Synthesis and Biological Evaluation of 2-Thioxopyrimidin-4(1H)-one Derivatives as Potential Non-Nucleoside HIV-1 Reverse Transcriptase Inhibitors
Abstract
:1. Introduction
2. Results and Discussion
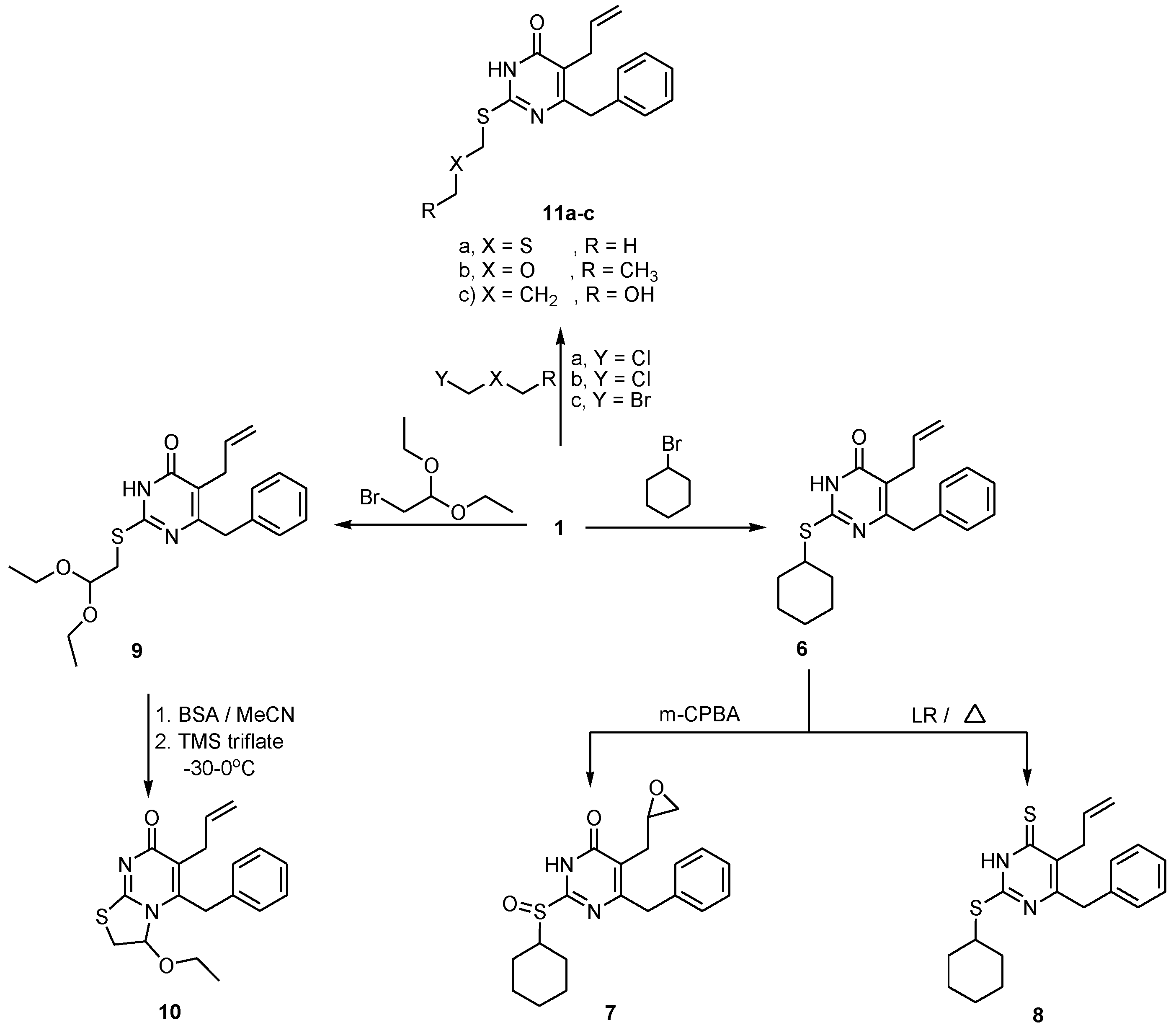
2.1. Chemistry
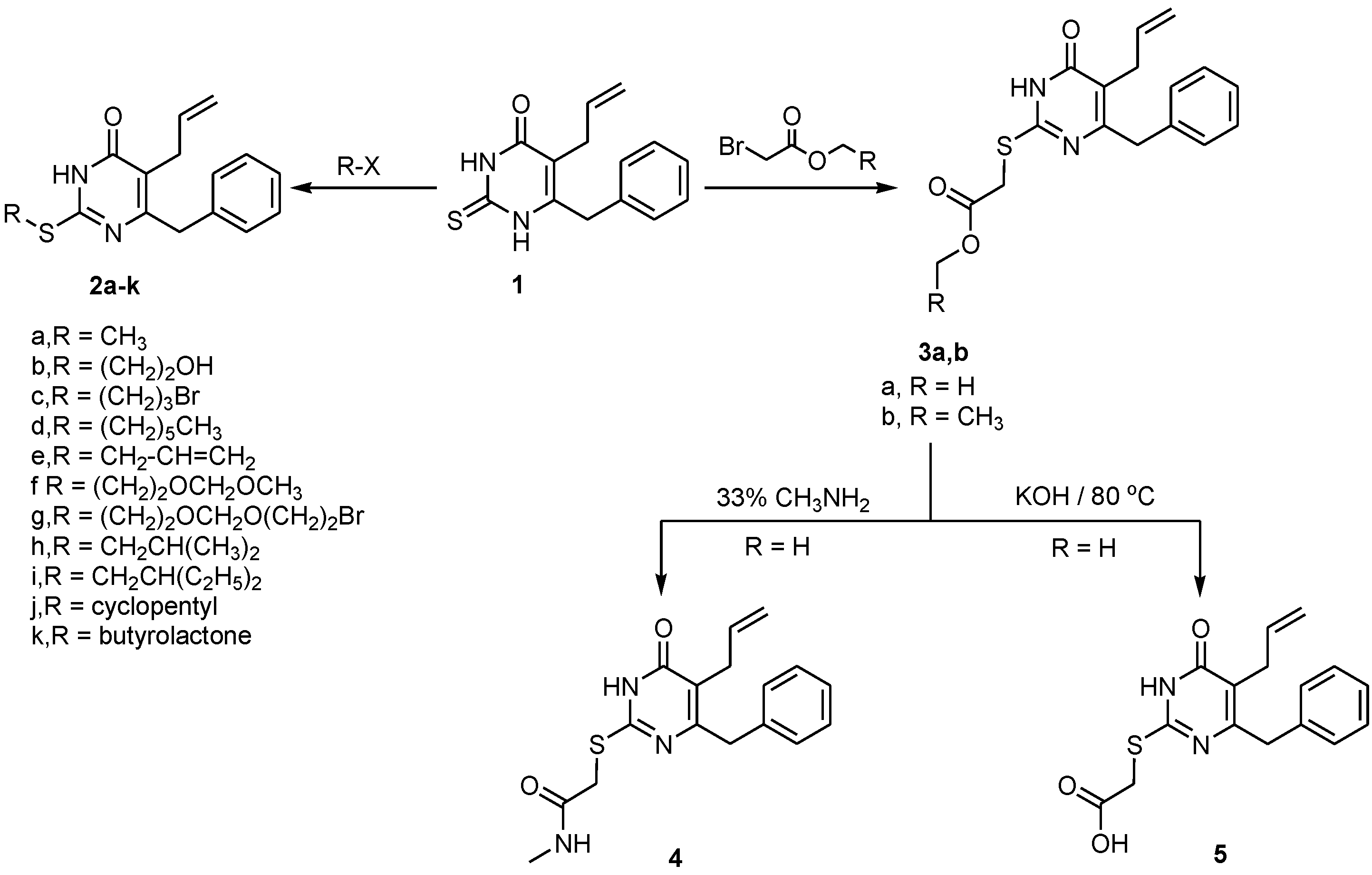
2.2. Pharmacological Screening
| Compound | IC50 a (µM) | CC50 b (µM) | SI c |
|---|---|---|---|
| 1 | – | – | – |
| 2a | 26.8 | >100 | >3 |
| 2c | – | – | – |
| 2d | 7.0 | 35.0 | >5 |
| 2e | 2.5 | 25.0 | >10 |
| 2h | 1.0 | >100 | >100 |
| 2j | 2.6 | >100 | >38 |
| 2k | – | – | – |
| 3a | 35.0 | >100 | >2 |
| 3b | – | – | – |
| 4 | – | – | – |
| 8 | 3.9 | >100 | >25 |
| 9 | – | – | – |
| 11a | 3.16 | >100 | >31 |
| 11c | 0.32 | >100 | >312 |
| MKC-442 | 0.005 | 141 | 28,000 |
| AZT | 0.04 | 52 | 1,300 |
3. Experimental Section
3.1. Chemistry
3.2. Pharmacological Screening
3.2.1. Virus and cells
3.2.2. Inhibition of Human Immunodeficiency Virus Type 1 (HIV-1) Replication
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Meadows, D.C.; Gervay-Hague, J. Current developments in HIV chemotherapy. J. Chem. Med. Chem. 2006, 1, 16–29. [Google Scholar]
- Castro, H.C.; Loureiro, L.I.; Pujol-Luz, M.; Souza, A.M.; Albuquerque, M.G.; Santos, D.O.; Cabral, L.M.; Frugulhetti, I.C.; Rodrigues, C.R. HIV-1 reverse transcriptase: A therapeutical target in the spotlight. Curr. Med. Chem. 2006, 3, 313–324. [Google Scholar]
- Tronchet, J.M.; Seman, M. Nonnucleoside inhibitors of HIV-1 reverse transcriptase: From the biology of reverse transcription to molecular design. Curr. Top. Med. Chem. 2003, 3, 1496–1511. [Google Scholar]
- Zhan, P.; Liu, X.; Li, Z.; Pannecouque, C.; de Clercq, E. Design strategies of novel NNRTIs to overcome drug resistance. Curr. Med. Chem. 2009, 16, 3903–3917. [Google Scholar]
- De Béthune, M. Non-nucleoside reverse transcriptase inhibitors (NNRTIs), their discovery, development, and use in the treatment of HIV-1 infection. Antivir. Res. 2010, 85, 75–90. [Google Scholar]
- Botta, M.; Artico, M.; Massa, S.; Gambacorta, A.; Marogniu, M.E.; Pani, A.; la Colla, P. Synthesis, antimicrobial and antiviral activities of isotrimethoprim and some related derivatives. Eur. J. Med. Chem. 1992, 27, 251–257. [Google Scholar]
- Artico, M.; Massa, S.; Mai, A. 3,4-Dihydro-2-alkyloxy-6-benzyl-4-oxoypyrimidines (DABOs): A new class of specific inhibitors of human immunodeficiency virus type 1. Antivir. Chem. Chemother. 1993, 4, 361–368. [Google Scholar]
- Massa, S.; Mai, A.; Artico, M.; Sbardella, G.; Tramontano, E.; Loi, A.; Scano, P.; la Colla, P. Synthesis and antiviral activity of new 3,4-dihydro-2-alkoxy-6-benzyl-4-oxopyrimidines (DABOs), specific inhibitors of human immunodeficiency virus type 1. Antivir. Chem. Chemother. 1995, 6, 1–8. [Google Scholar]
- Mai, A.; Artico, M.; Sbardella, G.; Massa, S.; Loi, A.G.; Tramontano, E.; Scano, P.; la Colla, P. Synthesis and anti-HIV-1 activity of thio analogues of dihydroalkoxybenzyloxopyrimidines. J. Med. Chem. 1995, 38, 3258–3263. [Google Scholar]
- Mai, A.; Artico, M.; Sbardella, G.; Quartarone, S.; Massa, S.; Loi, A.G.; de Montis, A.; Scintu, F.; Putzolu, M.; la Colla, P. Dihydro(alkylthio)(naphthylmethyl)oxopyrimidines: Novel non-nucleoside reverse transcriptase inhibitors of the S-DABO series. J. Med. Chem. 1997, 40, 1447–1454. [Google Scholar]
- Mai, A.; Artico, M.; Sbardella, G.; Massa, S.; Novellino, E.; Greco, G.; Loi, A.G.; Tramontano, E.; Marongiu, M.E.; la Colla, P. 5-Alkyl-2-(alkylthio)-6-(2,6-dihalophenylmethyl)-3, 4-dihydropyrimidin-4(3H)-ones: Novel potent and selective dihydro-alkoxy-benzyl-oxopyrimidine derivatives. J. Med. Chem. 1999, 42, 619–627. [Google Scholar]
- Quaglia, M.; Mai, A.; Sbardella, G.; Artico, M.; Ragno, R.; Massa, S.; del Piano, D.; Setzu, G.; Doratiotto, S.; Cotichini, V. Chiral resolution and molecular modeling investigation of rac-2-cyclopentylthio-6-[1-(2,6-difluorophenyl)ethyl]-3,4-dihydro-5-methylpyrimidin-4-(3H)-one (MC-1047), a potent anti-HIV-1 reverse transcriptase agent of the DABO class. Chirality 2001, 13, 75–80. [Google Scholar]
- Sbardella, G.; Mai, A.; Artico, M.; Chimenti, P.; Massa, S.; Loddo, R.; Marongiu, M.E.; la Collat, P.; Pani, A. Structure-activity relationship studies on new DABOS: Effect of substitutions at pyrimidine C-5 and C-6 positions on anti-HIV-1 activity. Antivir. Chem. Chemother. 2001, 12, 37–50. [Google Scholar]
- Sbardella, G.; Mai, A.; Artico, M.; Massa, S.; Marceddu, T.; Vargiu, L.; Marongiu, M.; la Colla, P. Does the 2-methylthiomethyl substituent really confer high anti-HIV-1 activity to S-DABOs. Med. Chem. Res. 2000, 10, 30–39. [Google Scholar]
- Artico, A. Selected non-nucleoside reverse transcriptase inhibitors (NNRTIs): The DABOs family. Drugs Future 2002, 27, 159–165. [Google Scholar]
- Ragno, R.; Mai, A.; Sbardella, S.; Artico, M.; Massa, S.; Musiu, C.; Mura, M.; Marceddu, T.; Cadeddu, A.; la Colla, P. Computer-aided design, synthesis, and anti-HIV-1 activity in vitro of 2-alkylamino-6-[1-(2,6-difluorophenyl)alkyl]-3,4-dihydro-5-alkylpyrimidin-4(3H)-ones as novel potent non-nucleoside reverse transcriptase inhibitors, also active against the Y181C variant. J. Med. Chem. 2004, 47, 928–934. [Google Scholar]
- He, Y.; Chen, F.; Sun, G.; Wang, Y.; de Clercq, E.; Balzarini, J.; Pannecouque, C. Alkyl-2-[(aryl and alkyloxylcarbonylmethyl)thio]-6-(1-naphthylmethyl) pyrimidin-4(3H)-ones as an unique HIV reverse transcriptase inhibitors of S-DABO series. Bioorg. Med. Chem. Lett. 2004, 14, 3173–3176. [Google Scholar]
- Mai, A.; Artico, M.; Ragno, R.; Sbardella, G.; Massa, S.; Musiu, C.; Mura, M.; Marturana, F.; Cadeddu, A.; Maga, G.; et al. 5-Alkyl-2-alkylamino-6-(2,6-difluorophenylalkyl)-3,4-dihydropyrimidin-4(3H)-ones, a new series of potent, broad-spectrum non-nucleoside reverse transcriptase inhibitors belonging to the DABO family. Bioorg. Med. Chem. 2005, 13, 2065–2077. [Google Scholar]
- Manetti, F.; Jose, A.E.; Clotet-Codina, I.; Armand-Ugon, M.; Maga, G.; Crespan, E.; Cancio, R.; Mugnaini, C.; Bernardini, C.; Togninelli, A.; et al. Parallel solution-phase and microwave-assisted synthesis of new S-DABO derivatives endowed with subnanomolar anti-HIV-1 activity. J. Med. Chem. 2005, 48, 8000–8008. [Google Scholar]
- Mugnaini, C.; Manetti, F.; Este, J.; Clotet-Codina, I.; Maga, G.; Cancio, R.; Botta, M.; Corelli, F. Synthesis and biological investigation of S-aryl-S-DABO derivatives as HIV-1 inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 3541–3544. [Google Scholar]
- Togninelli, A.; Carmi, C.; Petricci, E.; Mugnaini, C.; Massa, S.; Corelli, F.; Botta, M. Solution-phase parallel synthesis of S-DABO analogues. Tetrahedron Lett. 2006, 47, 65–67. [Google Scholar]
- Ji, L.; Chen, F.; de Clercq, E.; Balzarini, J.; Pannecouque, C. Synthesis and anti-HIV-1 activity evaluation of 5-alkyl-2-alkylthio-6-(arylcarbonyl or α-cyanoarylmethyl)-3,4-dihydropyrimidin-4(3H)-ones as novel non-nucleoside HIV-1 reverse transcriptase inhibitors. J. Med. Chem. 2007, 50, 1778–1786. [Google Scholar]
- Radi, M.; Contemori, L.; Castagnolo, D.; Spinosa, R.; Este, J.; Massa, S.; Botta, M. A versatile route to C-6 arylmethyl-functionalized S-DABO and related analogues. Org. Lett. 2007, 9, 3157–3160. [Google Scholar]
- Mugnaini, C.; Alongi, M.; Togninelli, A.; Gevariya, H.; Brizzi, A.; Manetti, F.; Bernardini, C.; Angeli, L.; Tafi, A.; Bellucci, L.; et al. Dihydro-alkylthio-benzyl-oxopyrimidines as inhibitors of reverse transcriptase: Synthesis and rationalization of the biological data on both wild-type enzyme and relevant clinical mutants. J. Med. Chem. 2007, 50, 6580–6595. [Google Scholar]
- Wang, Y.; Chen, F.; de Clercq, E.; Balzarini, J.; Pannecouque, C. Synthesis and biological evaluation of novel 6-substituted 5-alkyl-2-(arylcarbonylmethylthio)pyrimidin-4(3H)-ones as potent non-nucleoside HIV-1 reverse transcriptase inhibitors. Bioorg. Med. Chem. 2008, 16, 3887–3894. [Google Scholar]
- Mai, A.; Artico, M.; Rotili, D.; Tarantino, D.; Clotet-Codina, I.; Armand-Ugon, M.; Ragno, R.; Simeoni, S.; Sbardella, G.; Nawrozkij, M.B.; et al. Synthesis and biological properties of novel 2-aminopyrimidin-4(3H)-ones highly potent against HIV-1 mutant strains. J. Med. Chem. 2007, 50, 5412–5424. [Google Scholar]
- Mai, A.; Sbardella, G.; Artico, M.; Ragno, R.; Massa, S.; Novellino, E.; Greco, G.; Lavecchia, A.; Musiu, C.; la Colla, M.; et al. Structure-based design, synthesis, and biological evaluation of conformationally restricted novel 2-alkylthio-6-[1-(2,6-difluorophenyl)alkyl]-3,4-dihydro-5-alkylpyrimidin-4(3H)-ones as non-nucleoside inhibitors of HIV-1 reverse transcriptase. J. Med. Chem. 2001, 44, 2544–2554. [Google Scholar]
- He, Y.P.; Chen, F.E.; Yu, X.J.; Wang, Y.P.; de Clercq, E.; Balzarini, J.; Pannecouque, C. Nonnucleoside HIV-1 reverse transcriptase inhibitors; part 3. Synthesis and antiviral activity of 5-alkyl-2-[(aryl and alkyloxyl-carbonylmethyl)thio]-6-(1-naphthylmethyl) pyrimidin-4(3H)-ones. Bioorg. Chem. 2004, 32, 536–548. [Google Scholar]
- Sun, G.F.; Kuang, Y.Y.; Chen, F.E.; de Clercq, E.; Balzarini, J.; Pannecouque, C. Non-nucleoside HIV reverse transcriptase inhibitors, Part 6[1]: Synthesis and anti-HIV activity of novel 2-[(arylcarbonylmethyl)thio]-6-arylthio DABO analogues. Arch. Pham. Chem. Life Sci. 2005, 338, 457–461. [Google Scholar]
- Lene, P.; Thomas, H.; Nagy, K.; Per, T.; Erik, P.; Claus, N. Synthesis of new MKC-442 analogus containing alkenyl chains or reactive functionalities at C-5. Monatshefte für Chemie 2002, 133, 1031–1043. (In German) [Google Scholar]
- Pedersen, O.S.; Pedersen, E.B. Nonnucleoside reverse transcriptase inhibitors: The NNRTI boom. Antivir. Chem. Chemother. 1999, 10, 285–293. [Google Scholar]
- Petersen, L.; Pedersen, E.B.; Nielsen, C. Three routes for the synthesis of 6 benzyl-1-ethoxymethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidine-5-carbaldehyde. Synthesis 2001, 4, 559–564. [Google Scholar]
- Danel, K.; Larsen, E.; Pedersen, E.B. Easy synthesis of 5,6-disubstituted acyclouridine derivatives. Synthesis 1995, 26, 934–936. [Google Scholar]
- Nielsen, C.M.; Bygbjerg, I.C.; Vestergaard, B.F. Detection of HIV antigens in eluates from whole blood collected on filterpaper. Lancet 1987, 1, 566–567. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalifa, N.M.; Al-Omar, M.A. Synthesis and Biological Evaluation of 2-Thioxopyrimidin-4(1H)-one Derivatives as Potential Non-Nucleoside HIV-1 Reverse Transcriptase Inhibitors. Int. J. Mol. Sci. 2014, 15, 20723-20735. https://doi.org/10.3390/ijms151120723
Khalifa NM, Al-Omar MA. Synthesis and Biological Evaluation of 2-Thioxopyrimidin-4(1H)-one Derivatives as Potential Non-Nucleoside HIV-1 Reverse Transcriptase Inhibitors. International Journal of Molecular Sciences. 2014; 15(11):20723-20735. https://doi.org/10.3390/ijms151120723
Chicago/Turabian StyleKhalifa, Nagy M., and Mohamed A. Al-Omar. 2014. "Synthesis and Biological Evaluation of 2-Thioxopyrimidin-4(1H)-one Derivatives as Potential Non-Nucleoside HIV-1 Reverse Transcriptase Inhibitors" International Journal of Molecular Sciences 15, no. 11: 20723-20735. https://doi.org/10.3390/ijms151120723






