Association of the miR-149 Rs2292832 Polymorphism with Papillary Thyroid Cancer Risk and Clinicopathologic Characteristics in a Chinese Population
Abstract
:1. Introduction
2. Results
2.1. Summary of Clinicopathologic Characteristics
| Characteristics | PTC (n = 838) | BN (n = 495) | Control (n = 1006) | PPTC vs. Con | PBN vs. Con |
|---|---|---|---|---|---|
| Age | |||||
| <45 | 365 (43.6) | 180 (36.4) | 435 (43.2) | 0.89 | 0.01 |
| ≥45 | 473 (56.4) | 315 (63.6) | 571 (56.8) | ||
| Age (mean ± SD) | 46.30 ± 11.02 | 48.64 ± 11.75 | 47.20 ± 12.29 | 0.11 | 0.03 |
| Gender | |||||
| Male | 227 (27.1) | 126 (25.5) | 310 (30.8) | 0.08 | 0.03 |
| Female | 611 (72.9) | 369 (74.5) | 696 (69.2) | ||
| Tumor size | |||||
| Mean (cm) | 1.26 ± 0.97 | – | – | ||
| ≤10 mm | 387 (46.2) | – | – | ||
| >10 mm | 451 (53.8) | – | – | ||
| Invasion | |||||
| Negative | 700 (83.5) | – | – | ||
| Minimal extension | 82 (9.8) | – | – | ||
| Advanced disease (T4a) | 49 (5.8) | – | – | ||
| Advanced disease (T4b) | 7 (0.8) | – | – | ||
| Multifocal (%) | 234 (27.9) | – | – | ||
| Bilateral (%) | 151 (18.0) | – | – | ||
| T stage | |||||
| T1–T2 | 690 (82.3) | – | – | ||
| T3–T4 | 148 (17.7) | – | – | ||
| Central neck lymph node metastasis (%) | 415 (49.5) | – | – | ||
| Lateral neck lymph node metastasis (%) | 184 (22.0) | – | – | ||
2.2. Association between Rs2292832 Polymorphism and Risk of Thyroid Tumor
| Rs2292832 | PTC (n = 838) | BN (n = 495) | Control (n = 1006) | OR (95% CIs) a PTC vs. Con | p Value | OR (95% CIs) BN vs. Con | p Value |
|---|---|---|---|---|---|---|---|
| Genotype | |||||||
| TT | 379 (45.3) | 216 (43.6) | 496 (49.3) | 1 (reference) | 0.01 | 1 (reference) | 0.12 |
| TC | 354 (42.2) | 227 (45.9) | 424 (42.1) | 1.09 (0.90–1.32) | 0.41 | 1.22 (0.97–1.53) | 0.09 |
| CC | 105 (12.5) | 52 (10.5) | 86 (8.6) | 1.60 (1.17–2.20) | 0.003 | 1.36 (0.93–2.00) | 0.11 |
| TC/CC D | 459 (54.7) | 279 (56.4) | 510 (50.7) | 1.17 (0.98–1.41) | 0.09 | 1.24 (1.01–1.55) | 0.05 |
| TT/TC R | 733 (87.5) | 443 (89.5) | 920 (91.4) | 1.54 (1.14–2.09) | 0.005 | 1.23 (0.86–1.78) | 0.26 |
| Allele | |||||||
| T | 0.66 | 0.66 | 0.70 | 1 (reference) | 1 (reference) | ||
| C | 0.34 | 0.34 | 0.30 | 1.10 (1.02–1.18) | 0.009 | 1.09 (1.00–1.18) | 0.05 |
2.3. Characteristics of Papillary Thyroid Cancer (PTC) Patients with Different Rs2292832 Single Nucleotide Polymorphism (SNP) Genotypes
| Characteristics | TT Genotype (n = 379) | TC Genotype (n = 354) | CC Genotype (n = 105) | p Value a |
|---|---|---|---|---|
| Gender | ||||
| Male | 102 (26.9) | 97 (27.4) | 28 (26.7) | 0.984 |
| Female | 277 (73.1) | 257 (72.6) | 77 (73.3) | |
| Age | ||||
| <45 | 170 (44.9) | 154 (43.5) | 41 (39.0) | 0.569 |
| ≥45 | 209 (55.1) | 200 (56.5) | 64 (61.0) | |
| Size | ||||
| ≤1 cm | 162 (42.7) | 182 (51.4) | 43 (41.0) | 0.032 |
| >1 cm | 217 (57.3) | 172 (48.6) | 62 (59.0) | |
| Size (cm) | 1.31 ± 0.99 | 1.21 ± 1.00 | 1.15 ± 0.76 | 0.219 |
| Invasion | ||||
| Yes | 71 (18.7) | 43 (12.1) | 24 (22.9) | 0.009 |
| No | 308 (81.3) | 311 (87.9) | 81 (77.1) | |
| Multifocal | ||||
| Yes | 109 (28.8) | 98 (27.7) | 27 (25.7) | 0.820 |
| No | 270 (71.2) | 256 (72.3) | 78 (74.3) | |
| Bilateral | ||||
| Yes | 65 (17.2) | 68 (19.2) | 18 (17.1) | 0.745 |
| No | 314 (82.8) | 286 (80.8) | 87 (82.9) | |
| With (PTC/BN) | ||||
| Yes | 45 (11.9) | 39 (11.0) | 16 (15.1) | 0.503 |
| No | 334 (88.1) | 315 (89.0) | 89 (84.9) | |
| pN+ (Level VI) | 49.6% | 50.3% | 46.7% | 0.808 |
| pN+ (lateral neck) | 23.7% | 20.6% | 20.0% | 0.519 |
| T stage | ||||
| I–II | 303 (79.9) | 306 (86.4) | 81 (77.1) | 0.023 |
| III–IV | 76 (20.1) | 48(13.6) | 24 (22.9) |
| Rs2292832 | Invasion | Odds Ratio a (95% CI) | p Value | T Stage | Odds Ratio (95% CI) | p Value | ||
|---|---|---|---|---|---|---|---|---|
| Negative (n = 700) | Positive (n = 138) | I–II (n = 690) | III–IV (n = 148) | |||||
| TT | 308 (44.0) | 71 (51.4) | 1 (reference) | 0.006 | 303 (43.9) | 76 (51.4) | 1 (reference) | 0.007 |
| TC | 311 (44.4) | 43 (31.2) | 0.61 (0.39–0.95) | 306 (44.3) | 48 (32.4) | 0.60 (0.37–0.97) | ||
| CC | 81 (11.6) | 24 (17.4) | 1.55 (0.89–2.72) | 81 (11.8) | 24 (16.2) | 1.56 (0.87–2.80) | ||
| TC/CC D | 392 (56.0) | 67 (48.6) | 0.79 (0.53–1.18) | 0.25 | 387 (56.1) | 72 (48.6) | 0.81 (0.53–1.21) | 0.30 |
| TT/TC R | 619 (88.4) | 114 (82.6) | 1.93 (1.13–3.28) | 0.015 | 609 (88.2) | 124 (83.8) | 1.92 (1.10–3.35) | 0.021 |
| Allele C | 33.8% | 33.0% | 1.03 (0.89–1.19) | 0.72 | 33.9% | 32.4% | 1.03 (0.88–1.20) | 0.70 |
2.4. No Significant Association between Rs2292832 and Short-Term Disease Persistence in PTC Patients
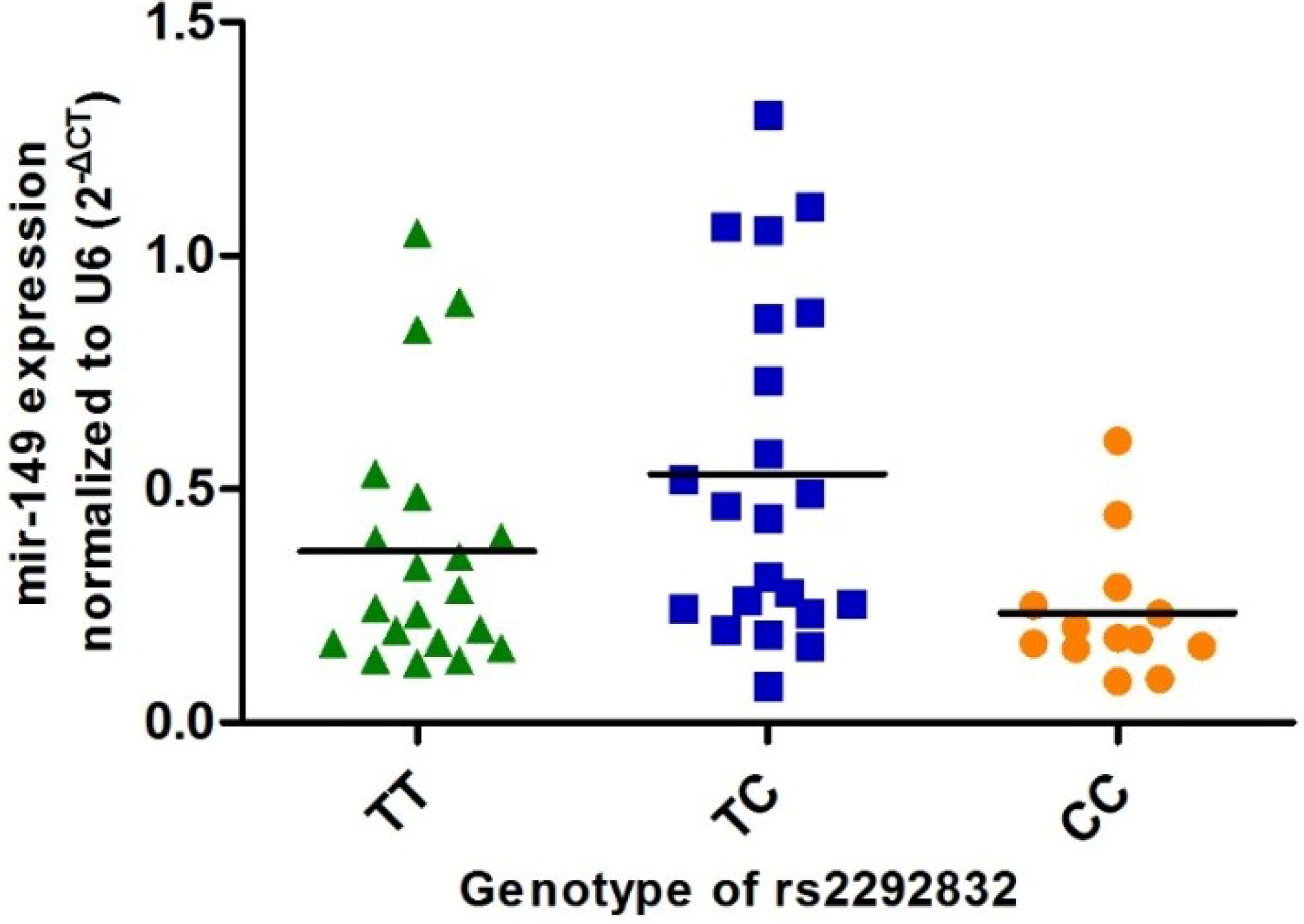
2.5. Association between Rs2292832 Polymorphism and Expression Level of Mature miR-149 in Thyroid Tissue
2.6. In Silico Screening for Target Genes of Hsa-mir-149-5p
| Gene | ID | Full Name | Function | Related Tumor Type |
|---|---|---|---|---|
| MPZ | NM_000530 | Myelin protein zero | Different expression | Bladder cancer |
| PTGIS | NM_000961 | Prostaglandin I2 (prostacyclin) synthase | Vascular related, Tumor progression | Colorectal cancer, Breast cancer |
| CREBL2 | NM_001310 | cAMP responsive element binding protein-like 2 | Suppressor gene | Hematologic malignancy |
| CD47 | NM_001777 | CD47 molecule | Immune and inflammation | Colorectal cancer Thyroid cancer |
| EIF5 | NM_001969 | Eukaryotic translation initiation factor 5 | Cell proliferation | Leukemia |
| PDGFRA | NM_006206 | Platelet-derived growth factor receptor, alpha polypeptide | Oncogene, Tumor progression | GIST, Gliomas, Thyroid cancer |
| KLHL3 | NM_017415 | Kelch-like family member 3 (Drosophila) | – | – |
| APPL2 | NM_018171 | Adaptor protein, phosphotyrosine interaction, PH domain and leucine zipper containing 2 | Apoptosis | Gliomas |
| UBFD1 | NM_019116 | Ubiquitin family domain containing 1 | – | – |
| CRTC2 | NM_181715 | CREB regulated transcription coactivator 2 | Metabolism | Breast cancer |
| PLAG1 | NM_001114635 | Pleiomorphic adenoma gene 1 | Oncogene | Salivary tumor |
| TOP1 | NM_003286 | Topoisomerase (DNA) I | Cell proliferation | Colorectal cancer |
3. Discussion
4. Materials and Methods
4.1. Study Subjects
4.2. DNA Extraction and Genotyping
4.3. qRT-PCR Analysis
4.4. Clinical Management
4.5. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Ezzat, S.; Asa, S.L. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat. Rev. Cancer 2006, 6, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Adjadj, E.; Schlumberger, M.; de Vathaire, F. Germ-line DNA polymorphisms and susceptibility to differentiated thyroid cancer. Lancet Oncol. 2009, 10, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, J.; Sulem, P.; Gudbjartsson, D.F.; Jonasson, J.G.; Sigurdsson, A.; Bergthorsson, J.T.; He, H.; Blondal, T.; Geller, F.; Jakobsdottir, M.; et al. Common variants on 9q22.33 and 14q13.3 predispose to thyroid cancer in European populations. Nat. Genet. 2009, 41, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, J.; Sulem, P.; Gudbjartsson, D.F.; Jonasson, J.G.; Masson, G.; He, H.; Jonasdottir, A.; Sigurdsson, A.; Stacey, S.N.; Johannsdottir, H.; et al. Discovery of common variants associated with low TSH levels and thyroid cancer risk. Nat. Genet. 2012, 44, 319–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuse, M.; Takahashi, M.; Mitsutake, N.; Nishihara, E.; Hirokawa, M.; Kawaguchi, T.; Rogounovitch, T.; Saenko, V.; Bychkov, A.; Suzuki, K.; et al. The FOXE1 and NKX2–1 loci are associated with susceptibility to papillary thyroid carcinoma in the Japanese population. J. Med. Genet. 2011, 48, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Kohler, A.; Chen, B.; Gemignani, F.; Elisei, R.; Romei, C.; Figlioli, G.; Cipollini, M.; Cristaudo, A.; Bambi, F.; Hoffmann, P.; et al. Genome-wide association study on differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 2013, 98, E1674–E1681. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, X.; Zhao, Y.; Tian, T.; Jin, G.; Shu, Y.; Chen, Y.; Xu, L.; Zen, K.; Zhang, C.; Shen, H. Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J. Clin. Oncol. 2010, 28, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Shi, J.; Budhu, A.; Yu, Z.; Forgues, M.; Roessler, S.; Ambs, S.; Chen, Y.; Meltzer, P.S.; Croce, C.M.; et al. MicroRNA expression, survival, and response to interferon in liver cancer. N. Engl. J. Med. 2009, 361, 1437–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, B.M.; Robles, A.I.; Harris, C.C. Genetic variation in microRNA networks: The implications for cancer research. Nat. Rev. Cancer 2010, 10, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Jazdzewski, K.; Murray, E.L.; Franssila, K.; Jarzab, B.; Schoenberg, D.R.; de la Chapelle, A. Common SNP in pre-mir-146a decreases mature mir expression and predisposes to papillary thyroid carcinoma. Proc. Natl. Acad. Sci. USA 2008, 105, 7269–7274. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.J.; Wang, Y.L.; Li, D.S.; Wang, Y.; Wang, X.F.; Zhu, Y.X.; Yang, Y.J.; Wang, Z.Y.; Ma, Y.Y.; Wu, Y.; et al. Association between the rs2910164 polymorphism in pre-mir-146a sequence and thyroid carcinogenesis. PLoS One 2013, 8, e56638. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Howarth, K.M.; Martin, L.; Gorman, M.; Mihai, R.; Moss, L.; Auton, A.; Lemon, C.; Mehanna, H.; Mohan, H.; et al. Thyroid cancer susceptibility polymorphisms: Confirmation of loci on chromosomes 9q22 and 14q13, validation of a recessive 8q24 locus and failure to replicate a locus on 5q24. J. Med. Genet. 2012, 49, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Min, K.T.; Jeon, Y.J.; Kwon, C.I.; Ko, K.H.; Park, P.W.; Hong, S.P.; Rim, K.S.; Kwon, S.W.; Hwang, S.G.; et al. Association study of microRNA polymorphisms with hepatocellular carcinoma in Korean population. Gene 2012, 504, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.H.; Rah, H.; Choi, Y.K.; Jeon, Y.J.; Min, K.T.; Kwack, K.; Hong, S.P.; Hwang, S.G.; Kim, N.K. Association of the mir-146aC>G, mir-149T>C, mir-196a2T>C, and mir-499A>G polymorphisms with gastric cancer risk and survival in the Korean population. Mol. Carcinog. 2013, 52, E39–E51. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhou, X.; Qiu, M.T.; Yin, R.; Wu, Y.Q.; Xu, L. Lack of association between hsa-mir-149 rs2292832 polymorphism and cancer risk: A meta-analysis of 12 studies. PLoS One 2013, 8, e73762. [Google Scholar] [CrossRef] [PubMed]
- Min, K.T.; Kim, J.W.; Jeon, Y.J.; Jang, M.J.; Chong, S.Y.; Oh, D.; Kim, N.K. Association of the mir-146aC>G, 149C>T, 196a2C>T, and 499A>G polymorphisms with colorectal cancer in the Korean population. Mol. Carcinog. 2012, 51, E65–E73. [Google Scholar] [PubMed]
- Hu, Z.; Liang, J.; Wang, Z.; Tian, T.; Zhou, X.; Chen, J.; Miao, R.; Wang, Y.; Wang, X.; Shen, H. Common genetic variants in pre-microRNAs were associated with increased risk of breast cancer in Chinese women. Hum. Mutat. 2009, 30, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, G.; Wei, S.; Niu, J.; El-Naggar, A.K.; Sturgis, E.M.; Wei, Q. Genetic variants in selected pre-microRNA genes and the risk of squamous cell carcinoma of the head and neck. Cancer 2010, 116, 4753–4760. [Google Scholar] [PubMed]
- Tu, H.F.; Liu, C.J.; Chang, C.L.; Wang, P.W.; Kao, S.Y.; Yang, C.C.; Yu, E.H.; Lin, S.C.; Chang, K.W. The association between genetic polymorphism and the processing efficiency of mir-149 affects the prognosis of patients with head and neck squamous cell carcinoma. PLoS One 2012, 7, e51606. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, A.; Huck, B.; Keller, B.; Strotbek, M.; Schmid, S.; Boerries, M.; Busch, H.; Muller, D.; Olayioye, M.A. miR-149 functions as a tumor suppressor by controlling breast epithelial cell migration and invasion. Cancer Res. 2014, 74, 5256–5265. [Google Scholar] [CrossRef] [PubMed]
- Oster, B.; Linnet, L.; Christensen, L.L.; Thorsen, K.; Ongen, H.; Dermitzakis, E.T.; Sandoval, J.; Moran, S.; Esteller, M.; Hansen, T.F.; et al. Non-CpG island promoter hypomethylation and mir-149 regulate the expression of SRPX2 in colorectal cancer. Int. J. Cancer 2013, 132, 2303–2315. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Zhao, W.; Xiong, J.; Cao, R. miR-149 inhibits non-small-cell lung cancer cells EMT by targeting FOXM1. Biochem. Res. Int. 2013, 2013, 506731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, P.; Dykstra, M.; Gelebart, P.; Williams, D.; Ingham, R.; Adewuyi, E.E.; Lai, R.; McMullen, T. Platelet-derived growth factor receptor-α promotes lymphatic metastases in papillary thyroid cancer. J. Pathol. 2012, 228, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, S.K.; Park, H.J.; Chung, D.H.; Park, H.K.; Lee, J.S.; Kwon, K.H.; Chung, J.H. PDGFRA promoter polymorphisms are associated with the risk of papillary thyroid cancer. Mol. Med. Rep. 2012, 5, 1267–1270. [Google Scholar] [PubMed]
- Nannini, M.; Biasco, G.; Astolfi, A.; Pantaleo, M.A. An overview on molecular biology of KIT/PDGFRA wild type (WT) gastrointestinal stromal tumours (GIST). J. Med. Genet. 2013, 50, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Velghe, A.I.; van Cauwenberghe, S.; Polyansky, A.A.; Chand, D.; Montano-Almendras, C.P.; Charni, S.; Hallberg, B.; Essaghir, A.; Demoulin, J.B. PDGFRA alterations in cancer: Characterization of a gain-of-function V536E transmembrane mutant as well as loss-of-function and passenger mutations. Oncogene 2014, 33, 2568–2576. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Veiga, I.; Ribeiro, F.R.; Vieira, J.; Pinto, C.; Pinheiro, M.; Mesquita, B.; Santos, C.; Soares, M.; Dinis, J.; et al. Chromosome copy number changes carry prognostic information independent of KIT/PDGFRA point mutations in gastrointestinal stromal tumors. BMC Med. 2010, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Wei, G.; Wu, J.; Fang, D.; Liao, Z.; Xiao, H.; Li, M.; Li, Y. Down-regulation of mir-218-2 and its host gene SLIT3 cooperate to promote invasion and progression of thyroid cancer. J. Clin. Endocrinol. Metab. 2013, 98, E1334–E1344. [Google Scholar] [CrossRef] [PubMed]
- Abend, M.; Pfeiffer, R.M.; Ruf, C.; Hatch, M.; Bogdanova, T.I.; Tronko, M.D.; Hartmann, J.; Meineke, V.; Mabuchi, K.; Brenner, A.V. Iodine-131 dose-dependent gene expression: Alterations in both normal and tumour thyroid tissues of post-Chernobyl thyroid cancers. Br. J. Cancer 2013, 109, 2286–2294. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Feng, S.H.; Guo, S.C.; Wei, W.J.; Li, D.S.; Wang, Y.; Wang, X.; Wang, Z.Y.; Ma, Y.Y.; Jin, L.; et al. Confirmation of papillary thyroid cancer susceptibility loci identified by genome-wide association studies of chromosomes 14q13, 9q22, 2q35 and 8p12 in a Chinese population. J. Med. Genet. 2013, 50, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, M.; Qian, J.; Yang, Y.; Li, S.; Lu, D.; Yu, S.; Meng, W.; Ye, W.; Jin, L. Rationales, design and recruitment of the Taizhou Longitudinal Study. BMC Public Health 2009, 9, 223. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, W.-J.; Lu, Z.-W.; Li, D.-S.; Wang, Y.; Zhu, Y.-X.; Wang, Z.-Y.; Wu, Y.; Wang, Y.-L.; Ji, Q.-H. Association of the miR-149 Rs2292832 Polymorphism with Papillary Thyroid Cancer Risk and Clinicopathologic Characteristics in a Chinese Population. Int. J. Mol. Sci. 2014, 15, 20968-20981. https://doi.org/10.3390/ijms151120968
Wei W-J, Lu Z-W, Li D-S, Wang Y, Zhu Y-X, Wang Z-Y, Wu Y, Wang Y-L, Ji Q-H. Association of the miR-149 Rs2292832 Polymorphism with Papillary Thyroid Cancer Risk and Clinicopathologic Characteristics in a Chinese Population. International Journal of Molecular Sciences. 2014; 15(11):20968-20981. https://doi.org/10.3390/ijms151120968
Chicago/Turabian StyleWei, Wen-Jun, Zhong-Wu Lu, Duan-Shu Li, Yu Wang, Yong-Xue Zhu, Zhuo-Ying Wang, Yi Wu, Yu-Long Wang, and Qing-Hai Ji. 2014. "Association of the miR-149 Rs2292832 Polymorphism with Papillary Thyroid Cancer Risk and Clinicopathologic Characteristics in a Chinese Population" International Journal of Molecular Sciences 15, no. 11: 20968-20981. https://doi.org/10.3390/ijms151120968




