Association Study Identifying a New Susceptibility Gene (AUTS2) for Schizophrenia
Abstract
:1. Introduction
2. Results
| Makers | Allele Frequency (%) | p-Value a | OR b (95% CI) | Genotype Frequency (%) | p-Value a | HWE p-Value | Models | OR b (95% CI) | p-Value a | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNPs | ID | ||||||||||||
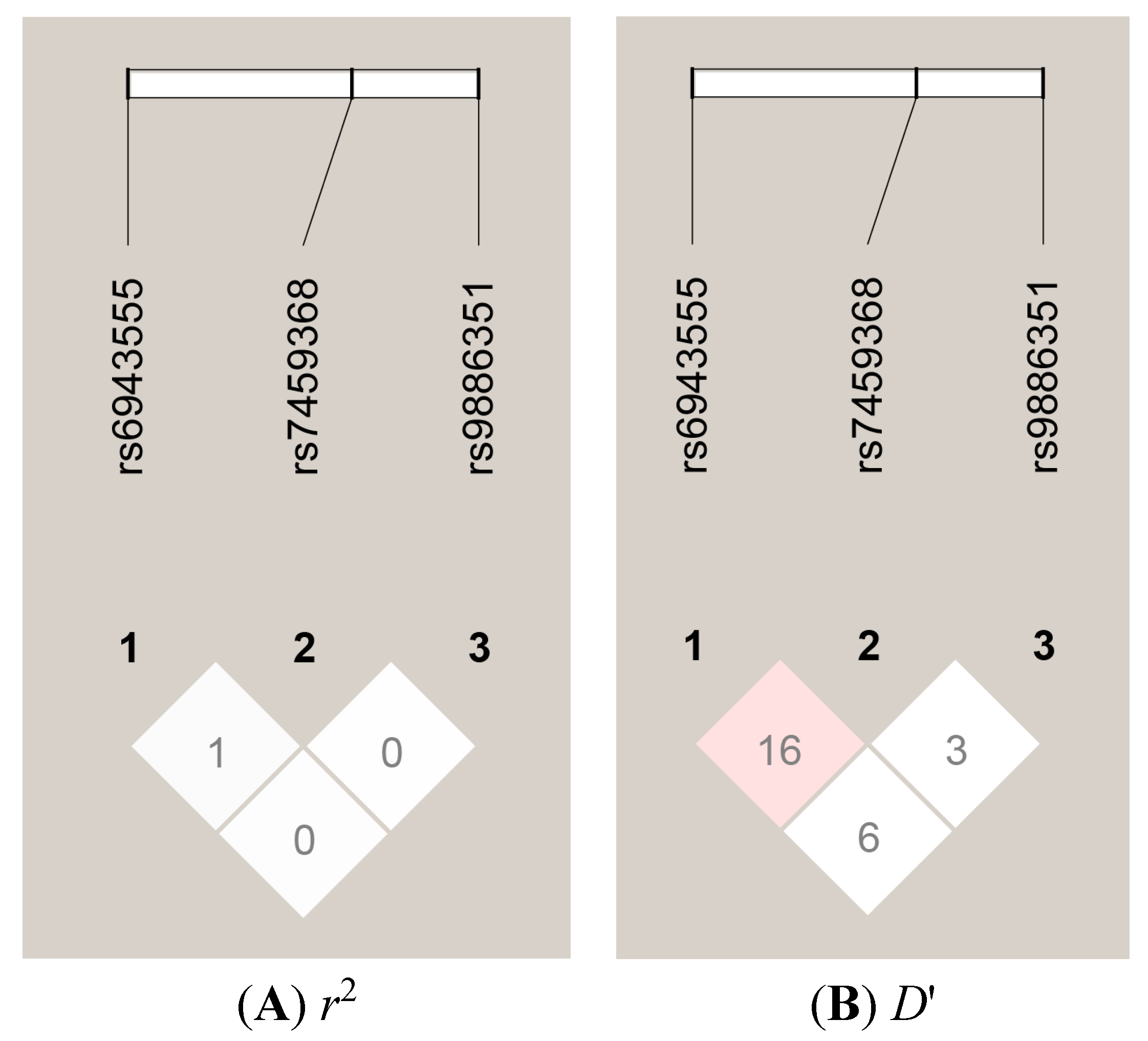
| SNP1 | rs6943555 | A | T | 0.097 | AA | AT | TT | 0.001 | 0.496 | ||||
| SCZ | 33.0 | 77.0 | 1.191 | 7.8 | 50.5 | 41.7 | Dominant | 1.363 (0.848–2.191) | 0.001 | ||||
| CTR | 29.3 | 70.7 | (0.969–1.463) | 10.4 | 37.9 | 51.7 | Recessive | 0.734 (0.456–1.180) | 0.201 | ||||
| Addictive | 1.197 (0.971–1.475) | 0.093 | |||||||||||
| SNP2 | rs7459368 | G | A | 0.720 | GG | AG | AA | 0.809 | 0.954 | ||||
| SCZ | 17.4 | 82.6 | 1.047 | 2.7 | 29.5 | 67.8 | Dominant | 1.117 (0.495–2.523) | 0.809 | ||||
| CTR | 16.8 | 83.2 | (0.813–1.349) | 3.0 | 27.6 | 69.4 | Recessive | 0.895 (0.396–2.021) | 0.789 | ||||
| Addictive | 1.048 (0.813–1.351) | 0.719 | |||||||||||
| SNP3 | rs9886351 | G | A | 0.681 | GG | AG | AA | 0.803 | 0.114 | ||||
| SCZ | 27.4 | 72.6 | 0.956 | 8.3 | 38.3 | 53.4 | Dominant | 1.028 (0.632–1.672) | 0.803 | ||||
| CTR | 26.6 | 73.4 | (0.771–1.185) | 8.5 | 36.1 | 55.4 | Recessive | 1.067 (0.662–1.719) | 0.789 | ||||
| Addictive | 1.030 (0.825–1.286) | 0.794 | |||||||||||
| Marks and Sex | Allele Frequency (%) | p-Value a | OR b (95% CI) | Genotype Frequency (%) | p-Value a | ||||
|---|---|---|---|---|---|---|---|---|---|
| SNP1 | A | T | AA | AT | TT | ||||
| F | SCZ | 35.0 | 65.0 | 0.284 | 1.251 (0.881–1.538) | 8.3 | 53.4 | 38.3 | 0.001 |
| CTR | 30.0 | 70.0 | 12.3 | 35.4 | 52.3 | ||||
| M | SCZ | 31.1 | 68.9 | 0.399 | 1.140 (0.84–1.547) | 7.8 | 41.1 | 51.0 | 0.223 |
| CTR | 28.4 | 71.6 | 7.4 | 47.5 | 45.1 | ||||
| SNP2 | G | A | GG | AG | AA | ||||
| F | SCZ | 17.7 | 82.3 | 0.453 | 0.874 (0.615–1.242) | 3.4 | 28.6 | 68.0 | 0.641 |
| CTR | 15.8 | 84.2 | 2.1 | 27.6 | 70.4 | ||||
| M | SCZ | 17.2 | 82.8 | 0.764 | 1.058 (0.733–1.525) | 2.0 | 30.4 | 67.6 | 0.395 |
| CTR | 18 | 82.0 | 4.2 | 27.6 | 68.2 | ||||
| SNP3 | G | A | GG | AG | AA | ||||
| F | SCZ | 30.1 | 69.9 | 0.119 | 0.791 (0.589–1.062) | 9.2 | 40.8 | 50.0 | 0.283 |
| CTR | 25.1 | 74.9 | 7.5 | 35.3 | 57.2 | ||||
| M | SCZ | 25.2 | 74.8 | 0.318 | 1.174 (0.857–1.608) | 7.3 | 35.8 | 56.9 | 0.596 |
| CTR | 28.4 | 71.6 | 9.9 | 37.0 | 53.1 | ||||
3. Discussion
4. Materials and Methods
4.1. Single-Nucleotide Polymorphism (SNP) Selection
4.2. Genotyping
| SNPs | Primers (5'–3') | Sizes (bp) | T (°C) | Enzymes/Regions | T (°C) |
|---|---|---|---|---|---|
| SNP1 | F: 5'-TGGGTGTTGGAAGAGTTTTGA-3'; R: 5'-ATACAGTATACATAAACATTGGAAAAGAGGGAA-3' | 196 | 60 | Hinf1, G▼ANTC | 37 |
| SNP2 | F: 5'-AAAGTTCTGGACAGTGGTGCTC-3'; R: 5'-TTCTGACAGTGCGTAAAGGTTG-3' | 257 | 65 | Msp1, C▼CGG | 37 |
| SNP3 | F: 5'-GGTGGAAAATAAGCCAGTATGC-3'; R: 5'-TAGGAAAATGGATTAAACGTAGGAG-3' | 221 | 65 | Hinf1, G▼ANTC | 37 |
4.3. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fatemi, S.H.; Folsom, T.D. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr. Bull. 2009, 35, 528–548. [Google Scholar]
- Goldman, A.L.; Pezawas, L.; Mattay, V.S.; Fischl, B.; Verchinski, B.A.; Zoltick, B.; Weinberger, D.R.; Meyer-Lindenberg, A. Heritability of brain morphology related to schizophrenia: A large-scale automated magnetic resonance imaging segmentation study. Biol. Psychiatry 2008, 63, 475–483. [Google Scholar]
- Zhang, C.; Fang, Y.; Xie, B.; Cheng, W.; Du, Y.; Wang, D.; Yu, S. DNA methyltransferase 3B gene increases risk of early onset schizophrenia. Neurosci. Lett. 2009, 462, 308–311. [Google Scholar]
- Wirgenes, K.V.; Sonderby, I.E.; Haukvik, U.K.; Mattingsdal, M.; Tesli, M.; Athanasiu, L.; Sundet, K.; Rossberg, J.I.; Dale, A.M.; Brown, A.A.; et al. Tcf4 sequence variants and mrna levels are associated with neurodevelopmental characteristics in psychotic disorders. Transl. Psychiatry 2012, 2, e112. [Google Scholar]
- Need, A.C.; Ge, D.; Weale, M.E.; Maia, J.; Feng, S.; Heinzen, E.L.; Shianna, K.V.; Yoon, W.; Kasperaviciute, D.; Gennarelli, M.; et al. A genome-wide investigation of SNPs and CNVs in schizophrenia. PLoS Genet. 2009, 5, e1000373. [Google Scholar]
- O’Donovan, M.C.; Craddock, N.; Norton, N.; Williams, H.; Peirce, T.; Moskvina, V.; Nikolov, I.; Hamshere, M.; Carroll, L.; Georgieva, L.; et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat. Genet. 2008, 40, 1053–1055. [Google Scholar]
- Walsh, T.; McClellan, J.M.; McCarthy, S.E.; Addington, A.M.; Pierce, S.B.; Cooper, G.M.; Nord, A.S.; Kusenda, M.; Malhotra, D.; Bhandari, A.; et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science 2008, 320, 539–543. [Google Scholar]
- Williams, H.J.; Owen, M.J.; O’Donovan, M.C. New findings from genetic association studies of schizophrenia. J. Hum. Genet. 2009, 54, 9–14. [Google Scholar]
- Lencz, T.; Morgan, T.V.; Athanasiou, M.; Dain, B.; Reed, C.R.; Kane, J.M.; Kucherlapati, R.; Malhotra, A.K. Converging evidence for a pseudoautosomal cytokine receptor gene locus in schizophrenia. Mol. Psychiatry 2007, 12, 572–580. [Google Scholar]
- Sullivan, P.F.; Lin, D.; Tzeng, J.Y.; van den Oord, E.; Perkins, D.; Stroup, T.S.; Wagner, M.; Lee, S.; Wright, F.A.; Zou, F.; et al. Genomewide association for schizophrenia in the catie study: Results of stage 1. Mol. Psychiatry 2008, 13, 570–584. [Google Scholar]
- Yue, W.H.; Wang, H.F.; Sun, L.D.; Tang, F.L.; Liu, Z.H.; Zhang, H.X.; Li, W.Q.; Zhang, Y.L.; Zhang, Y.; Ma, C.C.; et al. Genome-wide association study identifies a susceptibility locus for schizophrenia in hanchinese at 11p11.2. Nat. Genet. 2011, 43, 1228–1231. [Google Scholar]
- Ripke, S.; Sanders, A.R.; Kendler, K.S.; Levinson, D.F.; Sklar, P.; Holmans, P.A.; Lin, D.Y.; Duan, J.; Ophoff, R.A.; Andreassen, O.A.; et al. Genome-wide association study identifies five new schizophrenia loci. Nat. Genet. 2011, 43, 969–976. [Google Scholar]
- Guan, F.; Wei, S.; Feng, J.; Zhang, C.; Xing, B.; Zhang, H.; Gao, C.; Yang, H.; Li, S. Association study of a new schizophrenia susceptibility locus of 10q24.32–33 in a hanchinese population. Schizophr. Res. 2012, 138, 63–68. [Google Scholar]
- Bedogni, F.; Hodge, R.D.; Nelson, B.R.; Frederick, E.A.; Shiba, N.; Daza, R.A.; Hevner, R.F. Autism susceptibility candidate 2 (AUTS2) encodes a nuclear protein expressed in developing brain regions implicated in autism neuropathology. Gene Expr. Patterns 2010, 10, 9–15. [Google Scholar]
- Pennacchio, L.A.; Ahituv, N.; Moses, A.M.; Prabhakar, S.; Nobrega, M.A.; Shoukry, M.; Minovitsky, S.; Dubchak, I.; Holt, A.; Lewis, K.D.; et al. In vivo enhancer analysis of human conserved non-coding sequences. Nature 2006, 444, 499–502. [Google Scholar]
- Amarillo, I.E.; Li, W.L.; Li, X.; Vilain, E.; Kantarci, S. De novo single exon deletion of AUTS2 in a patient with speech and language disorder: A review of disrupted AUTS2 and further evidence for its role in neurodevelopmental disorders. Am. J. Med. Genet. 2014, 164, 958–965. [Google Scholar]
- Bakkaloglu, B.; O’Roak, B.J.; Louvi, A.; Gupta, A.R.; Abelson, J.F.; Morgan, T.M.; Chawarska, K.; Klin, A.; Ercan-Sencicek, A.G.; Stillman, A.A.; et al. Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. Am. J. Hum. Genet. 2008, 82, 165–173. [Google Scholar]
- Ben-David, E.; Granot-Hershkovitz, E.; Monderer-Rothkoff, G.; Lerer, E.; Levi, S.; Yaari, M.; Ebstein, R.P.; Yirmiya, N.; Shifman, S. Identification of a functional rare variant in autism using genome-wide screen for monoallelic expression. Hum. Mol. Genet. 2011, 20, 3632–3641. [Google Scholar]
- Huang, X.L.; Zou, Y.S.; Maher, T.A.; Newton, S.; Milunsky, J.M. A de novo balanced translocation breakpoint truncating the autism susceptibility candidate 2 (AUTS2) gene in a patient with autism. Am. J. Med. Genet. 2010, 152, 2112–2114. [Google Scholar]
- Schumann, G.; Coin, L.J.; Lourdusamy, A.; Charoen, P.; Berger, K.H.; Stacey, D.; Desrivieres, S.; Aliev, F.A.; Khan, A.A.; Amin, N.; et al. Genome-wide association and genetic functional studies identify autism susceptibility candidate 2 gene (AUTS2) in the regulation of alcohol consumption. Proc. Natl. Acad. Sci. USA 2011, 108, 7119–7124. [Google Scholar]
- Chen, Y.H.; Liao, D.L.; Lai, C.H.; Chen, C.H. Genetic analysis of AUTS2 as a susceptibility gene of heroin dependence. Drug Alcohol Depend. 2013, 128, 238–242. [Google Scholar]
- Chojnicka, I.; Gajos, K.; Strawa, K.; Broda, G.; Fudalej, S.; Fudalej, M.; Stawinski, P.; Pawlak, A.; Krajewski, P.; Wojnar, M.; et al. Possible association between suicide committed under influence of ethanol and a variant in the AUTS2 gene. PLoS One 2013. [Google Scholar] [CrossRef]
- Burbach, J.P.; van der Zwaag, B. Contact in the genetics of autism and schizophrenia. Trends Neurosci. 2009, 32, 69–72. [Google Scholar]
- Qin, P. The impact of psychiatric illness on suicide: Differences by diagnosis of disorders and by sex and age of subjects. J. Psychiatr. Res. 2011, 45, 1445–1452. [Google Scholar]
- Chambers, R.A.; Krystal, J.H.; Self, D.W. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol. Psychiatry 2001, 50, 71–83. [Google Scholar]
- Green, A.I.; Salomon, M.S.; Brenner, M.J.; Rawlins, K. Treatment of schizophrenia and comorbid substance use disorder. Curr. Drug Targets CNS Neurol. Disord. 2002, 1, 129–139. [Google Scholar]
- Smith, M.J.; Wang, L.; Cronenwett, W.; Goldman, M.B.; Mamah, D.; Barch, D.M.; Csernansky, J.G. Alcohol use disorders contribute to hippocampal and subcortical shape differences in schizophrenia. Schizophr. Res. 2011, 131, 174–183. [Google Scholar]
- Huang, Y.Z.; Zhan, Z.Y.; Li, X.Y.; Wu, S.R.; Sun, Y.J.; Xue, J.; Lan, X.Y.; Lei, C.Z.; Zhang, C.L.; Jia, Y.T.; et al. SNP and haplotype analysis reveal IGF2 variants associated with growth traits in chineseqinchuan cattle. Mol. Biol. Rep. 2014, 41, 591–598. [Google Scholar]
- Sultana, R.; Yu, C.E.; Yu, J.; Munson, J.; Chen, D.; Hua, W.; Estes, A.; Cortes, F.; de la Barra, F.; Yu, D.; et al. Identification of a novel gene on chromosome 7q11.2 interrupted by a translocation breakpoint in a pair of autistic twins. Genomics 2002, 80, 129–134. [Google Scholar]
- Girirajan, S.; Brkanac, Z.; Coe, B.P.; Baker, C.; Vives, L.; Vu, T.H.; Shafer, N.; Bernier, R.; Ferrero, G.B.; Silengo, M.; et al. Relative burden of large cnvs on a range of neurodevelopmental phenotypes. PLoS Genet. 2011, 7, e1002334. [Google Scholar]
- Nagamani, S.C.; Erez, A.; Ben-Zeev, B.; Frydman, M.; Winter, S.; Zeller, R.; el-Khechen, D.; Escobar, L.; Stankiewicz, P.; Patel, A.; et al. Detection of copy-number variation in AUTS2 gene by targeted exonic array cgh in patients with developmental delay and autistic spectrum disorders. Eur. J. Hum. Genet. 2013, 21, 343–346. [Google Scholar]
- Elia, J.; Gai, X.; Xie, H.M.; Perin, J.C.; Geiger, E.; Glessner, J.T.; D’Arcy, M.; deBerardinis, R.; Frackelton, E.; Kim, C.; et al. Rare structural variants found in attention-deficit hyperactivity disorder are preferentially associated with neurodevelopmental genes. Mol. Psychiatry 2010, 15, 637–646. [Google Scholar]
- Mefford, H.C.; Muhle, H.; Ostertag, P.; von Spiczak, S.; Buysse, K.; Baker, C.; Franke, A.; Malafosse, A.; Genton, P.; Thomas, P.; et al. Genome-wide copy number variation in epilepsy: Novel susceptibility loci in idiopathic generalized and focal epilepsies. PLoS Genet. 2010, 6, e1000962. [Google Scholar]
- Talkowski, M.E.; Rosenfeld, J.A.; Blumenthal, I.; Pillalamarri, V.; Chiang, C.; Heilbut, A.; Ernst, C.; Hanscom, C.; Rossin, E.; Lindgren, A.M.; et al. Sequencing chromosomal abnormalities reveals neurodevelopmental loci that confer risk across diagnostic boundaries. Cell 2012, 149, 525–537. [Google Scholar]
- De Bakker, P.I.; Yelensky, R.; Pe’er, I.; Gabriel, S.B.; Daly, M.J.; Altshuler, D. Efficiency and power in genetic association studies. Nat. Genet. 2005, 37, 1217–1223. [Google Scholar]
- NCBI. Available online: http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=6943555 (accessed on 21 October 2014).
- Ke, X.Y.; Cardon, L.R. Efficient selective screening of haplotype tag SNPS. Bioinformatics 2003, 19, 287–288. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar]
- Shi, Y.Y.; He, L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005, 15, 97–98. [Google Scholar]
- Guan, F.L.; Zhang, C.; Wei, S.G.; Zhang, H.B.; Gong, X.M.; Feng, J.L.; Gao, C.G.; Su, R.; Yang, H.M.; Li, S.B. Association of PDE4B polymorphisms and schizophrenia in Northwestern Han Chinese. Hum. Genet. 2012, 131, 1047–1056. [Google Scholar]
- Gabriel, R.; von Glasow, R.; Sander, R.; Andreae, M.; Crutzen, P.J. Bromide content of sea-salt aerosol particles collected over the Indian Ocean during INDOEX 1999. J. Geophys. Res. 2002, 107, 8032. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Xu, Y.-H.; Wei, S.-G.; Zhang, H.-B.; Fu, D.-K.; Feng, Z.-F.; Guan, F.-L.; Zhu, Y.-S.; Li, S.-B. Association Study Identifying a New Susceptibility Gene (AUTS2) for Schizophrenia. Int. J. Mol. Sci. 2014, 15, 19406-19416. https://doi.org/10.3390/ijms151119406
Zhang B, Xu Y-H, Wei S-G, Zhang H-B, Fu D-K, Feng Z-F, Guan F-L, Zhu Y-S, Li S-B. Association Study Identifying a New Susceptibility Gene (AUTS2) for Schizophrenia. International Journal of Molecular Sciences. 2014; 15(11):19406-19416. https://doi.org/10.3390/ijms151119406
Chicago/Turabian StyleZhang, Bao, Yue-Hong Xu, Shu-Guang Wei, Hong-Bo Zhang, Dong-Ke Fu, Zu-Fei Feng, Fang-Lin Guan, Yong-Sheng Zhu, and Sheng-Bin Li. 2014. "Association Study Identifying a New Susceptibility Gene (AUTS2) for Schizophrenia" International Journal of Molecular Sciences 15, no. 11: 19406-19416. https://doi.org/10.3390/ijms151119406





