Molecular Characterization of the BMP7 Gene and Its Potential Role in Shell Formation in Pinctada martensii
Abstract
:1. Introduction
2. Results
2.1. Molecular Cloning and Sequence Analysis of BMP7 from Pinctada martensii (Pm-BMP7)
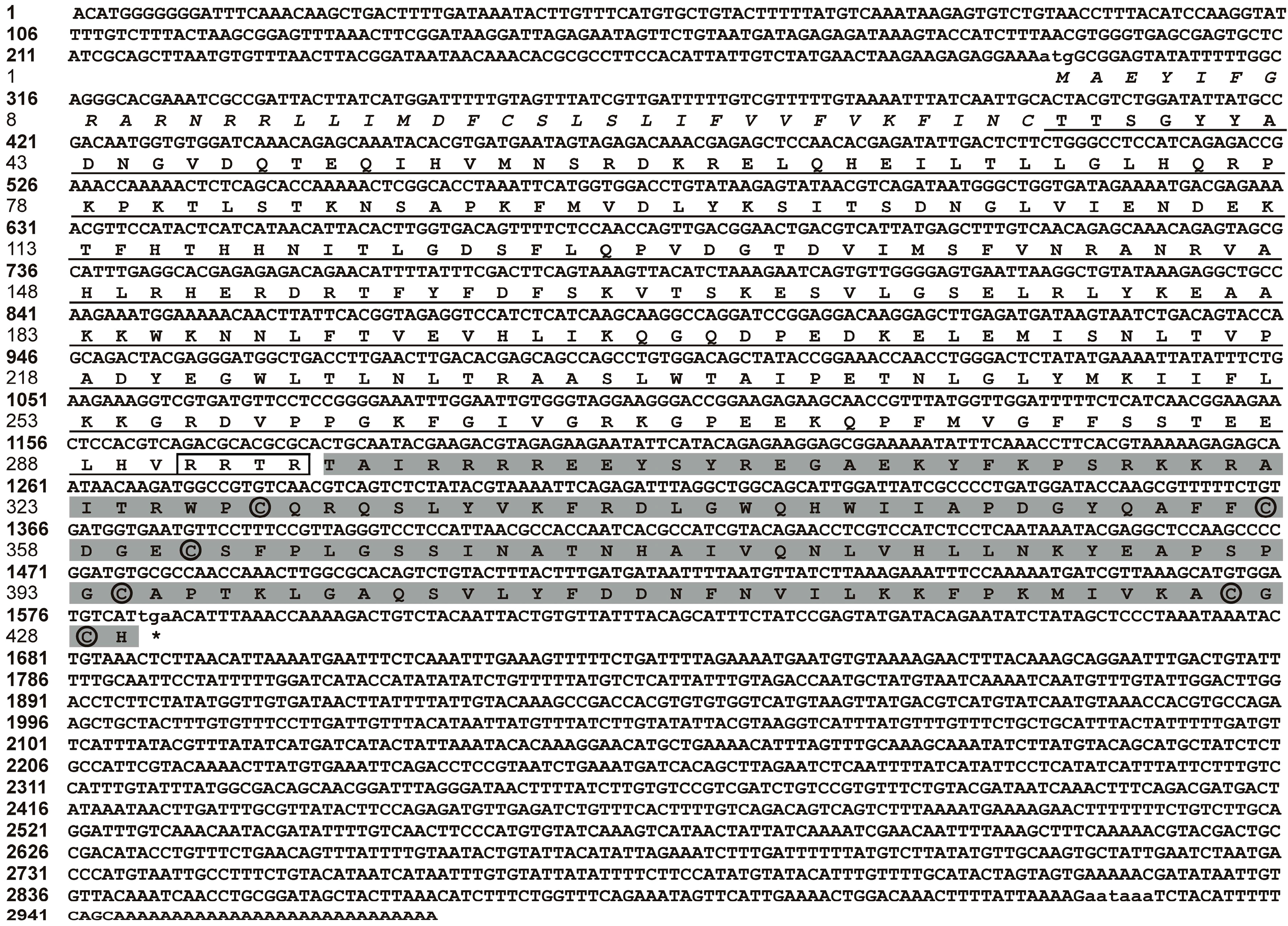
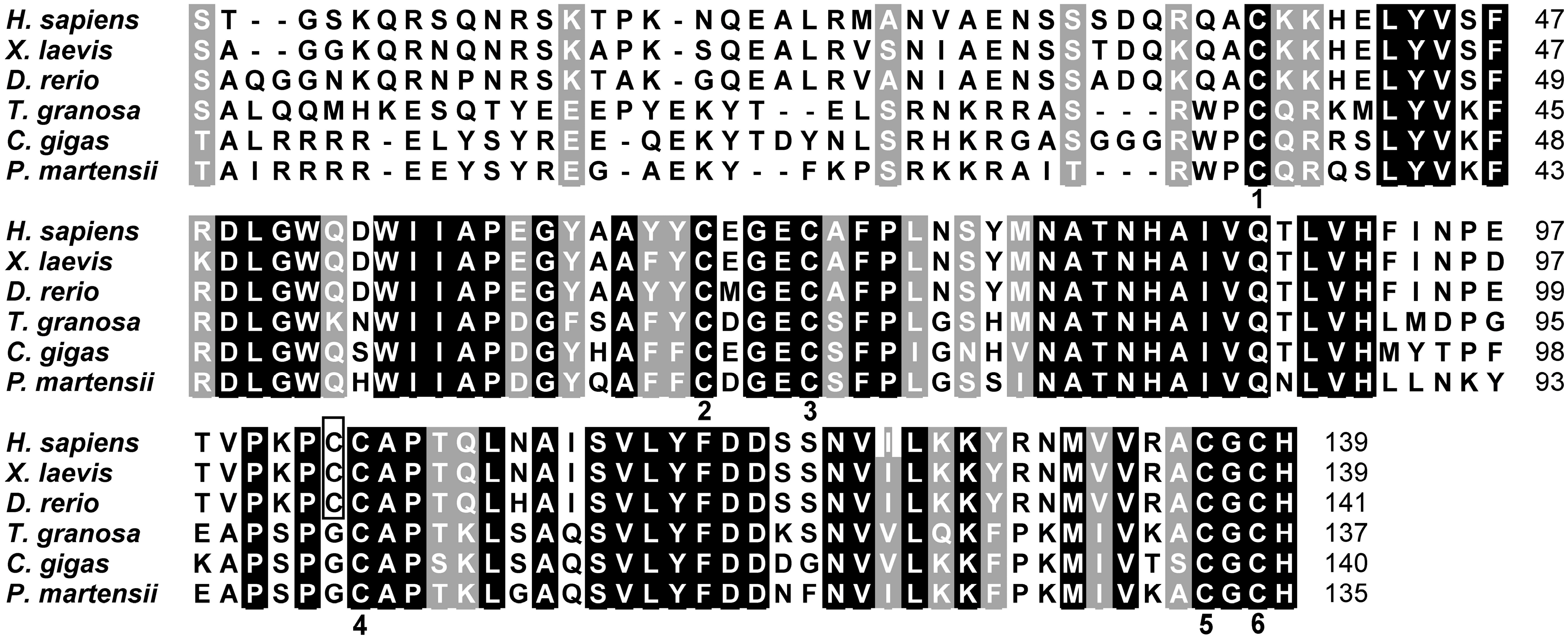
2.2. Homologous and Structural Analysis of Pm-BMP7
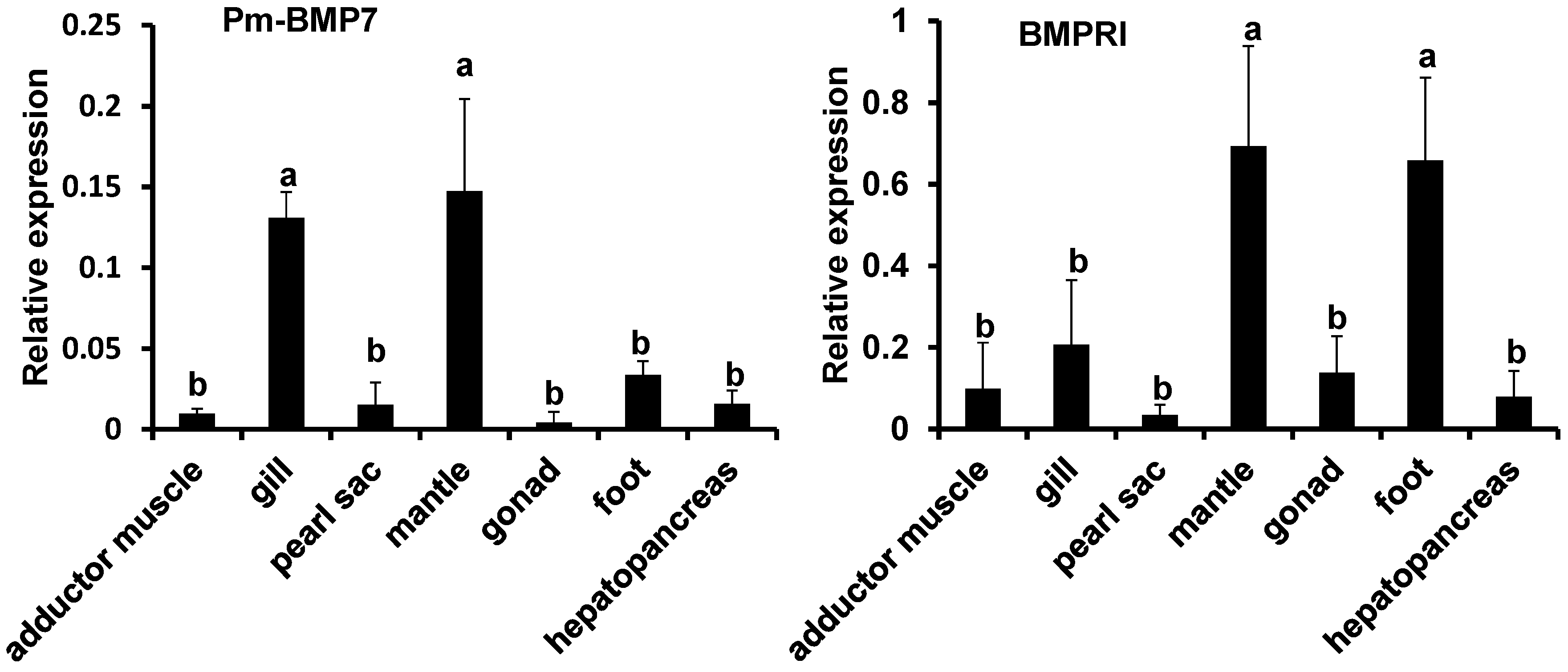
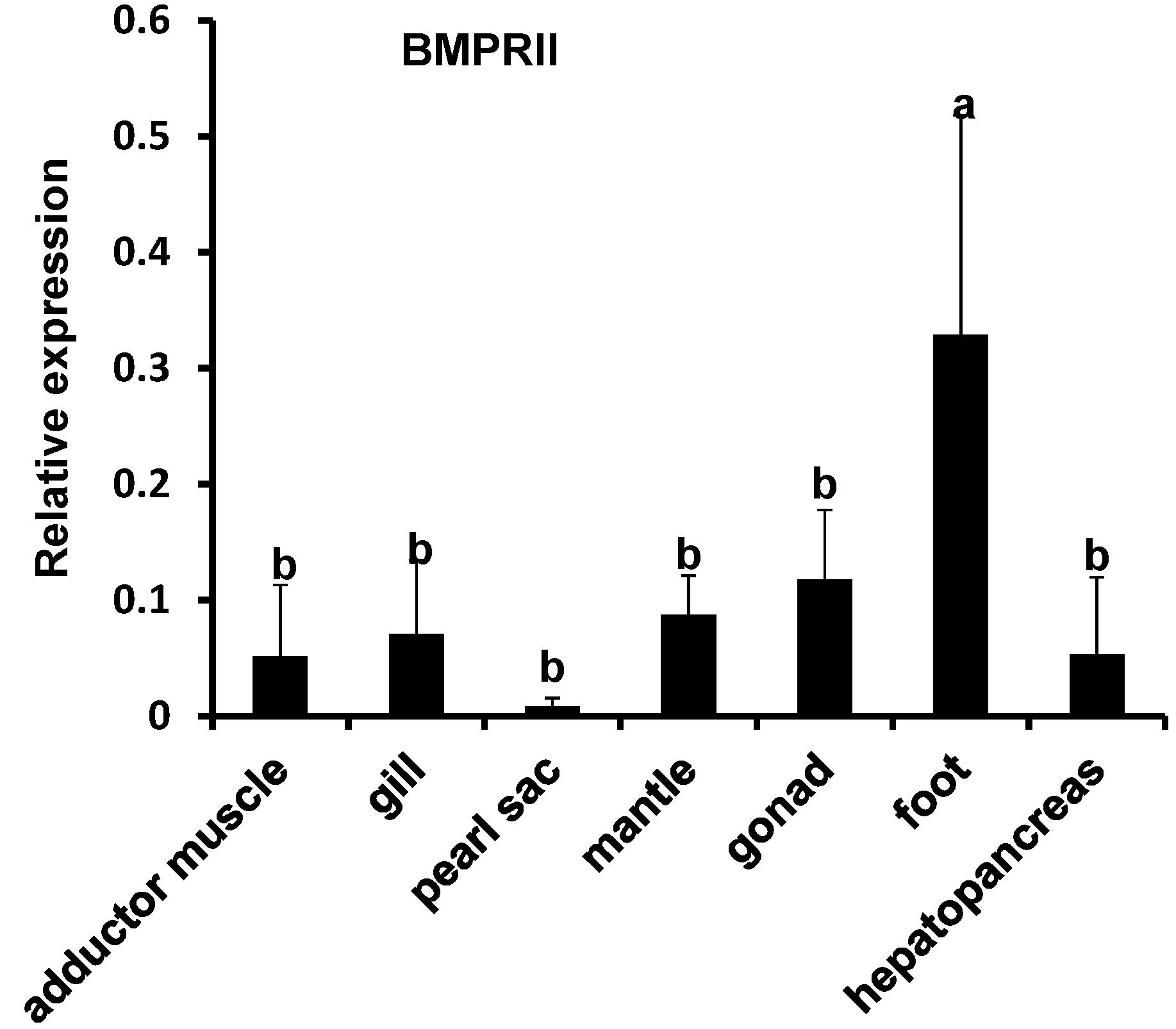
2.3. Expression Analysis of Pm-BMP7 and Its Receptors

2.4. Functions of Pm-BMP7 in Shell Formation
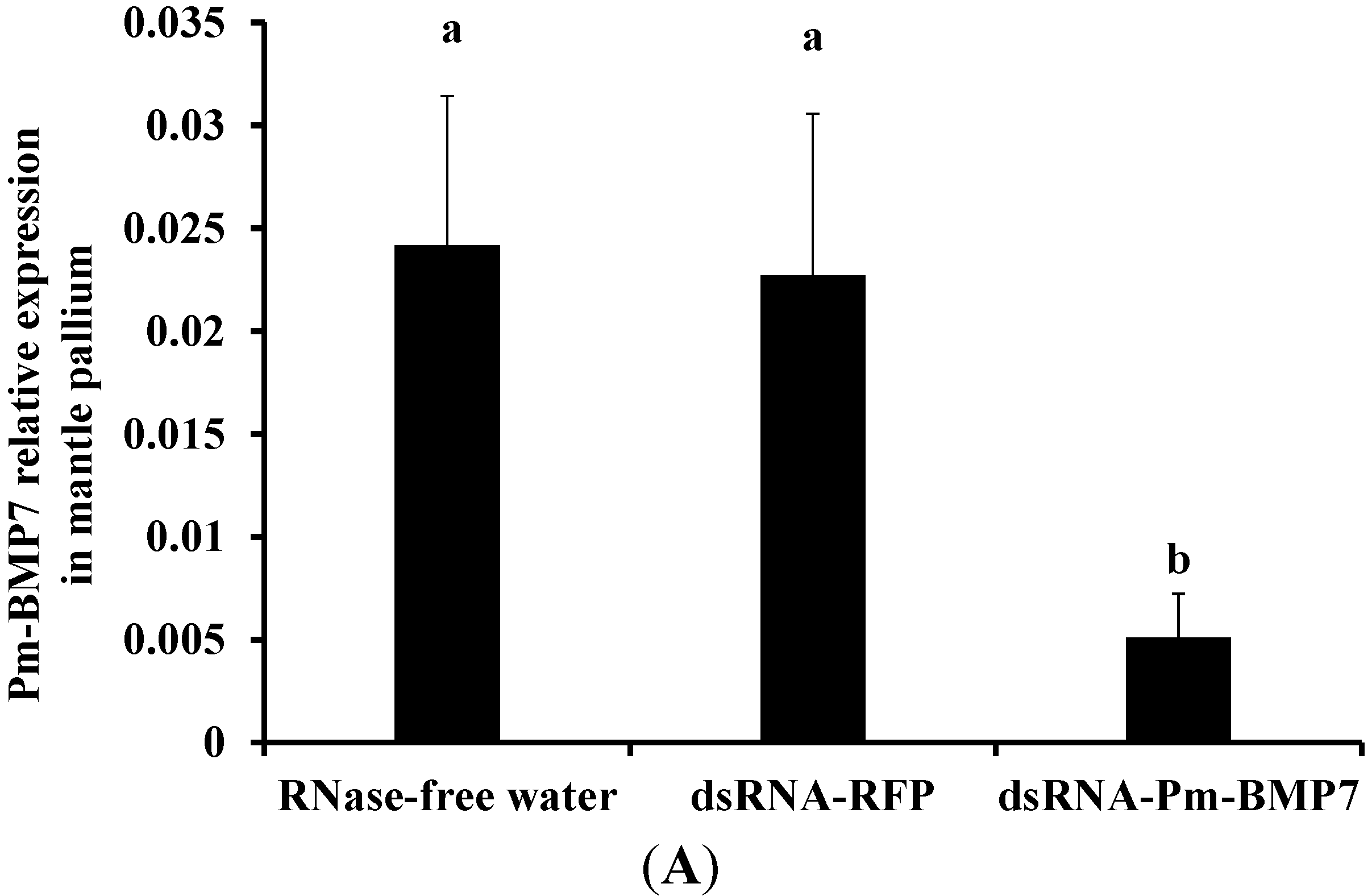
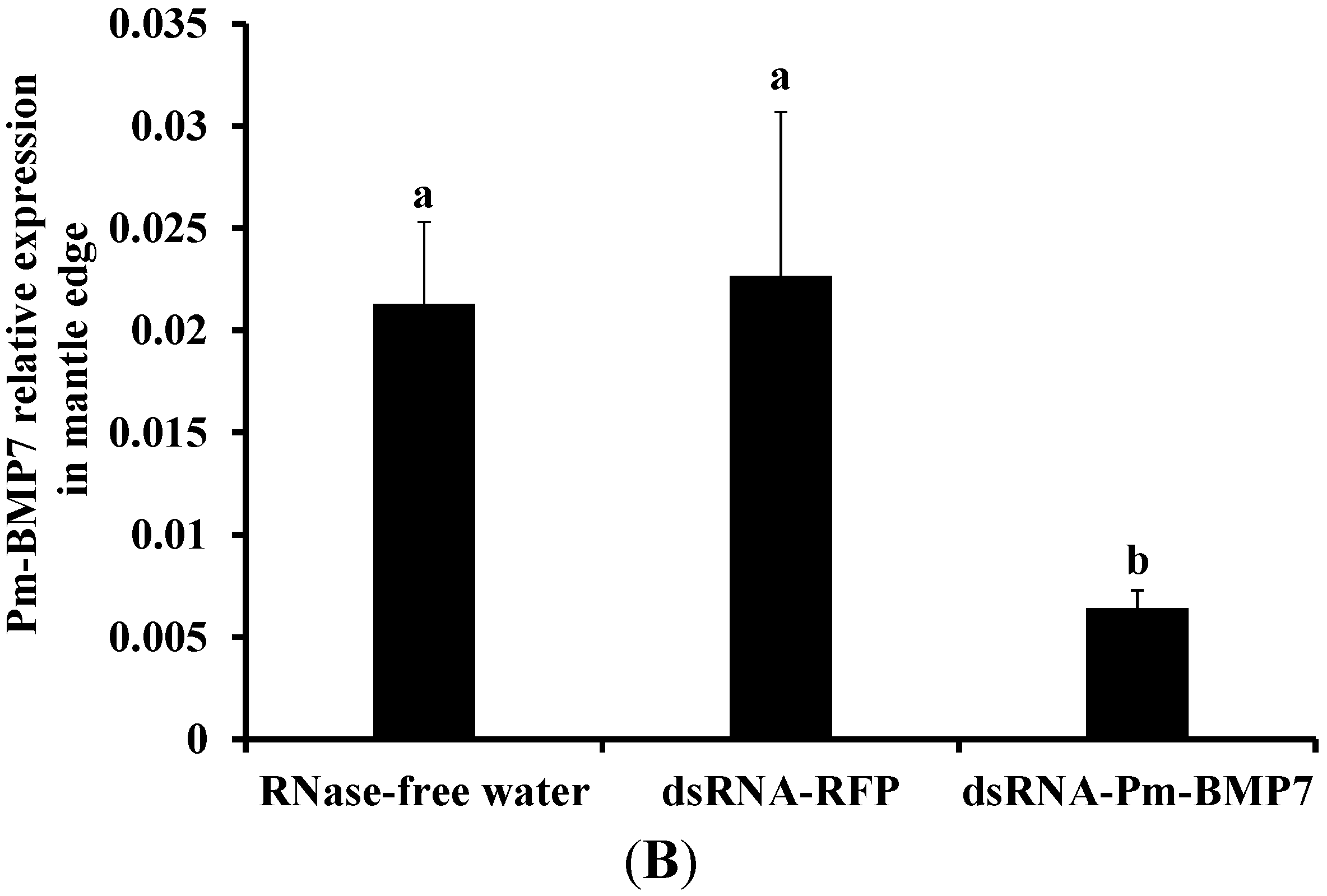
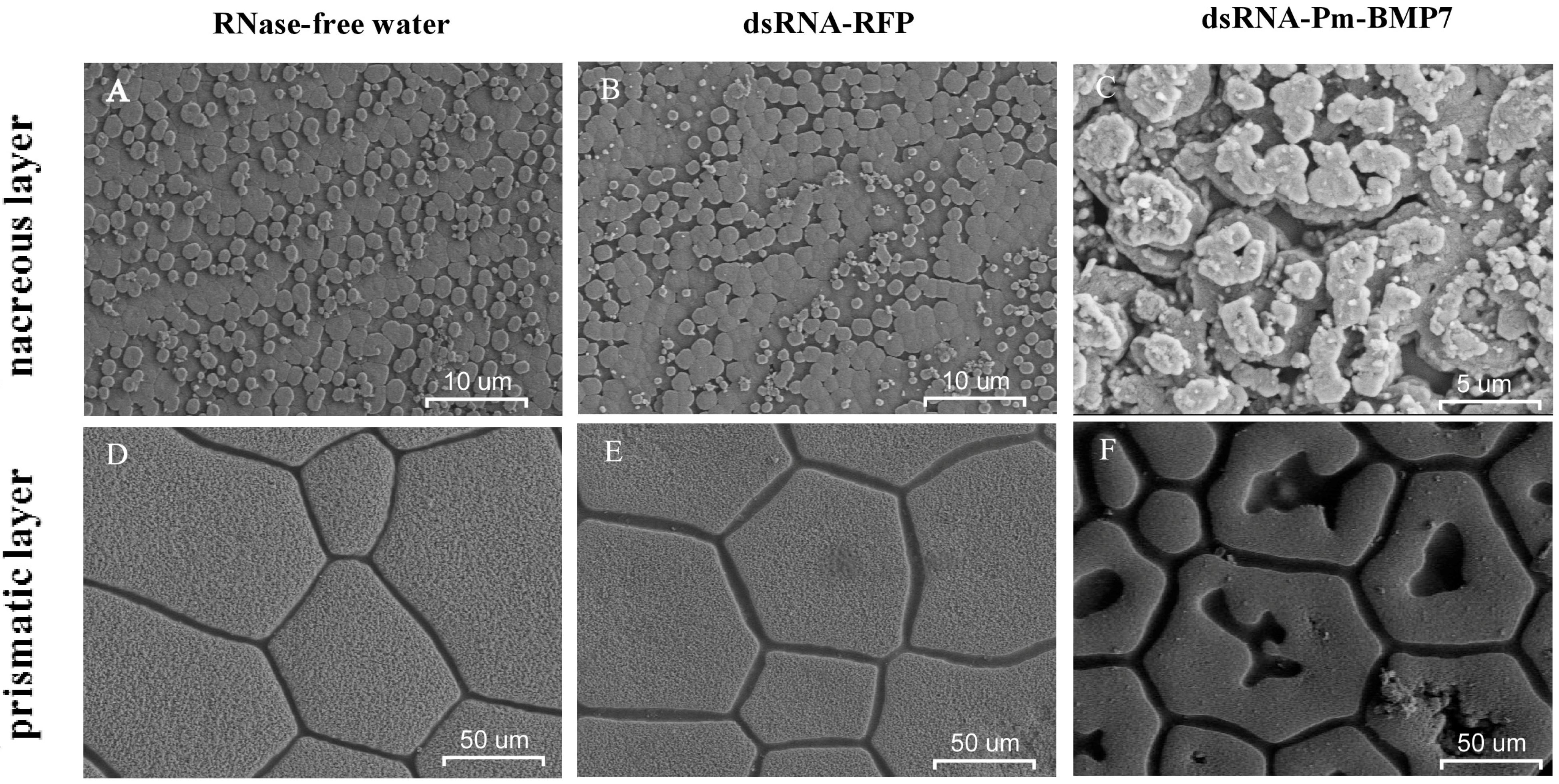
3. Discussion
4. Experimental Section
4.1. Experimental Animals, RNA Extraction and cDNA Synthesis
4.2. Rapid Amplification of cDNA Ends (RACE)
| Primer Name | Primer Sequence (From 5' to 3') | Application |
|---|---|---|
| BMP7-5' outer | TTAAGCTGCGATGAGCACTCGCTCACC | RACE |
| BMP7-5' inner | GAGCACTCGCTCACCCACGTTAAAGAT | RACE |
| BMP7-3' outer | TCCTCCATTAACGCCACCAATCACGC | RACE |
| BMP7-3' inner | TCAATAAATACGAGGCTCCAAGCCCC | RACE |
| BMP7-F | GCGCACTGCAATACGAAGA | qRT-PCR |
| BMP7-R | GGAGGACCCTAACGGAAAG | qRT-PCR |
| BMPRI-F | AAGGCAAAGATGGAGGAAC | qRT-PCR |
| BMPRI-R | TCTCGTGGAACTGGGTGAT | qRT-PCR |
| BMPRII-F | GAGACAAGTTTAAGCCTACCGT | qRT-PCR |
| BMPRII-R | GAATCCCAAGTCACCTATCACA | qRT-PCR |
| β-actin-F | GTGTAAGGCGGGGTTTGCT | qRT-PCR |
| β-actin-R | GGGTCCTTCAGCGTTAGTATCTT | qRT-PCR |
| dsRNA-BMP7-F | GCGTAATACGACTCACTATAGGGTCTAAAGAATCAGTGTTGGGGAGTG | RNAi |
| dsRNA-BMP7-R | GCGTAATACGACTCACTATAGGGGTTAATGGAGGACCCTAACGGAAAG | RNAi |
| dsRNA-RFP-F | GCGTAATACGACTCACTATAGGGGAGCTGGTTTAGTGAACCGTCAGA | RNAi |
| dsRNA-RFP-R | GCGTAATACGACTCACTATAGGGAAAACCTCTACAAATGTGGTATGGC | RNAi |
4.3. DNA Sequencing and Sequence Analysis
4.4. Quantitative Real-Time PCR (qRT-PCR) Assay
4.5. RNA Interference (RNAi) Experiment
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Weiner, S.; Traub, W.; Parker, S. Macromolecules in mollusc shells and their functions in biomineralization (and discussion). Philos. Trans. R. Soc. Lond. B 1984, 304, 425–434. [Google Scholar] [CrossRef]
- Marin, F.; Luquet, G. Molluscan shell proteins. Comptes Rendus Palevol 2004, 3, 469–492. [Google Scholar] [CrossRef]
- Fang, D.; Xu, G.; Hu, Y.; Pan, C.; Xie, L.; Zhang, R. Identification of genes directly involved in shell formation and their functions in pearl oyster, Pinctada fucata. PLoS One 2011, 6, e21860. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.T.; Xiang, L.X.; Shao, J.Z. Bone morphogenetic protein. Biochem. Biophys. Res. Commun. 2007, 362, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Fact. 2004, 22, 233–241. [Google Scholar] [CrossRef]
- Bragdon, B.; Moseychuk, O.; Saldanha, S.; King, D.; Julian, J.; Nohe, A. Bone morphogenetic proteins: A critical review. Cell Signal. 2011, 23, 609–620. [Google Scholar] [CrossRef]
- Hwang, S.; Partin, J.S.; Lennarz, W.J. Characterization of a homolog of human bone morphogenetic protein 1 in the embryo of the sea urchin, Strongylocentrotus purpuratus. Development 1994, 120, 559–568. [Google Scholar] [PubMed]
- Shih, L.-J.; Chen, C.; Chen, C.-P.; Hwang, S.-P. Identification and characterization of bone morphogenetic protein 2/4 gene from the starfish Archaster typicus. Comp. Biochem. Physiol. B 2002, 131, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, C.; Lanfear, R.; Ray, R.P. Rapid evolution of a novel signalling mechanism by concerted duplication and divergence of a BMP ligand and its extracellular modulators. Dev. Genes Evol. 2010, 220, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Wharton, K.A.; Thomsen, G.H.; Gelbart, W.M. Drosophila 60a gene, another transforming growth factor β family member, is closely related to human bone morphogenetic proteins. Proc. Natl. Acad. Sci. USA 1991, 88, 9214–9218. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, T.; Hanashita, T.; Toriyama, M.; Takagi, R.; Akashika, T.; Higashikubo, N. Gene cloning and biochemical characterization of the BMP-2 of Pinctada fucata. Biosci. Biotechnol. Biochem. 2008, 72, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Nederbragt, A.J.; van Loon, A.E.; Dictus, W.J. Expression of Patella vulgata orthologs of engrailed and dpp-BMP2/4 in adjacent domains during molluscan shell development suggests a conserved compartment boundary mechanism. Dev. Biol. 2002, 246, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Takeuchi, T.; Sarashina, I.; Endo, K. Expression patterns of engrailed and dpp in the gastropod Lymnaea stagnalis. Dev. Genes Evol. 2008, 218, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Freitas, T.C.; Jung, E.; Pearce, E.J. A bone morphogenetic protein homologue in the parasitic flatworm, Schistosoma mansoni. Int. J. Parasitol. 2009, 39, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Zoccola, D.; Moya, A.; Beranger, G.E.; Tambutte, E.; Allemand, D.; Carle, G.F.; Tambutte, S. Specific expression of BMP2/4 ortholog in biomineralizing tissues of corals and action on mouse BMP receptor. Mar. Biotechnol. 2009, 11, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, B.; Broun, M.; Blitz, I.L.; Bode, H.R. Hybmp5-8b, a BMP5-8 orthologue, acts during axial patterning and tentacle formation in hydra. Dev. Biol. 2004, 267, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.E.; Binder, M.; von Lintig, J.; Guo, Y.W.; Wang, X.; Kaandorp, J.A.; Wiens, M.; Schroder, H.C. Interaction of the retinoic acid signaling pathway with spicule formation in the marine sponge Suberites domuncula through activation of bone morphogenetic protein-1. Biochim. Biophys. Acta 2011, 1810, 1178–1194. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, Q.; Jiao, Y.; Huang, R.; Deng, Y.; Wang, H.; Du, X. Identification of genes potentially related to biomineralization and immunity by transcriptome analysis of pearl sac in pearl oyster Pinctada martensii. Mar. Biotechnol. 2012, 14, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Nelsen, S.M.; Christian, J.L. Site-specific cleavage of BMP4 by furin, PC6, and PC7. J. Biol. Chem. 2009, 284, 27157–27166. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, D.M. The TGF-β superfamily: New members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994, 8, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Wozney, J.M. Overview of bone morphogenetic proteins. Spine 2002, 27, S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, R.; Meurer, S.K. Bone morphogenetic protein-7 in focus: A member of the transforming growth factor-β superfamily is implicated in the maintenance of liver health. Hepatology 2007, 45, 1324–1325. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Wang, N.; Inoue, H.; Maeyama, K.; Okamoto, K.; Nagai, K.; Kondo, H.; Hirono, I.; Asakawa, S.; Watabe, S. Deep sequencing of ESTs from nacreous and prismatic layer producing tissues and a screen for novel shell formation-related genes in the pearl oyster. PLoS One 2011, 6, e21238. [Google Scholar] [CrossRef] [PubMed]
- Celeste, A.J.; Iannazzi, J.A.; Taylor, R.C.; Hewick, R.M.; Rosen, V.; Wang, E.A.; Wozney, J.M. Identification of transforming growth factor β family members present in bone-inductive protein purified from bovine bone. Proc. Natl. Acad. Sci. USA 1990, 87, 9843–9847. [Google Scholar] [CrossRef] [PubMed]
- Ozkaynak, E.; Rueger, D.C.; Drier, E.A.; Corbett, C.; Ridge, R.J.; Sampath, T.K.; Oppermann, H. OP-1 cDNA encodes an osteogenic protein in the TGF-β family. EMBO J. 1990, 9, 2085. [Google Scholar] [PubMed]
- Silve, C.; Lopez, E.; Vidal, B.; Smith, D.C.; Camprasse, S.; Camprasse, G.; Couly, G. Nacre initiates biomineralization by human osteoblasts maintained in vitro. Calcif. Tissue Int. 1992, 51, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Lamghari, M.; Almeida, M.; Berland, S.; Huet, H.; Laurent, A.; Milet, C.; Lopez, E. Stimulation of bone marrow cells and bone formation by nacre: In vivo and in vitro studies. Bone 1999, 25, 91S–94S. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, M. The water-soluble matrix fraction from the nacre of Pinctada maxima produces earlier mineralization of MC3T3-E1 mouse pre-osteoblasts. Comp. Biochem. Physiol. B 2003, 135, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pereira Mouriès, L.; Almeida, M.-J.; Milet, C.; Berland, S.; Lopez, E. Bioactivity of nacre water-soluble organic matrix from the bivalve mollusk Pinctada maxima in three mammalian cell types: Fibroblasts, bone marrow stromal cells and osteoblasts. Comp. Biochem. Physiol. B 2002, 132, 217–229. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, F.; Luo, S.; Jiao, Y.; Deng, Y.; Du, X.; Huang, R.; Wang, Q.; Chen, W. Molecular Characterization of the BMP7 Gene and Its Potential Role in Shell Formation in Pinctada martensii. Int. J. Mol. Sci. 2014, 15, 21215-21228. https://doi.org/10.3390/ijms151121215
Yan F, Luo S, Jiao Y, Deng Y, Du X, Huang R, Wang Q, Chen W. Molecular Characterization of the BMP7 Gene and Its Potential Role in Shell Formation in Pinctada martensii. International Journal of Molecular Sciences. 2014; 15(11):21215-21228. https://doi.org/10.3390/ijms151121215
Chicago/Turabian StyleYan, Fang, Shaojie Luo, Yu Jiao, Yuewen Deng, Xiaodong Du, Ronglian Huang, Qingheng Wang, and Weiyao Chen. 2014. "Molecular Characterization of the BMP7 Gene and Its Potential Role in Shell Formation in Pinctada martensii" International Journal of Molecular Sciences 15, no. 11: 21215-21228. https://doi.org/10.3390/ijms151121215




