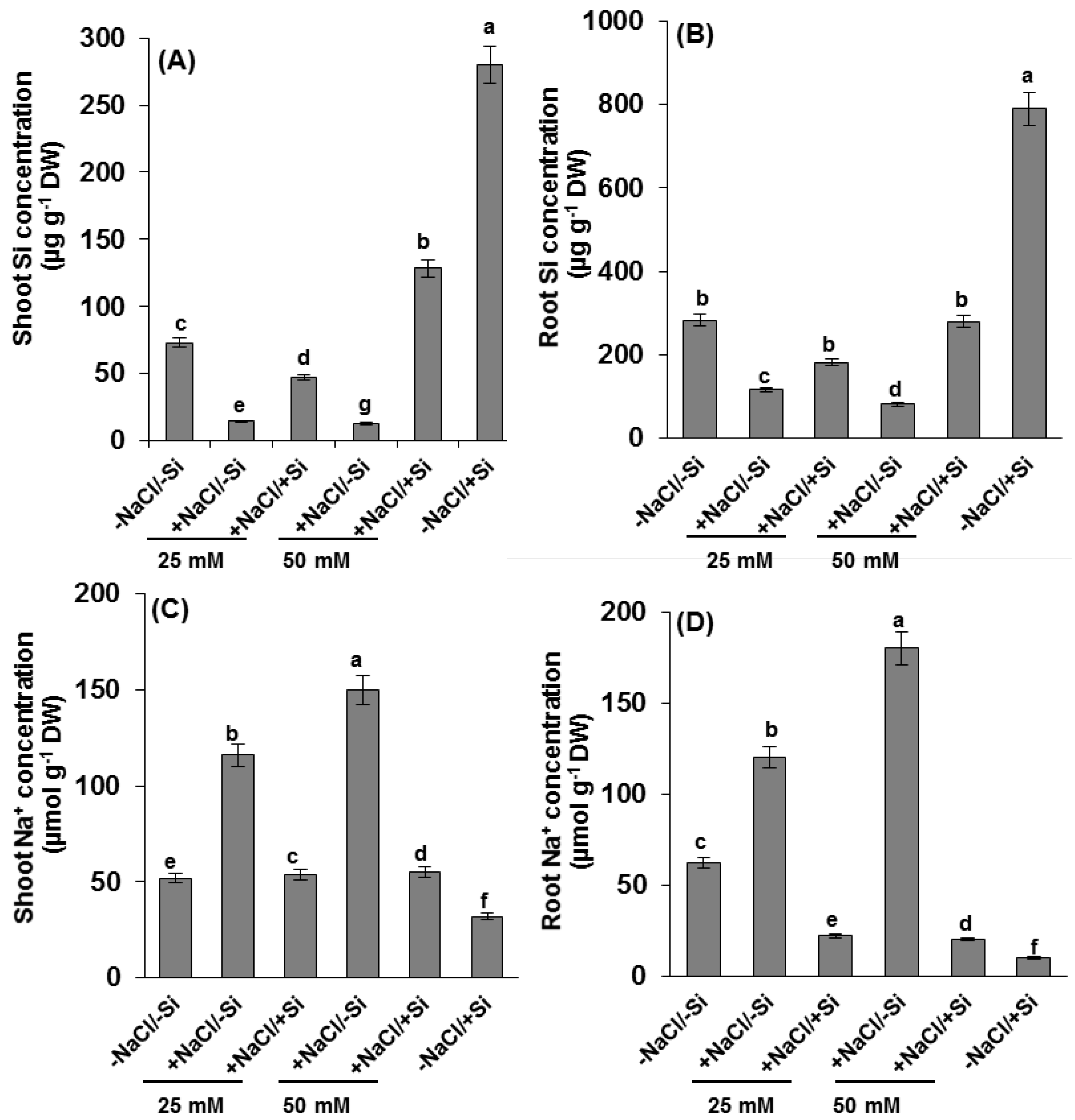
2.2. Silicon and Na+ Concentration
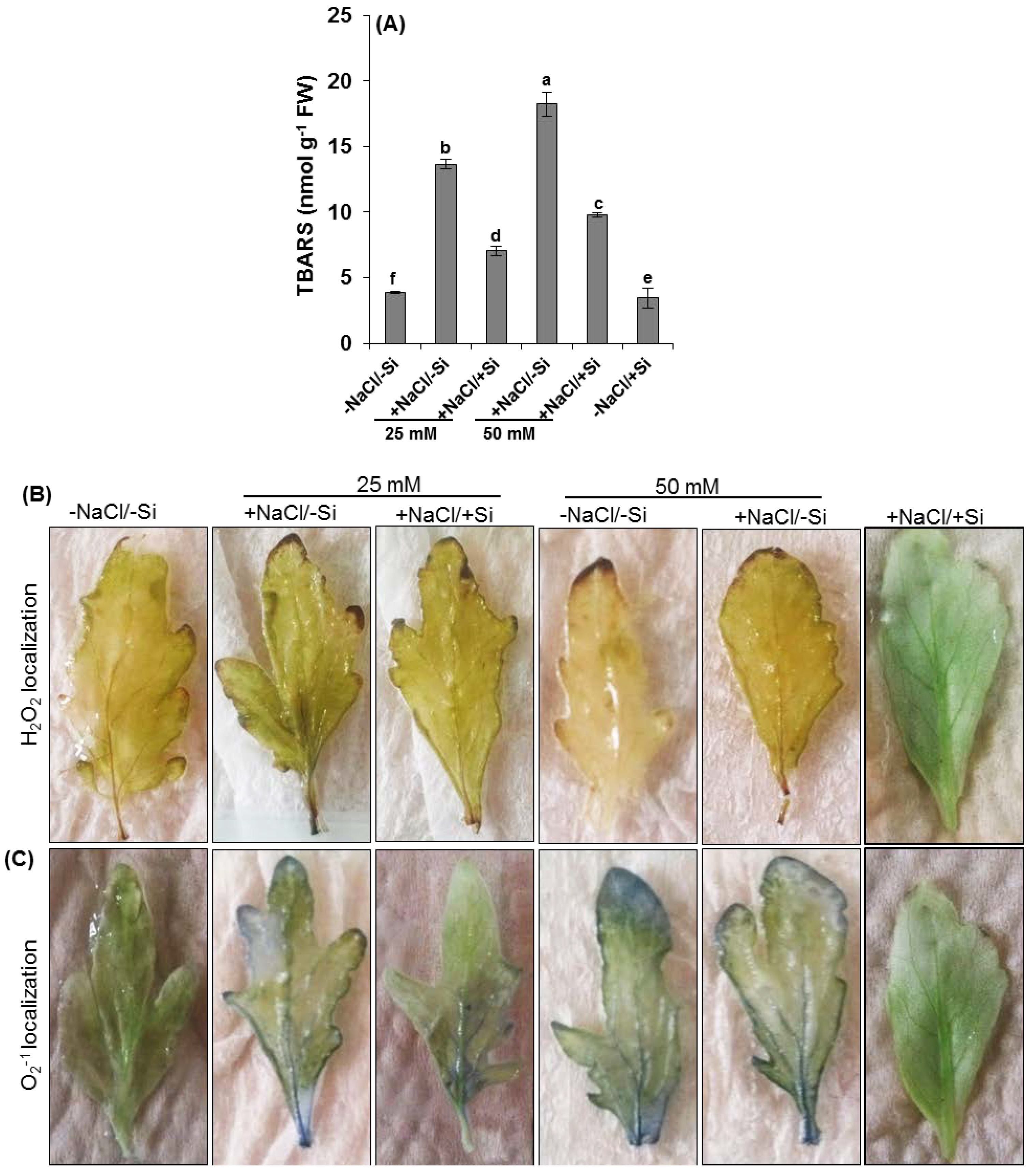
2.3. Oxidative Damage
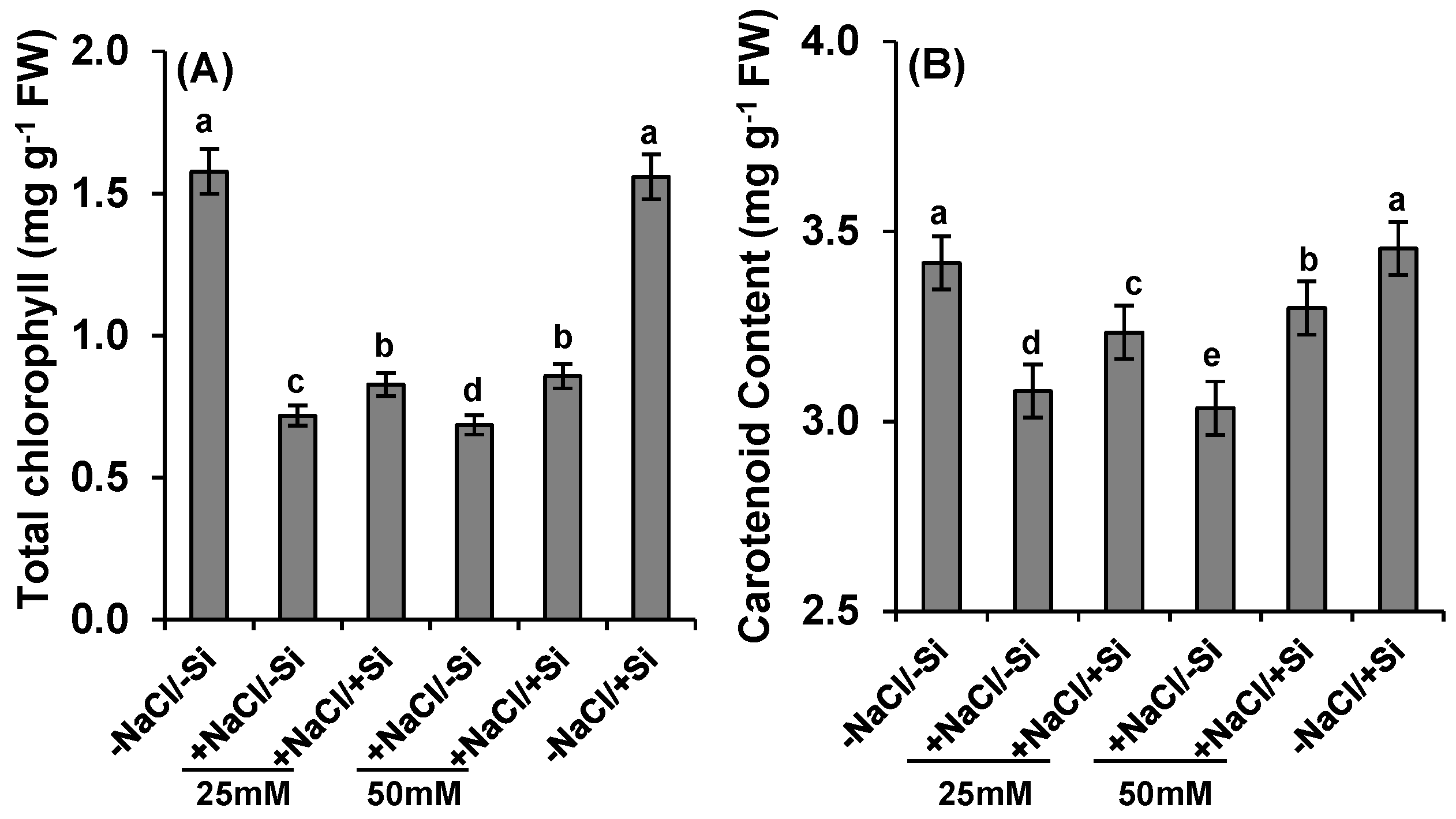
2.4. Pigment Concentration
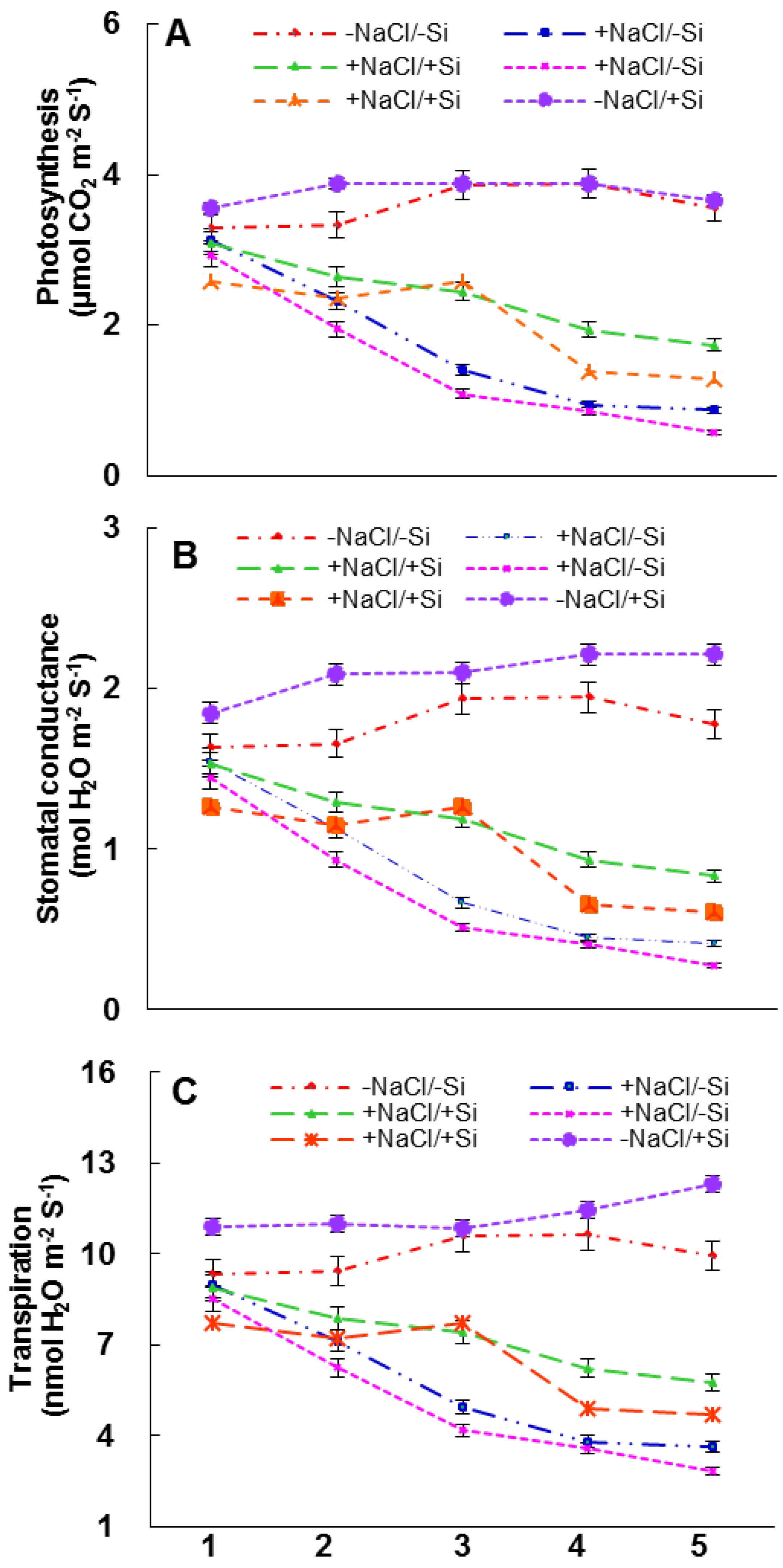
2.5. Photosynthetic Measurements
2.6. 1D BN-PAGE (First Dimensional Blue-Native Polyacrylamide Gel Electrophoresis)
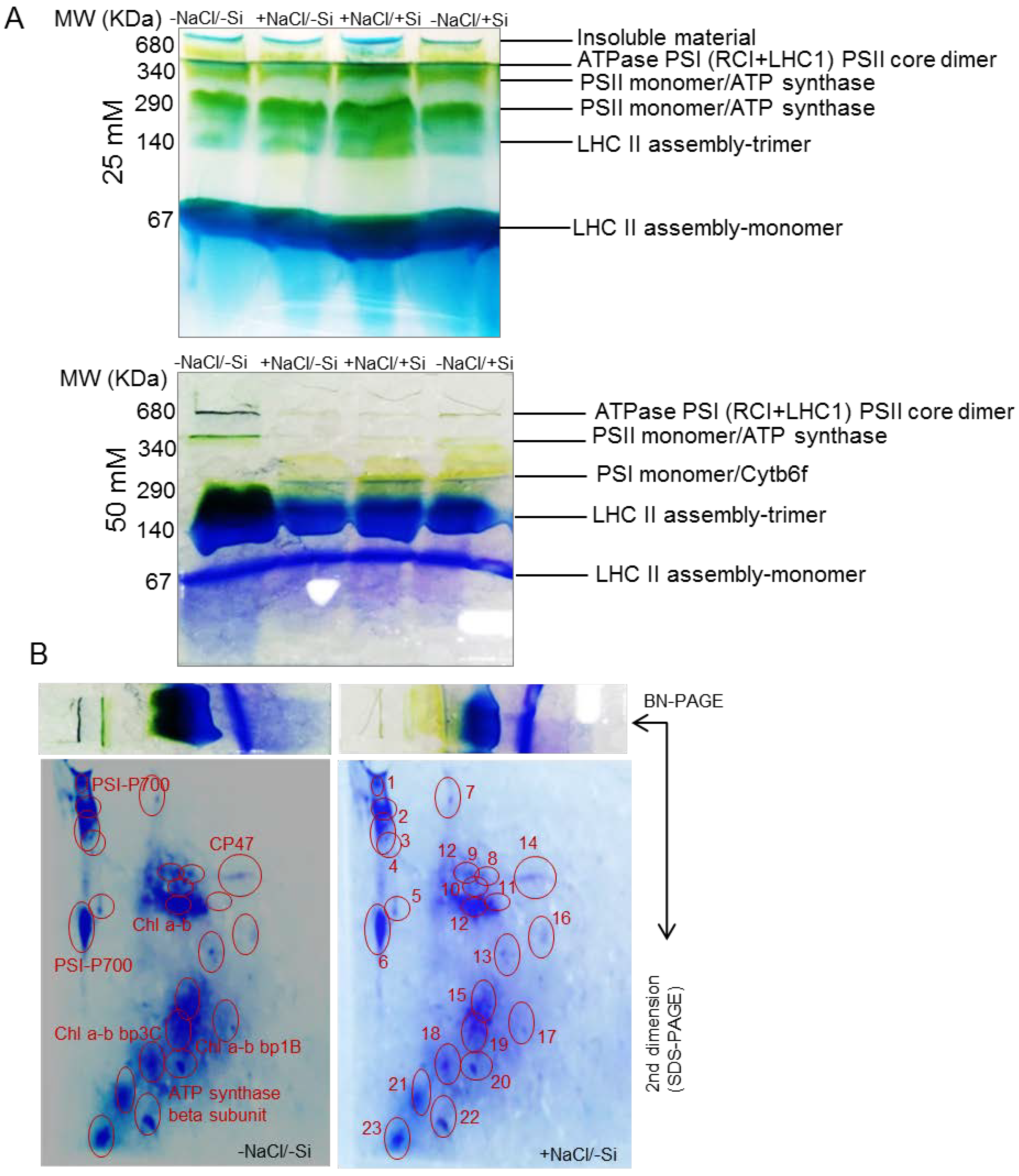
| Spot No. | Protein Identification | Plant Species | MASCOT Ion Score | NCBI Accession Number | Peptides | Sequence Coverage (%) |
|---|---|---|---|---|---|---|
| 1 | Self-incompatibility ribonuclease | Solanum peruvianum | 32 | K4AZ78 | K.RWPQLK.H | 12 |
| 2 | Photosystem I P700 chlorophyll a apoprotein A2 | Solanum lycopersicum | 29 | Q2MIA1 | R.TPLANLIR.W | 18 |
| 3 | Non-specific lipid-transfer protein | Solanum lycopersicum | 36 | K4CLX6 | MTFSKMQK.I | 26 |
| 4 | CEL4 = CELLULASE 4 | Solanum lycopersicum | 47 | Q9S948 | R.GSYSNGLRK.V | 68 |
| 5 | Glucose-6-phosphate isomerase | Solanum lycopersicum | 33 | K4BU47 | K.SGGTPETR.N | 63 |
| 6 | Photosystem I P700 chlorophyll a apoprotein A2 | Solanum lycopersicum | 61 | Q2MIA1 | R.TPLANLIR.W | 26 |
| 7 | Uncharacterized protein | Solanum lycopersicum | 42 | K4CNV4 | R.HEATQER.D | 62 |
| 8 | Oxygen-evolving enhancer protein 1, chloroplastic | Solanum lycopersicum | 74 | P23322 | R.VPFLFTIK.Q | 39 |
| 9 | Oxygen-evolving enhancer protein 1, chloroplastic | Solanum lycopersicum | 68 | P23322 | R.VPFLFTIK.Q | 41 |
| 10 | Oxygen-evolving enhancer protein 1, chloroplastic | Solanum lycopersicum | 288 | P23322 | R.GSSFLDPK.G | 58 |
| 11 | Photosystem I P700 chlorophyll a apoprotein A2 | Solanum lycopersicum | 69 | Q2MIA1 | R.TPLANLIR.W | 28 |
| 12 | Chlorophyll a-b binding protein 1B | Solanum lycopersicum | 50 | P07370 | K.FGEAVWFK.A | 20 |
| 13 | Chlorophyll a-b binding protein 1B | Solanum lycopersicum | 46 | P07370 | K.FGEAVWFK.A | 36 |
| 14 | Photosystem II CP47 chlorophyll apoprotein | Solanum lycopersicum | 38 | Q2MI75 | R.TGKPSLDLPK.I | 36 |
| 15 | Chlorophyll a-b binding protein 3C, chloroplastic | Solanum lycopersicum | 37 | P07369 | K.FGEAVWFK.A | 36 |
| 16 | Photosystem II CP47 chlorophyll apoprotein | Solanum lycopersicum | 57 | Q2MI75 | R.TPLANLIR.W | 71 |
| 17 | Chloroplast sedoheptulose-1,7-bisphosphatase | Solanum lycopersicum | 44 | C5IU71 | K.VVDLLAPYR.R | 71 |
| 18 | Chlorophyll a-b binding protein 3C, chloroplastic | Solanum lycopersicum | 69 | P07369 | K.FGEAVWFK.A | 71 |
| 19 | Chlorophyll a-b binding protein 1B, chloroplastic | Solanum lycopersicum | 57 | P07370 | K.VVDLLAPYR.R | 71 |
| 20 | Photosystem I P700 chlorophyll a apoprotein A2 | Solanum lycopersicum | 61 | Q2MIA1 | R.TPLANLIR.W | 26 |
| 21 | ATP synthase subunit beta, chloroplastic | Solanum lycopersicum | 57 | Q9SCB5 | K.VVDLLAPYR.R | 23 |
| 22 | Chlorophyll a-b binding protein 3C, chloroplastic | Solanum lycopersicum | 69 | P07369 | K.FGEAVWFK.A | 41 |
| 23 | Chlorophyll a-b binding protein 1B, chloroplastic | Solanum lycopersicum | 69 | P07370 | K.FGEAVWFK.A | 41 |
2.7. 2D BN-SDS-PAGE
2.8. Relationships among Descriptive Parameters with Si Concentration
| Treatments | FW | DW | TBARS | CL | CR | NPR | TR | SC | |
|---|---|---|---|---|---|---|---|---|---|
| Si | (25 mM) | 0.768 ** | 0.354 * | −0.894 *** | 0.031 | 0.068 | 0.536 * | 0.516 * | 0.219 |
| (50 mM) | 0.568 * | 0.903 *** | 0.935 *** | 0.219 | 0.042 | 0.943 *** | 0.933 *** | 0.951 *** | |
3. Discussion
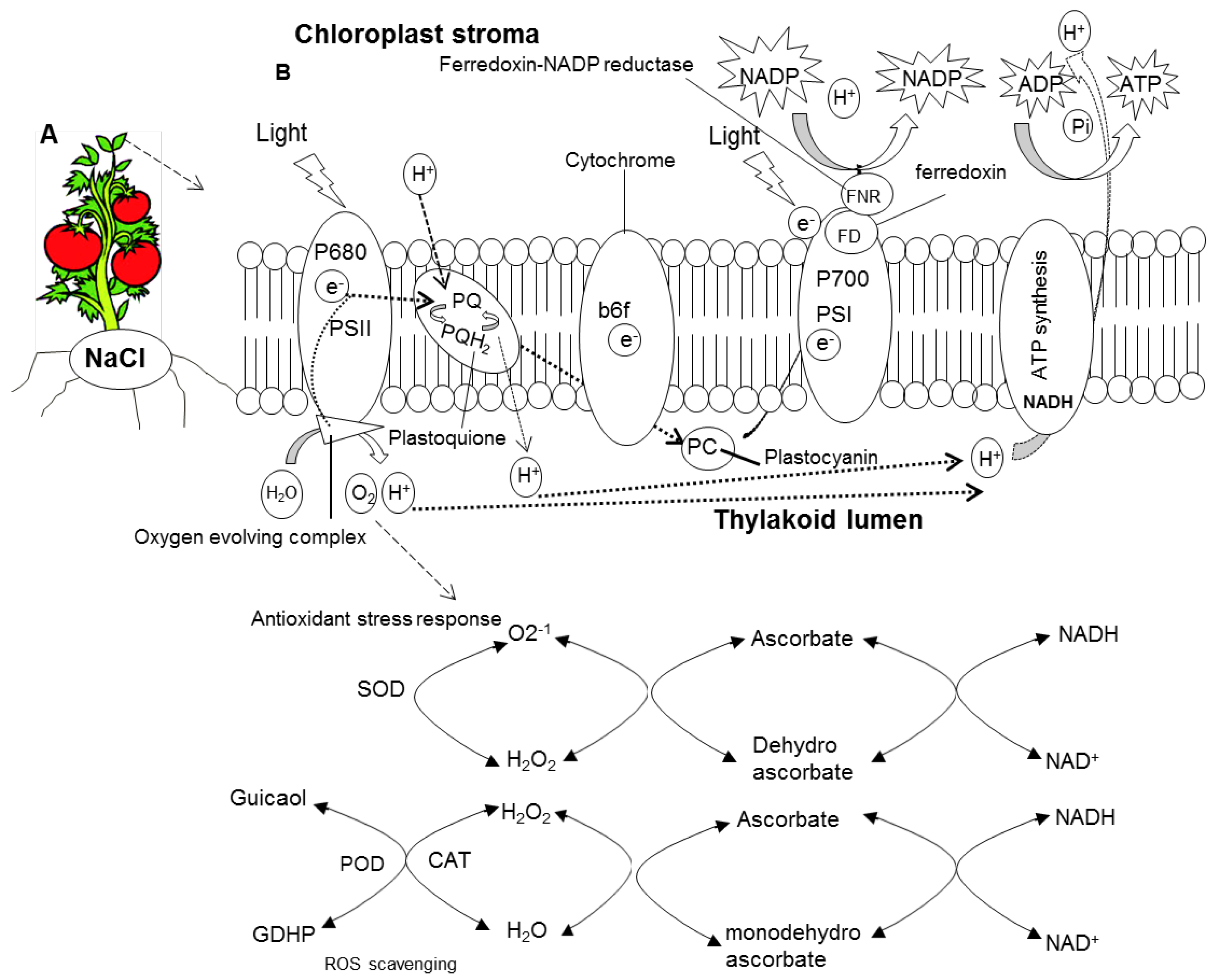
4. Experimental Section
4.1. Plant Material and Treatments
4.2. Assessment of Root Biomass
4.3. Thiobarbituric Acid Reactive Substances (TBARS) Determination and H2O2 and O2−1 Localizations
4.4. Estimation of Silicon and Na+ Content
4.5. Pigment Determination
4.6. Measurement of Photosynthetic Activity
4.7. 2D BN-SDS-PAGE
4.8. Image Analysis
4.9. Protein in Gel Digestion and Identification by Matrix Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry (MALDI-TOF-TOF-MS)
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ma, J.F.; Mitani, N.; Nagao, S.; Konishi, S.; Tamai, K.; Iwashita, T.; Yano, M. Characterization of the silicon system and molecular mapping of the silicon transporter gene in rice. Plant Physiol. 2004, 136, 3284–3289. [Google Scholar] [CrossRef]
- Fauteux, F.; Chain, F.; Belzile, F.; Menzies, J.G.; Belanger, R.R. The protective role of silicon in the Arabidopsis-powdery mildew pathosystem. Proc. Natl. Acad. Sci. USA 2006, 103, 17554–17559. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.P.; Tesson, B.; Norden-Krichmar, T.; Federowicz, S.; Hildebrand, M.; Allen, A.E. Whole transcriptome analysis of the silicon response of the diatom Thalassiosira pseudonana. BMC Genomics 2012, 13. [Google Scholar] [CrossRef]
- Romero-Aranda, M.R.; Jurado, O.; Cuartero, J. Silicon alleviates the deleterious salt effect on tomato plant growth by improving plant water status. J. Plant Physiol. 2005, 163, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Naranjo, E.; Andrades-Moreno, L.; Davy, A.J. Silicon alleviates deleterious effects of high salinity on the halophytic grass Spartina densiflora. Plant Physiol. Biochem. 2013, 63, 115–121. [Google Scholar] [CrossRef]
- Kim, Y.H.; Khan, A.L.; Kim, D.H.; Lee, S.Y.; Kim, K.M.; Waqas, M.; Jung, H.Y.; Shin, J.H.; Kim, J.G.; Lee, I.J. Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativa low silicon genes, and endogenous phytohormones. BMC Plant Biol. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E. Silicon. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 641–664. [Google Scholar] [CrossRef] [PubMed]
- Richmond, K.E.; Sussman, M. Got silicon? The non-essential beneficial plant nutrient. Curr. Opin. Plant Biol. 2003, 6, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Khan, A.L.; Waqas, M.; Jeong, H.J.; Kim, D.H.; Shin, J.S.; Kim, J.G.; Yeon, M.H.; Lee, I.J. Regulation of jasmonic acid biosynthesis by silicon application during physical injury to Oryza sativa L. J. Plant Res. 2014, 127, 525–532. [Google Scholar] [CrossRef]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Balakhnina, T.; Borkowska, A. Effects of silicon on plant resistance to environmental stresses: Review. Int. Agrophys. 2013, 27, 225–232. [Google Scholar] [CrossRef]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef]
- Sivanesan, I.; Park, S.W. The role of silicon in plant tissue culture. Front. Plant Sci. 2014. [Google Scholar] [CrossRef]
- Van Bockhaven, J.; DeVleesschauwer, D.; Höfte, M. Towards establishing broad-spectrum disease resistance in plants: Silicon leads the way. J. Exp. Bot. 2013, 64, 1281–1293. [Google Scholar] [CrossRef]
- Zörb, C.; Schmitt, S.; Mühling, K.H. Proteomic changes in maize roots after short-term adjustment to saline growth conditions. Proteomics 2010, 10, 4441–4449. [Google Scholar] [CrossRef]
- Song, S.Y.; Chen, Y.; Chen, J.; Dai, X.Y.; Zhang, W.H. Physiological mechanisms underlying OsNAC5-dependent tolerance of rice plants to abiotic stress. Planta 2011, 234, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, X.; Liang, M.; Tan, F.; Liang, W.; Chen, Y.; Lin, Y.; Huang, L.; Xing, J.; Chen, W. Proteomic analysis of salt-responsive proteins in the leaves of mangrove Kandelia candel during short-term stress. PLoS One 2014, 9, e83141. [Google Scholar] [CrossRef] [PubMed]
- Trappa, S.; Feificovab, D.; Rasmussena, N.F.; Gottwei, P.B. Plant uptake of NaCl in relation to enzyme kinetics and toxic effects. Environ. Exp. Bot. 2008, 64, 1–7. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Allakhverdiev, S.I.; Sakamoto, A.; Nishiyama, Y.; Inaba, M.; Murata, N. Ionic and osmotic effects of NaCl-induced inactivation of photosystems I and II in Synechococcus sp. Plant Physiol. 2000, 123, 1047–1056. [Google Scholar] [CrossRef]
- Askari, H.; Edqvist, J.; Hajheidari, M.; Kafi, M.; Salekdeh, G.H. Effects of salinity levels on proteome of Suaeda aegyptiaca leaves. Proteomics 2006, 6, 2542–2554. [Google Scholar] [CrossRef] [PubMed]
- Bandehagh, A.; Salekdeh, G.H.; Toorchi, M.; Mohammadi, A.; Komatsu, S. Comparative proteomic analysis of canola leaves under salinity stress. Proteomics 2011, 11, 1965–1975. [Google Scholar] [CrossRef]
- Kosova, K.; Vitamvas, P.; Prasil, I.T.; Renaut, J. Plant proteome changes under abiotic stress-contribution of proteomics studies to understanding plant stress response. J. Proteomics 2011, 74, 1301–1322. [Google Scholar] [CrossRef]
- Zhu, J.; Fu, X.; Koo, Y.D.; Zhu, J.K.; Jenney, F.E., Jr.; Adams, M.W.; Zhu, Y.; Shi, H.; Yun, D.J.; Hasegawa, P.M.; et al. An enhancer mutant of Arabidopsis salt overly sensitive 3 mediates both ion homeostasis and the oxidative stress response. Mol. Cell. Biol. 2007, 27, 5214–5224. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, S.; Zuchi, S. Adequate S supply protects barley plants from adverse effects of salinity stress by increasing thiol contents. Acta Physiol. Plant. 2013, 35, 175–181. [Google Scholar] [CrossRef]
- Chen, Y.E.; Yuan, S.; Du, J.B.; Xu, M.Y.; Zhang, Z.W.; Lin, H.H. Phosphorylation of photosynthetic antenna protein CP29 and photosystem II structure changes in monocotyledonous plants under environmental stresses. Biochemistry 2009, 48, 9757–9763. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liang, W.; Xing, J.; Tan, F.; Chen, Y.; Huang, L.; Cheng, C.L.; Chen, W. Dynamics of chloroplast proteome in salt-stressed mangrove Kandelia candel (L.) Druce. J. Proteome Res. 2013, 12, 5124–5136. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhang, H.; Wang, T.; Chen, S.X.; Dai, S.J. Proteomics-based investigation of salt-responsive mechanisms in plant roots. J. Proteomics 2013, 82, 230–253. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Rakwal, R.; Agrawal, G.K.; Jung, Y.H.; Shibato, J.; Jwa, N.S.; Iwahashi, Y.; Iwahashi, H.; Kim, D.H.; Shim, I.S.; et al. A hydroponic rice seedling culture model system for investigating proteome of salt stress in rice leaf. Electrophoresis 2005, 26, 4521–4539. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.; Flowers, T.; Moore, A.; Harpham, N. An accurate and reproducible method for proteome profiling of the effects of salt stress in the rice leaf lamina. J. Exp. Bot. 2006, 57, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Zaheer, S.; Ismail, S. Role of silicon in salt tolerance of wheat (Triticum aestivum L.). Plant Sci. 1992, 85, 43–50. [Google Scholar] [CrossRef]
- Liang, Y.C.; Chen, Q.; Liu, Q.; Zhang, W.H.; Ding, R.X. Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J. Plant Physiol. 2003, 160, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Stass, A.; Horst, W.J. Apoplastic binding of aluminum is involved in silicon-induced amelioration of aluminum toxicity in maize. Plant Physiol. 2004, 136, 3762–3770. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.C.; Wong, J.W.; Wei, L. Silicon-mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil. Chemosphere 2005, 58, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Cuartero, J.; Fernández-Muñoz, R. Tomato and salinity. Sci. Hort. 1999, 78, 83–125. [Google Scholar] [CrossRef]
- Zouni, A.; Witt, H.T.; Kern, J.; Fromme, P.; Krauss, N.; Saenger, W.; Orth, P. Crystal structure of photosystem II from Synechococcus elongatus at 3.8 Å resolution. Nature 2001, 409, 739–743. [Google Scholar] [CrossRef]
- D’ Amici, G.M.; Timperio, A.M.; Zolla, L. Coupling of native liquid Phase isoelectro-focusing and blue native polyacrylamide gel electrophoresis: A potent tool for native membrane multiprotein complex separation. J. Proteome Res. 2008, 7, 1326–1340. [Google Scholar] [CrossRef]
- Liang, Y.; Sun, W.; Zhu, Y.G.; Christie, P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: A review. Environ. Pollut. 2007, 147, 422–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zargar, S.M.; Nazir, M.; Agrawal, G.K.; Kim, D.W.; Rakwal, R. Silicon in plant tolerance against environmental stressors: towards crop improvement using Omics approaches. Curr. Proteomics 2010, 7, 135–143. [Google Scholar] [CrossRef]
- Vaculík, M.; Landberg, T.; Greger, M.; Luxová, M.; Stoláriková, M.; Lux, A. Silicon modifies root anatomy, and uptake and subcellular distribution of cadmium in young maize plants. Ann. Bot. 2012, 110, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, S.A.; Bhat, M.A. Biomineralisation of silicon and calcium in plants and its control: An overview. Plant 2014, 2, 6–13. [Google Scholar] [CrossRef]
- Li, J.; Frantz, J.; Leisner, S. Alleviation of copper toxicity in Arabidopsis thaliana by silicon addition to hydroponic solutions. J. Am. Soc. Hort. Sci. 2008, 133, 670–677. [Google Scholar]
- Khandekar, S.; Leisner, S. Soluble silicon modulates expression of Arabidopsis thaliana genes involved in copper stress. J. Plant Physiol. 2011, 168, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Blumwald, E. Sodium transport and salt tolerance in plants. Curr. Opin. Cell Biol. 2000, 12, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Sobhanian, H.; Aghaei, K.; Komatsu, S. Changes in the plant proteome resulting from salt stress: Towards the creation of salt-tolerant crops? J. Proteomics 2011, 4, 1323–1337. [Google Scholar] [CrossRef]
- Ranieri, A.; Castagna, A.; Baldan, B.; Soldatini, G.F. Iron deficiency differently affects peroxidase isoforms in sunflower. J. Exp. Bot. 2001, 52, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Qadir, S.; Qureshi, M.I.; Javed, S.; Abdin, M.Z. Genotypic variation in phytoremediation potential of Brassica juncea cultivars exposed to Cd-stress. Plant Sci. 2004, 167, 1171–1178. [Google Scholar] [CrossRef]
- Muneer, S.; Lee, B.R.; Bae, D.W.; Kim, T.H. Changes in expression of proteins involved in alleviation of Fe-deficiency by sulfur nutrition in Brassica napus L. Acta Physiol. Plant. 2013, 35, 3037–3045. [Google Scholar] [CrossRef]
- Muneer, S.; Lee, B.R.; Kim, K.Y.; Park, S.H.; Zhang, Q.; Kim, T.H. Involvement of sulphur nutrition in modulating iron deficiency response in photosynthetic organelles of oilseed rape (Brassica napus L.). Photosynth. Res. 2014, 119, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Tamai, K.; Konishi, S.; Fujiwara, T.; Katsuhara, M.; Yano, M. An efflux transporter of silicon in rice. Nature 2007, 448, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.A.; Hearst, J.E. Carotenoids 2: Genetics and molecular biology of carotenoid pigment biosynthesis. FASEB J. 1996, 10, 228–237. [Google Scholar] [PubMed]
- Zhang, Y.P.; Wang, Z.M.; Wu, Y.C.; Zhang, X. Stomatal characteristics of different green organs in wheat under different irrigation regimes. Acta Agron. Sin. 2006, 32, 70–75. [Google Scholar]
- Xu, Z.Z.; Zhou, G.S. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, W.; Nowakowska, J. Silicon and copper interaction in the growth of spring wheat seedlings. Biol. Plant. 1997, 39, 463–466. [Google Scholar] [CrossRef]
- Benavides, M.P.; Gallego, S.M.; Tomaro, M.L. Cadmium toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 21–34. [Google Scholar] [CrossRef]
- Hasan, S.A.; Fariduddin, Q.; Ali, B.; Hayat, S.; Ahmad, A. Cadmium: Toxicity and tolerance in plants. J. Environ. Biol. 1995, 30, 165–174. [Google Scholar]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Razavizadeh, R.; Ehsanpour, A.A.; Ahsan, N.; Komatsu, S. Proteome analysis of tobacco leaves under salt stress. Peptides 2009, 30, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.P.; Eubel, H.; O’Toole, N.; Millar, A. Combining proteomics of root and shoot mitochondria and transcript analysis to define constitutive and variable components in plant mitochondria. Phytochemistry 2011, 72, 1092–1108. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplast I. Kinetics and stoichiometry of fatty acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Hiscox, J.D.; Israclstam, G.F. A method for interaction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Arnon, J.D.; Israelstam, G.F. Copper enzymes in isolated chloroplast oxidase in Beta vulgaris. Plant Physiol. 1949, 42, 287–292. [Google Scholar]
- Kugler, M.; Jansch, L.; Kruft, V.; Schmitz, U.K.; Braun, H.P. Analysis of the chloroplast protein complexes by blue native polyacrylamide gel electrophoresis (BN-PAGE). Photosynth. Res. 1997, 53, 35–44. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muneer, S.; Park, Y.G.; Manivannan, A.; Soundararajan, P.; Jeong, B.R. Physiological and Proteomic Analysis in Chloroplasts of Solanum lycopersicum L. under Silicon Efficiency and Salinity Stress. Int. J. Mol. Sci. 2014, 15, 21803-21824. https://doi.org/10.3390/ijms151221803
Muneer S, Park YG, Manivannan A, Soundararajan P, Jeong BR. Physiological and Proteomic Analysis in Chloroplasts of Solanum lycopersicum L. under Silicon Efficiency and Salinity Stress. International Journal of Molecular Sciences. 2014; 15(12):21803-21824. https://doi.org/10.3390/ijms151221803
Chicago/Turabian StyleMuneer, Sowbiya, Yoo Gyeong Park, Abinaya Manivannan, Prabhakaran Soundararajan, and Byoung Ryong Jeong. 2014. "Physiological and Proteomic Analysis in Chloroplasts of Solanum lycopersicum L. under Silicon Efficiency and Salinity Stress" International Journal of Molecular Sciences 15, no. 12: 21803-21824. https://doi.org/10.3390/ijms151221803
APA StyleMuneer, S., Park, Y. G., Manivannan, A., Soundararajan, P., & Jeong, B. R. (2014). Physiological and Proteomic Analysis in Chloroplasts of Solanum lycopersicum L. under Silicon Efficiency and Salinity Stress. International Journal of Molecular Sciences, 15(12), 21803-21824. https://doi.org/10.3390/ijms151221803








