Structural Biology of DNA (6-4) Photoproducts Formed by Ultraviolet Radiation and Interactions with Their Binding Proteins
Abstract
:1. Introduction
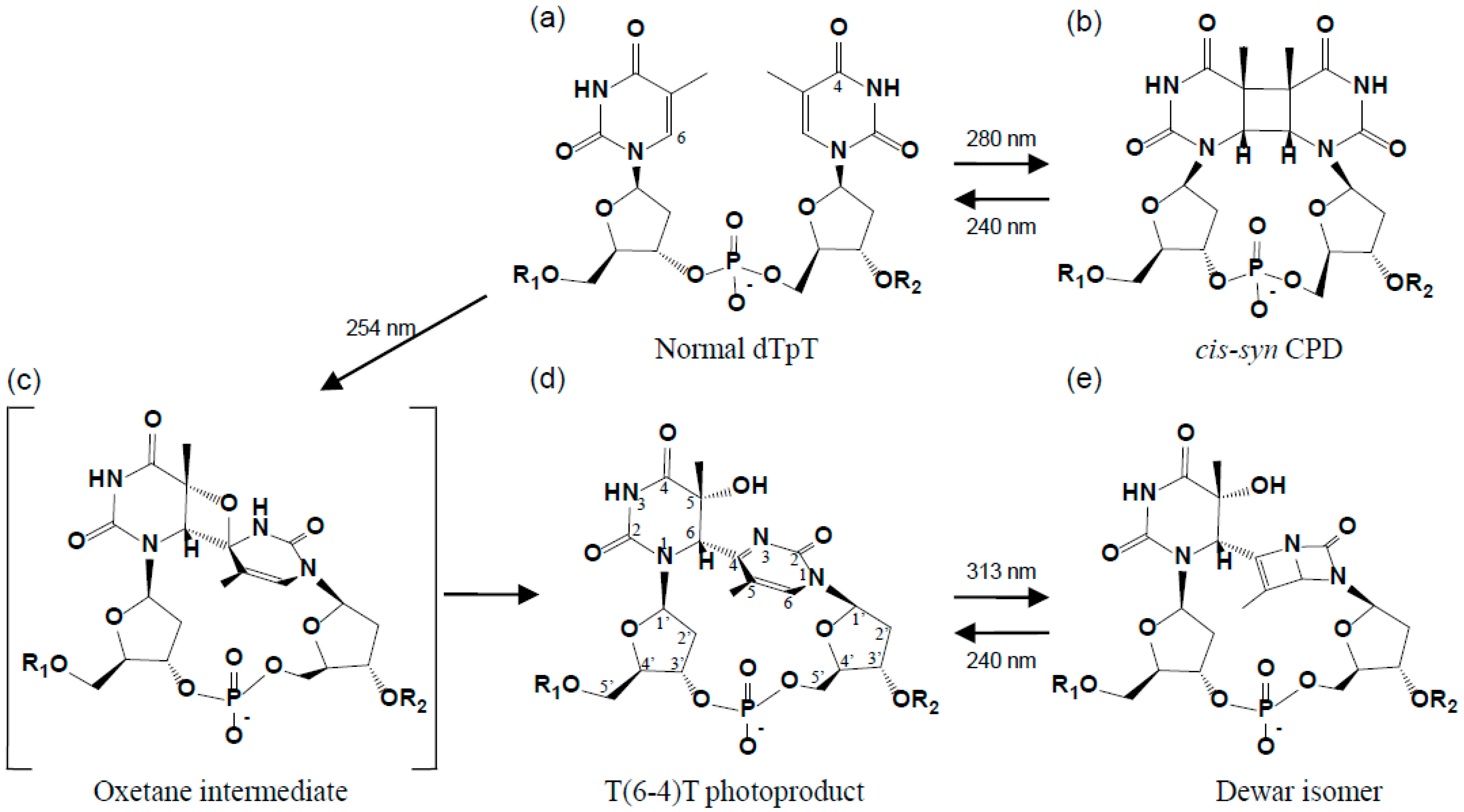
2. Structure of the (6-4) Photoproduct
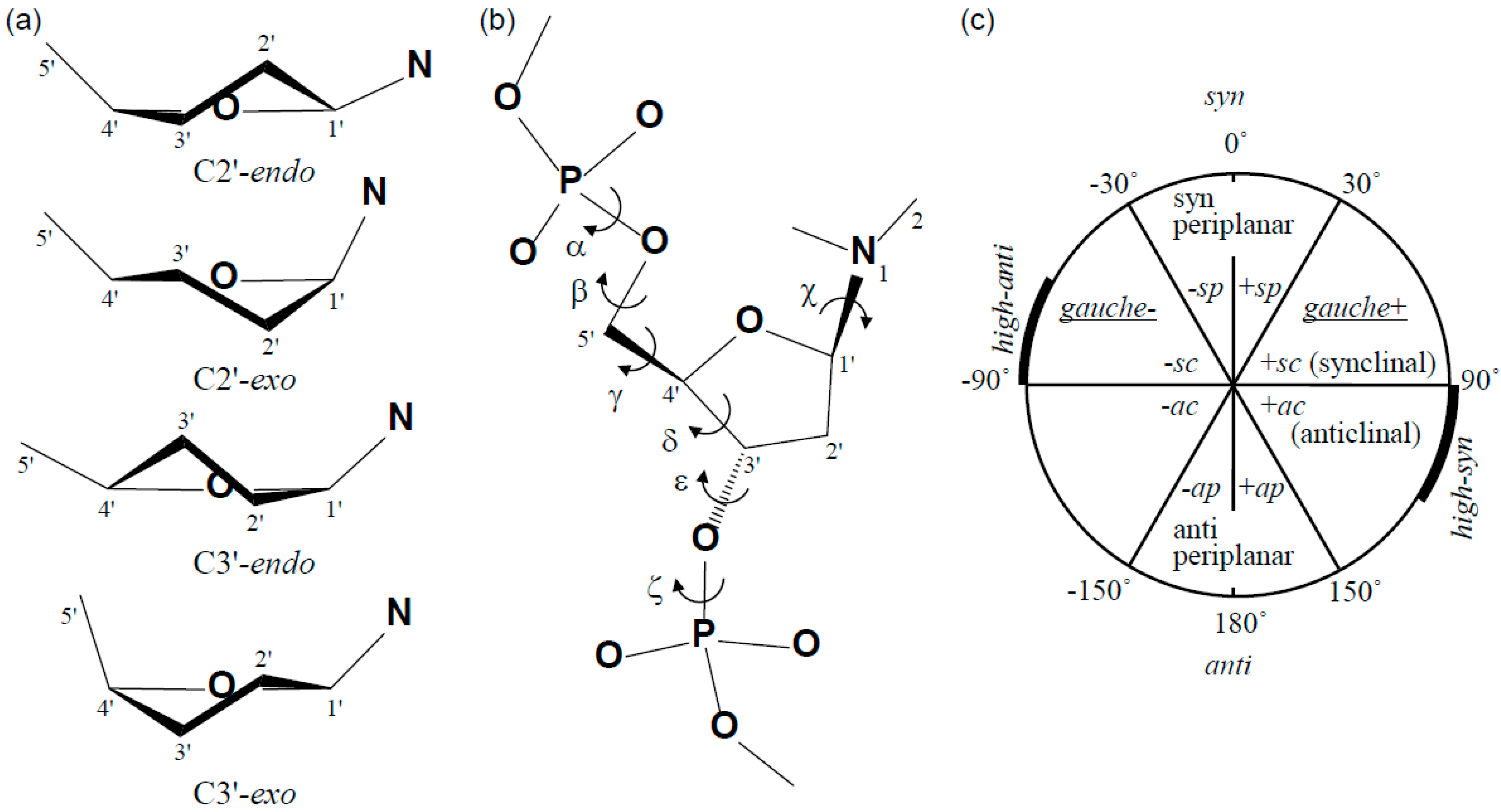
2.1. Structure of dT(6-4)T
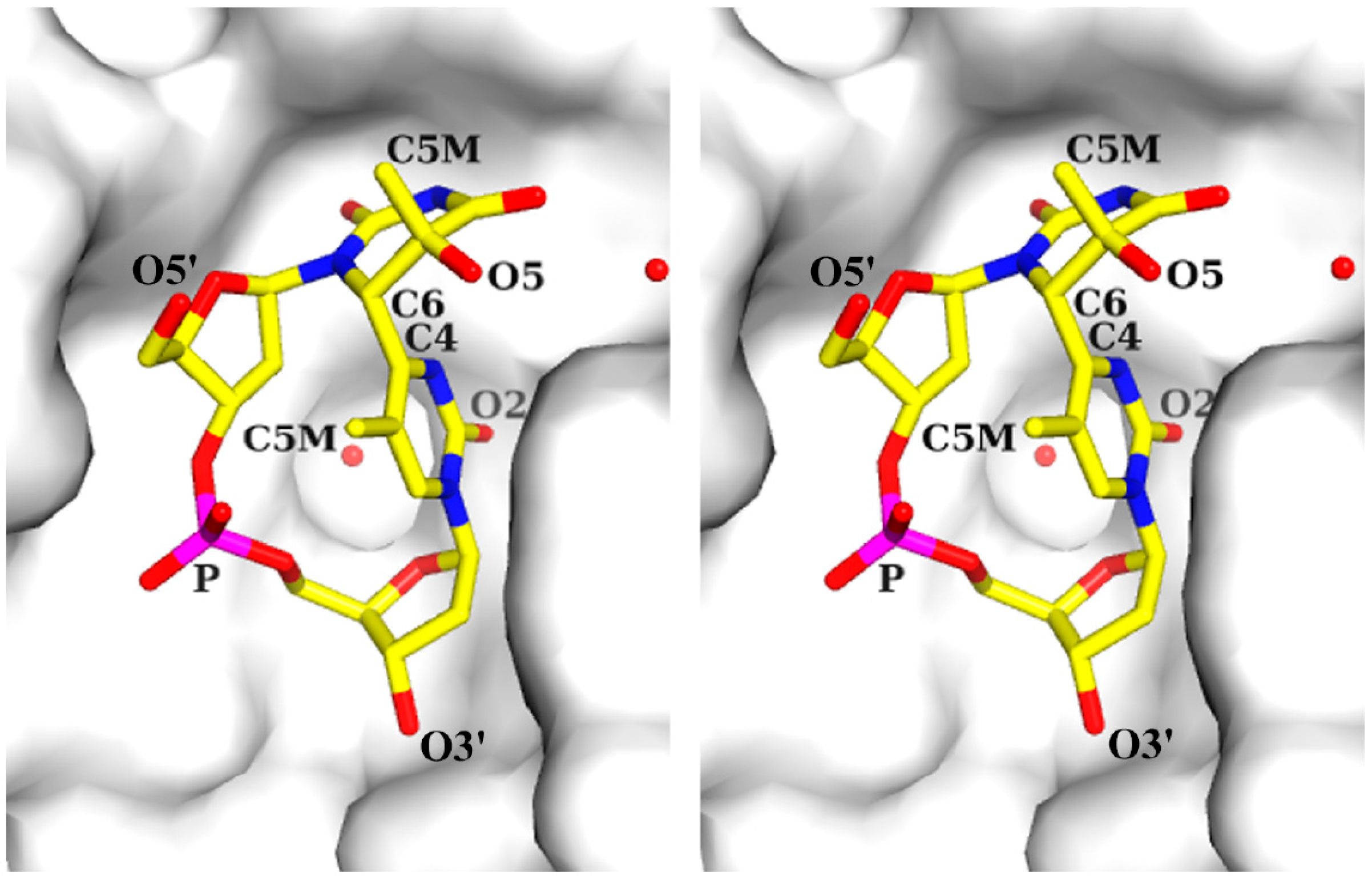
| Segment | Definition | Torsion Angle (°) | ||
|---|---|---|---|---|
| 64M-2 Fab dT(6-4)T a | dT(6-4)T a | B-DNA b | ||
| (6-4) Linkage c | N1−C6−C4#−C5# | −139 | − | |
| N1−C6−C4#−N3# | 39 | 27 | ||
| (C5 methyl)−C5−C6−C4# | −164 | −163 | ||
| 5'-T | ||||
| Glycosidic linkage | χ (O4'−C1'−N1−C2) | −135 | −138 | −117 |
| Backbone | γ (O5'−C5'−C4'−C3') | 103 | 56 | 54 |
| δ (C5'−C4'−C3'−O3') | 85 | 80 | 123 | |
| ε (C4'−C3'−O3'−P) | −110 | −107 | −169 | |
| ζ (C3'−O3'−P−O5') | −84 | −76 | −108 | |
| 3'-T | ||||
| Glycosidic linkage | χ (O4'−C1'−N1−C2) | −71 | −75 | −117 |
| Backbone | α (O3'−P−O5'−C5') | −71 | −96 | −63 |
| β (P−O5−C5'−C4') | −172 | −177 | 171 | |
| γ (O5'−C5'−C4'−C3') | 35 | 43 | 54 | |
| δ (C5'−C4'−C3'−O3') | 120 | 100 | 123 | |
2.2. Structure of Double-Stranded DNA Containing T(6-4)T

3. Recognition Mechanism of the (6-4) Photoproduct by Proteins
3.1. Interaction of Antibody Fab with the (6-4) Photoproduct
3.1.1. Fab Interaction with the T(6-4)T Segment
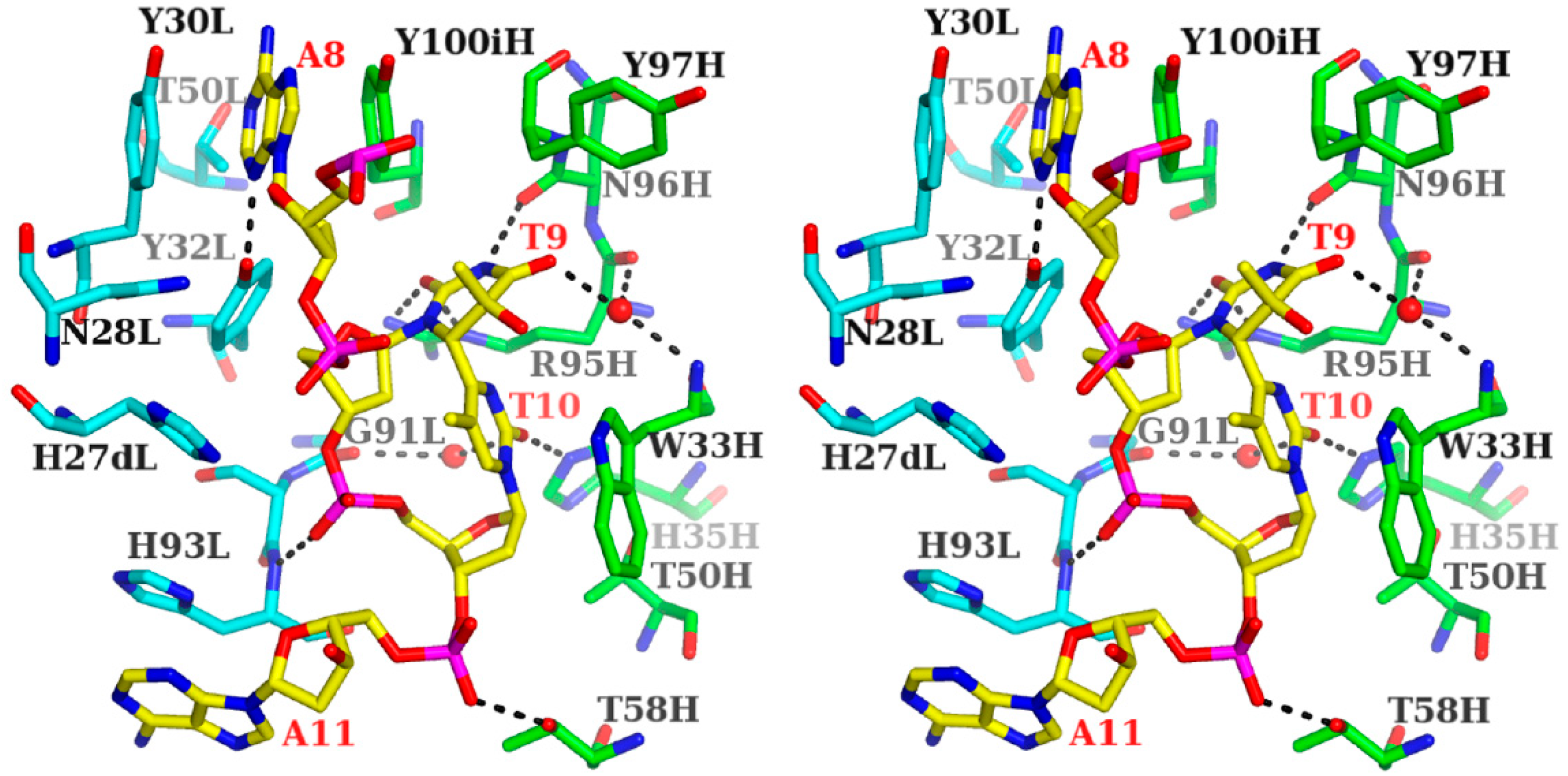
3.1.2. Fab Interaction with the Double-Stranded DNA (6-4) Photoproduct
3.2. Interaction between DNA (6-4) Photolyase and the (6-4) Photoproduct
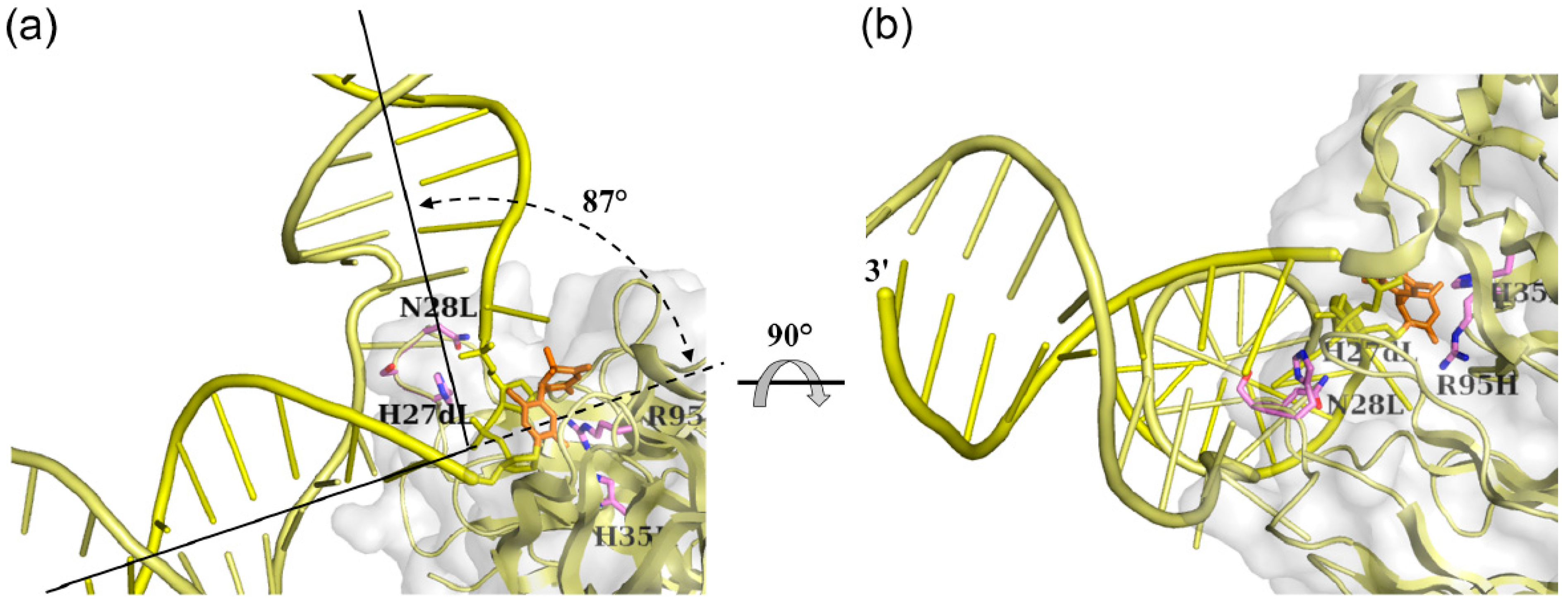
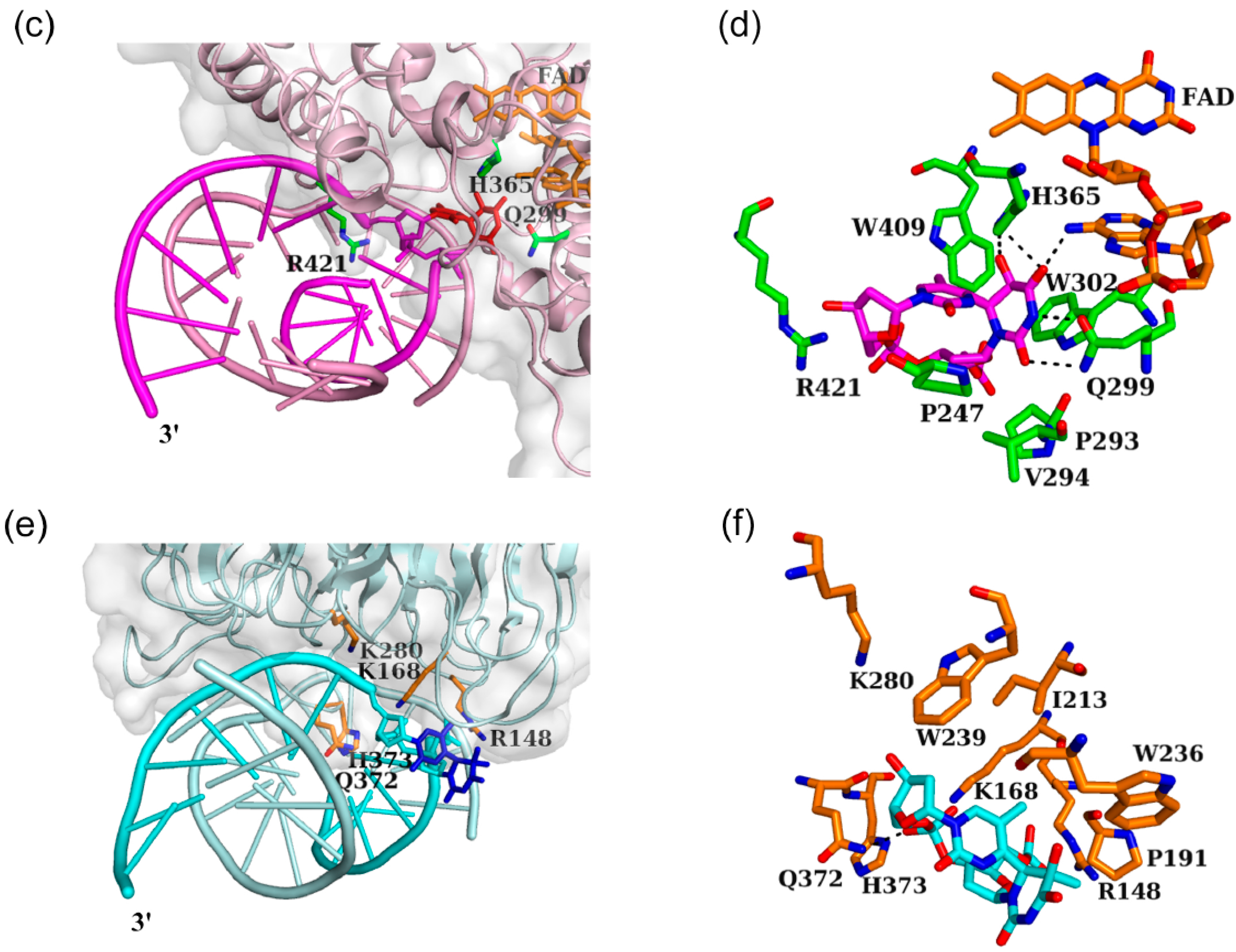
3.3. Interaction between Nucleotide Excision Repair Protein DDB1-DDB2 and the (6-4) Photoproduct
3.4. Distinctive Binding-Site Structures of Proteins Interacting with (6-4) Photoproducts
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lindahl, T. Instability and decay of the primary structure of DNA. Nature 1993, 362, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.L.; Nairn, R.S. The biology of the (6-4) photoproduct. Photochem. Photobiol. 1989, 49, 805–819. [Google Scholar] [CrossRef] [PubMed]
- LeClerc, J.E.; Borden, A.; Lawrence, C.W. The thymine-thymine pyrimidine-pyrimidone (6-4) ultraviolet light photoproduct is highly mutagenic and specifically induces 3' thymine-to-cytosine transitions in Escherichia coli. Proc. Natl. Acad. Sci. USA 1991, 88, 9685–9689. [Google Scholar] [CrossRef]
- Cleaver, J.E. Cancer in xeroderma pigmentosum and related disorders of DNA repair. Nat. Rev. Cancer 2005, 5, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Setlow, R.B. Repair deficient human disorders and cancer. Nature 1978, 271, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Weber, S. Light-driven enzymatic catalysis of DNA repair: A review of recent biophysical studies on photolyase. Biochim. Biophys. Acta 2005, 1707, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Benjdia, A. DNA photolyases and SP lyase: Structure and mechanism of light-dependent and independent DNA lyases. Curr. Opin. Struct. Biol. 2012, 22, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Nakane, M.; Hattori, T.; Matsunaga, T.; Ihara, M.; Nikaido, O. Simultaneous establishment of monoclonal antibodies specific for either cyclobutane pyrimidine dimer or (6-4) photoproduct from the same mouse immunized with ultraviolet-irradiated DNA. Photochem. Photobiol. 1991, 54, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Morioka, H.; Torizawa, T.; Kato, K.; Shimada, I.; Nikaido, O.; Ohtsuka, E. Specificities and rates of binding anti-(6-4) photoproduct antibody fragments to synthetic thymine photoproducts. J. Biochem. 1998, 123, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Torizawa, T.; Kato, K.; Kimura, Y.; Asada, T.; Kobayashi, H.; Komatsu, Y.; Morioka, H.; Nikaido, O.; Ohtsuka, E.; Shimada, I. 31P NMR study of the interactions between oligodeoxynucleotides containing (6-4) photoproduct and Fab fragments of monoclonal antibodies specific for (6-4) photoproduct. FEBS Lett. 1998, 429, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Mizutani, R.; Satow, Y.; Komatsu, Y.; Ohtsuka, E.; Nikaido, O. Crystal structure of the 64M-2 antibody Fab fragment in complex with a DNA dT(6-4)T photoproduct formed by ultraviolet radiation. J. Mol. Biol. 2000, 299, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Mizutani, R.; Satow, Y.; Sato, K.; Komatsu, Y.; Ohtsuka, E.; Nikaido, O. Structure of the DNA (6-4) photoproduct dTT(6-4)TT in complex with the 64M-2 antibody Fab fragment implies increased antibody-binding affinity by the flanking nucleotide. Acta Crystallogr. Sect. D Biol. Crystallogr. 2012, 68, 232–238. [Google Scholar] [CrossRef]
- Morioka, H.; Miura, H.; Kobayashi, H.; Koizumi, T.; Fujii, K.; Asano, K.; Matsunaga, T.; Nikaido, O.; Stewart, J.D.; Ohtsuka, E. Antibodies specific for (6-4) DNA photoproducts: cloning, antibody modeling and construction of a single-chain Fv derivative. Biochim. Biophys. Acta 1998, 1385, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Mizutani, R.; Satow, Y. Structure of a double-stranded DNA (6-4) photoproduct in complex with the 64M-5 antibody Fab. Acta. Crystallogr. Sect. D Biol. Crystallogr. 2013, 69, 504–512. [Google Scholar] [CrossRef]
- Todo, T.; Takemori, H.; Ryo, H.; Ihara, M.; Matsunaga, T.; Nikaido, O.; Sato, K.; Nomura, T. A new photoreactivating enzyme that specifically repairs ultraviolet light-induced (6-4) photoproducts. Nature 1993, 361, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, J.; Hsu, D.S.; Zhao, S.; Taylor, J.-S.; Sancar, A. Reaction mechanism of (6-4) photolyase. J. Biol. Chem. 1997, 272, 32580–32590. [Google Scholar] [CrossRef] [PubMed]
- Hitomi, K.; Kim, S.-T.; Iwai, S.; Harima, N.; Otoshi, E.; Ikenaga, M.; Todo, T. Binding and catalytic properties of Xenopus. (6-4) photolyase. J. Biol. Chem. 1997, 272, 32591–32598. [Google Scholar] [CrossRef] [PubMed]
- Sancar, A. Stucture and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem. Rev. 2003, 103, 2203–2237. [Google Scholar] [CrossRef] [PubMed]
- Selby, C.P.; Sancar, A. A cryptochrome/photolyase class of enzymes with single-stranded DNA-specific photolyase activity. Proc. Natl. Acad. Sci. USA 2006, 103, 17696–17700. [Google Scholar] [CrossRef] [PubMed]
- Hitomi, K.; Nakamura, H.; Kim, S.-T.; Mizukoshi, T.; Ishikawa, T.; Iwai, S.; Todo, T. Role of two histidines in the (6-4) photolyase reaction. J. Biol. Chem. 2001, 276, 10103–10109. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Z.; Tan, C.; Guo, X.; Wang, L.; Sancar, A.; Zhong, D. Dynamics and mechanism of repair of ultraviolet-induced (6-4) photoproduct by photolyase. Nature 2010, 466, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Sadeghian, K.; Bocola, M.; Merz, T.; Schütz, M. Theoretical study on the repair mechanism of the (6-4) photolesion by the (6-4) photolyase. J. Am. Chem. Soc. 2010, 132, 16285–16295. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.; Martin, R.; Iwai, S.; Plaza, P.; Brettel, K. Repair of the (6-4) photoproduct by DNA photolyase requires two photons. Angew. Chem. Int. Ed. 2013, 52, 7432–7436. [Google Scholar] [CrossRef]
- Faraji, S.; Dreuw, A. Physicochemical mechanism of light-driven DNA repair by (6-4) photolyases. Annu. Rev. Phys. Chem. 2014, 65, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Sancar, A. DNA excision repair. Annu. Rev. Biochem. 1996, 65, 43–81. [Google Scholar] [CrossRef] [PubMed]
- Gillet, L.C.J.; Schärer, O.D. Molecular mechanisms of mammalian global genome nucleotide excision repair. Chem. Rev. 2006, 106, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Maul, M.J.; Barends, T.R.M.; Glas, A.F.; Cryle, M.J.; Domratcheva, T.; Schneider, S.; Schlichting, I.; Carell, T. Crystal structure and mechanism of a DNA (6-4) photolyase. Angew. Chem. Int. Ed. 2008, 47, 10076–10080. [Google Scholar] [CrossRef]
- Glas, A.F.; Schneider, S.; Maul, M.J.; Hennecke, U.; Carell, T. Crystal structure of the T(6-4)C lesion in complex with a (6-4) DNA photolyase and repair of UV-induced (6-4) and Dewar photolesions. Chem. Eur. J. 2009, 15, 10387–10396. [Google Scholar] [CrossRef] [PubMed]
- Scrima, A.; Koníčková, R.; Czyzewski, B.K.; Kawasaki, Y.; Jeffrey, P.D.; Groisman, R.; Nakatani, Y.; Iwai, S.; Pavletich, N.P.; Thomä, N.H. Structural basis of UV DNA-damage recognition by the DDB1-DDB2 complex. Cell 2008, 135, 1213–1223. [Google Scholar] [CrossRef]
- Varghese, A.J.; Wang, S.Y. Thymine-thymine adduct as a photoproduct of thymine. Science 1968, 160, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Karle, I.L.; Wang, S.Y.; Varghese, A.J. Crystal and molecular structure of a thymine-thymine adduct. Science 1969, 164, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Karle, I.L. Crystal structure of a thymine-thymine adduct from irradiated thymine. Acta. Crystallogr. Sect. B Struct. Sci. 1969, 25, 2119–2126. [Google Scholar] [CrossRef]
- Rycyna, R.E.; Alderfer, J.L. UV irradiation of nucleic acids: formation, purification and solution conformational analysis of the “6–4 lesion” of dTpdT. Nucleic Acids Res. 1985, 13, 5949–5963. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.-S.; Garrett, D.S.; Wang, M.J. Models for the solution state structure of the (6-4) photoproduct of thymidylyl-(3'→5')-thymidine derived via a distance- and angle-constrained conformation search procedure. Biopolymers 1988, 27, 1571–1593. [Google Scholar] [CrossRef] [PubMed]
- PyMOL Home Page. Available online: http://www.pymol.org/ (accessed on 22 July 2014).
- Saenger, W. Principles of Nucleic Acid Structure; Springer-Verlag: New York, NY, USA, 1984; pp. 14–24. [Google Scholar]
- Drew, H.R.; Wing, R.M.; Takano, T.; Broka, C.; Tanaka, S.; Itakura, K.; Dickerson, R.E. Structure of a B-DNA dodecamer: Conformation and dynamics. Proc. Natl. Acad. Sci. USA 1981, 78, 2179–2183. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, G.; Vojtechovsky, J.; Clowney, L.; Brünger, A.T.; Berman, H.M. New parameters for the refinement of nucleic acid-containing structures. Acta. Crystallogr. Sect. D Biol. Crystallogr. 1996, 52, 57–64. [Google Scholar] [CrossRef]
- Kim, J.-K.; Choi, B.-S. The solution structure of DNA duplex-decamer containing the (6-4) photoproduct of thymidylyl (3'→5') thymidine by NMR and relaxation matrix refinement. Eur. J. Biochem. 1995, 228, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Patel, D.; Choi, B.-S. Contrasting structural impacts induced by cis-syn cyclobutane dimer and (6-4) adduct in DNA duplex decamers: implication in mutagenesis and repair activity. Photochem. Photobiol. 1995, 62, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Hwang, G.-S.; Choi, B.-S. Solution structure of a DNA decamer duplex containing the sTable 3' T G base pair of the pyrimidine(6-4)pyrimidone photoproduct [(6-4) adduct]: Implications for the highly specific 3'T→ C transition of the (6-4) adduct. Proc. Natl. Acad. Sci. USA 1999, 96, 6632–6636. [Google Scholar] [CrossRef] [PubMed]
- Kabat, E.A.; Wu, T.T.; Perry, H.M.; Gottesman, K.S.; Foeller, C. Sequences of Proteins of Immunological Interest; Public Health Service, National Institutes of Health: Bethesda, MD, USA, 1991.
- Otwinowski, Z.; Schevitz, R.W.; Zhang, R.-G.; Lawson, C.L.; Joachimiak, A.; Marmorstein, R.Q.; Luisi, B.F.; Sigler, P.B. Crystal structure of trp repressor/operator complex at atomic resolution. Nature 1988, 335, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Kato, J.; Morioka, H.; Stewart, J.D.; Ohtsuka, E. Tryptophan H33 plays an important role in pyrimidine (6-4) pyrimidone photoproduct binding by a high-affinity antibody. Protein Eng. 1999, 12, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Vassylyev, D.G.; Kashiwagi, T.; Mikami, Y.; Ariyoshi, M.; Iwai, S.; Ohtsuka, E.; Morikawa, K. Atomic model of a pyrimidine dimer excision repair enzyme complexed with a DNA substrate: Structural basis for damaged DNA recognition. Cell 1995, 83, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.-S.; Cohrs, M.P. DNA, light, and Dewar pyrimidinones: The structure and biological significance of TpT3. J. Am. Chem. Soc. 1987, 109, 2834–2835. [Google Scholar] [CrossRef]
- Taylor, J.-S.; Garrett, D.S.; Cohrs, M.P. Solution-state structure of the Dewar pyrimidinone photoproduct of thymidylyl-(3'→5')-thymidine. Biochemistry 1988, 27, 7206–7215. [Google Scholar] [CrossRef] [PubMed]
- Truglio, J.J.; Karakas, E.; Rhau, B.; Wang, H.; DellaVecchia, M.J.; Houten, B.V.; Kisker, C. Structural basis for DNA recognition and processing by UvrB. Nat. Struct. Mol. Biol. 2006, 13, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Mees, A.; Klar, T.; Gnau, P.; Hennecke, U.; Eker, A.P.M.; Carell, T.; Essen, L.O. Crystal structure of a photolyase bound to a CPD-like DNA lesion after in situ repair. Science 2004, 306, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokoyama, H.; Mizutani, R. Structural Biology of DNA (6-4) Photoproducts Formed by Ultraviolet Radiation and Interactions with Their Binding Proteins. Int. J. Mol. Sci. 2014, 15, 20321-20338. https://doi.org/10.3390/ijms151120321
Yokoyama H, Mizutani R. Structural Biology of DNA (6-4) Photoproducts Formed by Ultraviolet Radiation and Interactions with Their Binding Proteins. International Journal of Molecular Sciences. 2014; 15(11):20321-20338. https://doi.org/10.3390/ijms151120321
Chicago/Turabian StyleYokoyama, Hideshi, and Ryuta Mizutani. 2014. "Structural Biology of DNA (6-4) Photoproducts Formed by Ultraviolet Radiation and Interactions with Their Binding Proteins" International Journal of Molecular Sciences 15, no. 11: 20321-20338. https://doi.org/10.3390/ijms151120321




