α, ω-Cholesterol-Functionalized Low Molecular Weight Polyethylene Glycol as a Novel Modifier of Cationic Liposomes for Gene Delivery
Abstract
:1. Introduction
2. Results and Discussion
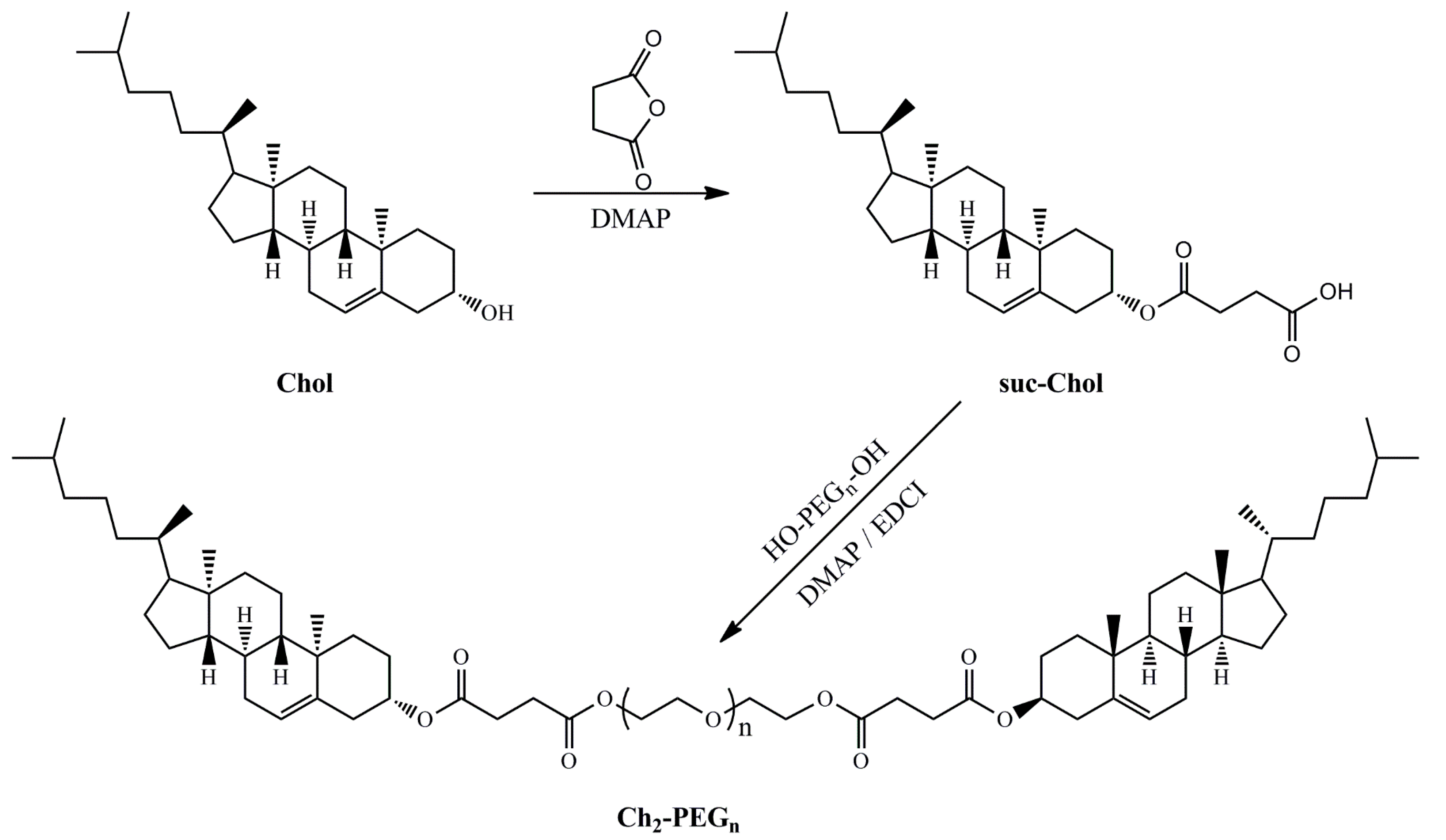
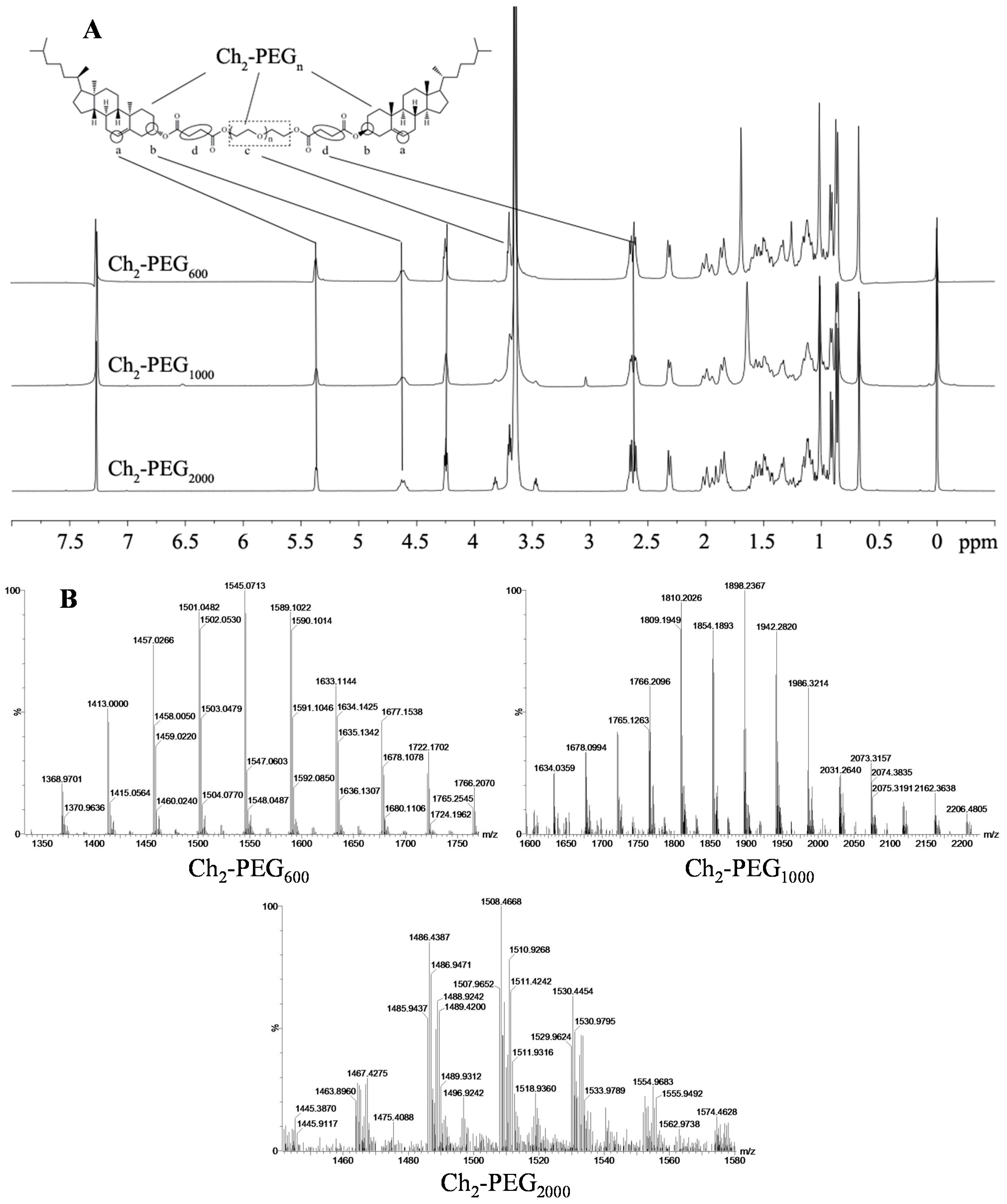
2.1. Synthesis and Identification of Ch2-PEGn
| Samples | Melting Point (°C) | Appearance (25 °C) |
|---|---|---|
| PEG600 → Ch2-PEG600 | 20 → 33 (↑) | Clear liquid → Clear semi-solid |
| PEG1000 → Ch2-PEG1000 | 33 → 40 (↑) | White paste → Clear solid |
| PEG2000 → Ch2-PEG2000 | 52 → 47.5 (↓) | White flake → White powder |
2.2. Physicochemical Properties of Ch2-PEGn-CLs

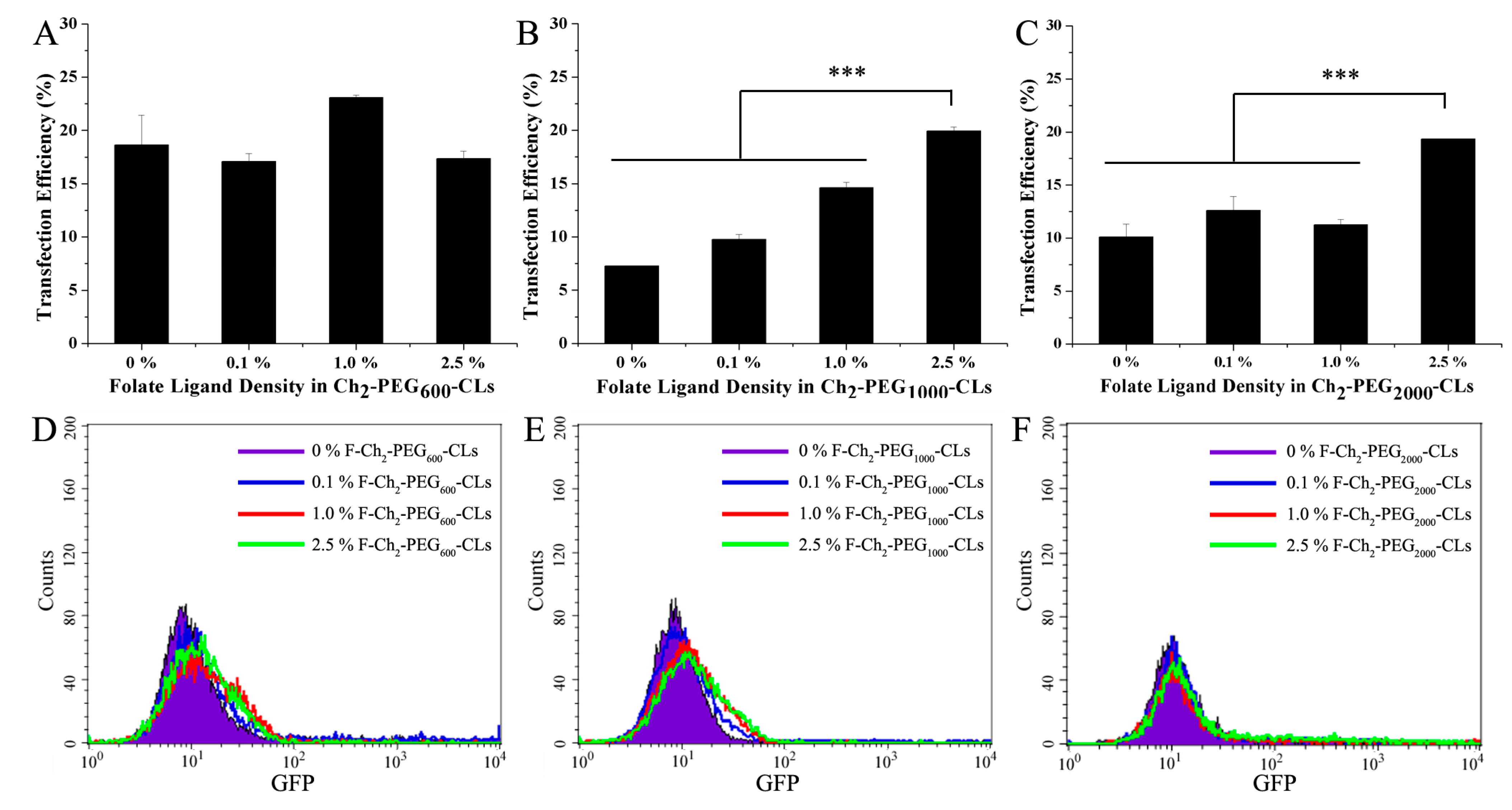
2.3. Gene Transfection Efficiencies of Ch2-PEGn-CLs


2.4. Gene Transfection Efficiencies of F-Ch2-PEGn-CLs with Introduction of a Folate Ligand
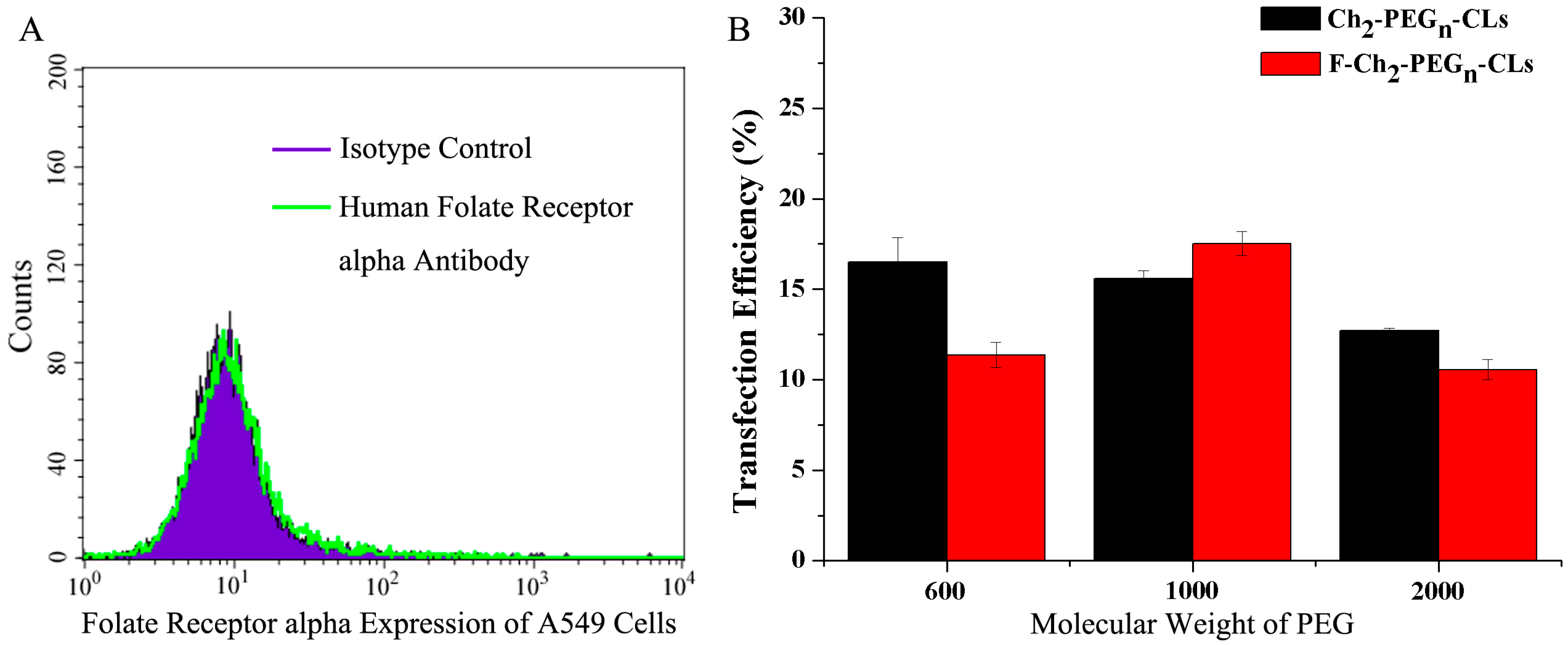
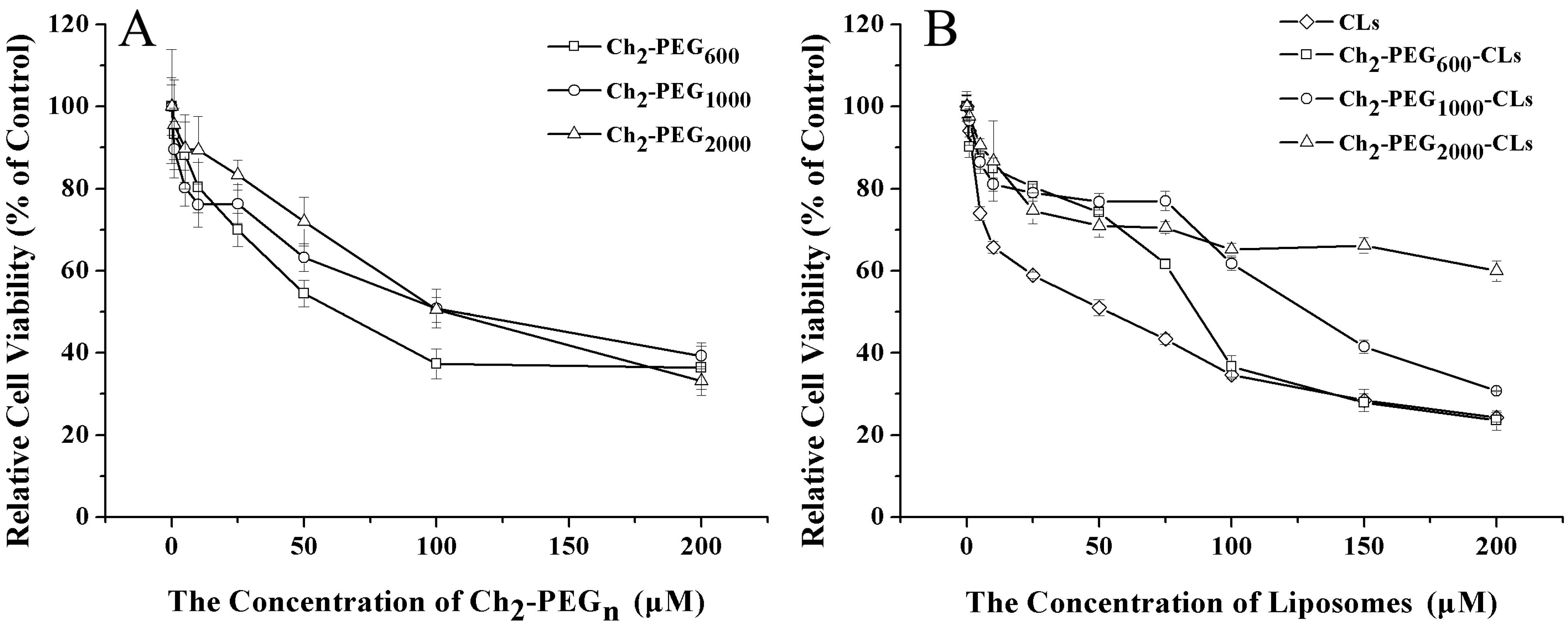
2.5 Cytotoxicity Evaluation
3. Experimental Section
3.1. Materials
3.2. Synthesis and Identification of Ch2-PEGn
3.2.1. Synthesis of Ch2-PEGn
3.2.2. 1H-NMR and Mass Spectra of Ch2-PEGn
3.2.3. Melting Point and Appearances
3.3. Preparation and Characterization of Cationic Liposomes
3.3.1. Preparation of Liposomes
3.3.2. Size and Zeta Potential Determination
3.4. Cell Culture
3.5. In Vitro Transfection Experiments
3.6. Cytotoxicity of Ch2-PEGn and Ch2-PEGn-CLs
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ikonen, E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell. Biol. 2008, 9, 125–138. [Google Scholar]
- Lingwood, D.; Simons, K. Lipid rafts as a membrane-organizing principle. Science 2010, 327, 46–50. [Google Scholar]
- He, Z.Y.; Wei, X.W.; Luo, M.; Luo, S.T.; Yang, Y.; Yu, Y.Y.; Chen, Y.; Ma, C.C.; Liang, X.; Guo, F.C.; et al. Folate-linked lipoplexes for short hairpin RNA targeting Claudin-3 delivery in ovarian cancer xenografts. J. Control. Release 2013, 172, 679–689. [Google Scholar]
- Porter, C. J.; Trevaskis, N. L.; Charman, W.N. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar]
- Ma, Q.; Li, B.; Yu, Y.; Zhang, Y.; Wu, Y.; Ren, W.; Zheng, Y.; He, J.; Xie, Y.; Song, X.; et al. Development of a novel biocompatible poly(ethylene glycol)-block-poly(γ-cholesterol-l-glutamate) as hydrophobic drug carrier. Int. J. Pharm. 2013, 445, 88–92. [Google Scholar]
- Tang, J.; Zhang, L.; Liu, Y.; Zhang, Q.; Qin, Y.; Yin, Y.; Yuan, W.; Yang, Y.; Xie, Y.; Zhang, Z.; et al. Synergistic targeted delivery of payload into tumor cells by dual-ligand liposomes co-modified with cholesterol anchored transferrin and TAT. Int. J. Pharm. 2013, 454, 31–40. [Google Scholar]
- Xu, L.; Wempe, M.F.; Anchordoquy, T.J. The effect of cholesterol domains on PEGylated liposomal gene delivery in vitro. Ther. Deliv. 2011, 2, 451–60. [Google Scholar]
- Li, J.M.; He, Z.Y.; Yu, S.; Li, S.Z.; Ma, Q.; Yu, Y.Y.; Zhang, J.L.; Li, R.; Zheng, Y.; He, G.; et al. Micelles based on methoxy poly(ethylene glycol)cholesterol conjugate for controlled and targeted drug delivery of a poorly water soluble drug. J. Biomed. Nanotechnol. 2012, 8, 809–17. [Google Scholar]
- Yu, Y.; He, Y.; Xu, B.; He, Z.; Zhang, Y.; Chen, Y.; Yang, Y.; Xie, Y.; Zheng, Y.; He, G.; et al. Self-assembled methoxy poly(ethylene glycol)cholesterol micelles for hydrophobic drug delivery. J. Pharm. Sci. 2013, 102, 1054–1062. [Google Scholar]
- Oba, M.; Miyata, K.; Osada, K.; Christie, R.J.; Sanjoh, M.; Li, W.; Fukushima, S.; Ishii, T.; Kano, M.R.; Nishiyama, N.; et al. Polyplex micelles prepared from omega-cholesteryl PEG-polycation block copolymers for systemic gene delivery. Biomaterials 2011, 32, 652–663. [Google Scholar]
- Xu, L.; Betker, J.; Yin, H.; Anchordoquy, T.J. Ligands located within a cholesterol domain enhance gene delivery to the target tissue. J. Control. Release 2012, 160, 57–63. [Google Scholar]
- He, Z.Y.; Chu, B.Y.; Wei, X.W.; Li, J.; Edwards, C.K., 3rd; Song, X.R.; He, G.; Xie, Y.M.; Wei, Y.Q.; Qian, Z.Y. Recent development of poly(ethylene glycol)-cholesterol conjugates as drug delivery systems. Int. J. Pharm. 2014, 469, 168–178. [Google Scholar]
- Meier, W.; Hotz, J.; GuntherAusborn, S. Vesicle and cell networks: Interconnecting cells by synthetic polymers. Langmuir 1996, 12, 5028–5032. [Google Scholar]
- Carrion, C.; Domingo, J.C.; de Madariaga, M.A. Preparation of long-circulating immunoliposomes using PEG-cholesterol conjugates: Effect of the spacer arm between PEG and cholesterol on liposomal characteristics. Chem. Phys. Lipids 2001, 113, 97–110. [Google Scholar]
- Rao, Z.; Taguchi, T. Spectroscopic studies on interactions between cholesterol-end capped polyethylene glycol and liposome. Colloids Surf. B Biointerfaces 2012, 97, 248–253. [Google Scholar]
- Diec, K.H.; Sokolowski, T.; Wittern, K.P.; Schreiber, J.; Meier, W. New liposome gels by self organization of vesicles and intelligent polymers. Cosmet. Toiletries 2002, 117, 55–62. [Google Scholar]
- Rao, Z.; Inoue, M.; Matsuda, M.; Taguchi, T. Quick self-healing and thermo-reversible liposome gel. Colloids Surf. B Biointerfaces 2011, 82, 196–202. [Google Scholar]
- Perez, N.; Whitcombe, M.J.; Vulfson, E.N. Surface imprinting of cholesterol on submicrometer core-shell emulsion particles. Macromolecules 2001, 34, 830–836. [Google Scholar]
- Petersen, H.; Fechner, P.M.; Martin, A.L.; Kunath, K.; Stolnik, S.; Roberts, C.J.; Fischer, D.; Davies, M.C.; Kissel, T. Polyethylenimine-graft-poly(ethylene glycol) copolymers: Influence of copolymer block structure on DNA complexation and biological activities as gene delivery system. Bioconjug. Chem. 2002, 13, 845–854. [Google Scholar]
- Savic, R.; Luo, L.; Eisenberg, A.; Maysinger, D. Micellar nanocontainers distribute to defined cytoplasmic organelles. Science 2003, 300, 615–618. [Google Scholar]
- Wang, Y.; Ke, C.Y.; Beh, C.W.; Liu, S.Q.; Goh, S.H.; Yang, Y.Y. The self-assembly of biodegradable cationic polymer micelles as vectors for gene transfection. Biomaterials 2007, 28, 5358–5368. [Google Scholar]
- Zhang, X.; Pan, S.R.; Hu, H.M.; Wu, G.F.; Feng, M.; Zhang, W.; Lu, X. Poly(ethylene glycol)-block-polyethylenimine copolymers as carriers for gene delivery: Effects of PEG molecular weight and PEGylation degree. J. Biomed. Mater. Res. A 2008, 84A, 795–804. [Google Scholar]
- Haak, C.S.; Bhayana, B.; Farinelli, W.A.; Anderson, R.R.; Haedersdal, M. The impact of treatment density and molecular weight for fractional laser-assisted drug delivery. J. Control. Release 2012, 163, 335–341. [Google Scholar]
- He, Z.; Yu, Y.; Zhang, Y.; Yan, Y.; Zheng, Y.; He, J.; Xie, Y.; He, G.; Wei, Y.; Song, X. Gene delivery with active targeting to ovarian cancer cells mediated by folate receptor α. J. Biomed. Nanotechnol. 2013, 9, 833–844. [Google Scholar]
- Heyes, J.; Hall, K.; Tailor, V.; Lenz, R.; MacLachlan, I. Synthesis and characterization of novel poly(ethylene glycol)-lipid conjugates suitable for use in drug delivery. J. Control. Release 2006, 112, 280–290. [Google Scholar]
- Gimpl, G.; Gehrig-Burger, K. Probes for studying cholesterol binding and cell biology. Steroids 2011, 76, 216–231. [Google Scholar]
- Photos, P.J.; Bacakova, L.; Discher, B.; Bates, F.S.; Discher, D.E. Polymer vesicles in vivo: Correlations with PEG molecular weight. J. Control. Release 2003, 90, 323–334. [Google Scholar]
- Han, X.; Liu, J.; Liu, M.; Xie, C.; Zhan, C.Y.; Gu, B.; Liu, Y.; Feng, L.L.; Lu, W.Y. 9-NC-loaded folate-conjugated polymer micelles as tumor targeted drug delivery system: Preparation and evaluation in vitro. Int. J. Pharm. 2009, 372, 125–131. [Google Scholar]
- He, Z.; Zheng, X.; Wu, X.; Song, X.; He, G.; Wu, W.; Yu, S.; Mao, S.; Wei, Y. Development of glycyrrhetinic acid-modified stealth cationic liposomes for gene delivery. Int. J. Pharm. 2010, 397, 147–154. [Google Scholar]
- Song, X.R.; Zheng, Y.; He, G.; Yang, L.; Luo, Y.F.; He, Z.Y.; Li, S.Z.; Li, J.M.; Yu, S.; Luo, X.; et al. Development of PLGA nanoparticles simultaneously loaded with vincristine and verapamil for treatment of hepatocellular carcinoma. J. Pharm. Sci. 2010, 99, 4874–4879. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.-C.; He, Z.-Y.; Xia, S.; Ren, K.; Hui, L.-W.; Qin, H.-X.; Tang, M.-H.; Zeng, J.; Song, X.-R. α, ω-Cholesterol-Functionalized Low Molecular Weight Polyethylene Glycol as a Novel Modifier of Cationic Liposomes for Gene Delivery. Int. J. Mol. Sci. 2014, 15, 20339-20354. https://doi.org/10.3390/ijms151120339
Ma C-C, He Z-Y, Xia S, Ren K, Hui L-W, Qin H-X, Tang M-H, Zeng J, Song X-R. α, ω-Cholesterol-Functionalized Low Molecular Weight Polyethylene Glycol as a Novel Modifier of Cationic Liposomes for Gene Delivery. International Journal of Molecular Sciences. 2014; 15(11):20339-20354. https://doi.org/10.3390/ijms151120339
Chicago/Turabian StyleMa, Cui-Cui, Zhi-Yao He, Shan Xia, Ke Ren, Li-Wei Hui, Han-Xiao Qin, Ming-Hai Tang, Jun Zeng, and Xiang-Rong Song. 2014. "α, ω-Cholesterol-Functionalized Low Molecular Weight Polyethylene Glycol as a Novel Modifier of Cationic Liposomes for Gene Delivery" International Journal of Molecular Sciences 15, no. 11: 20339-20354. https://doi.org/10.3390/ijms151120339






