Effect of Nanoparticles Exposure on Fractional Exhaled Nitric Oxide (FENO) in Workers Exposed to Nanomaterials
Abstract
:1. Introduction
2. Results
2.1. Participant Characteristics and FENO Values
2.2. Association of Determinants with FENO Values
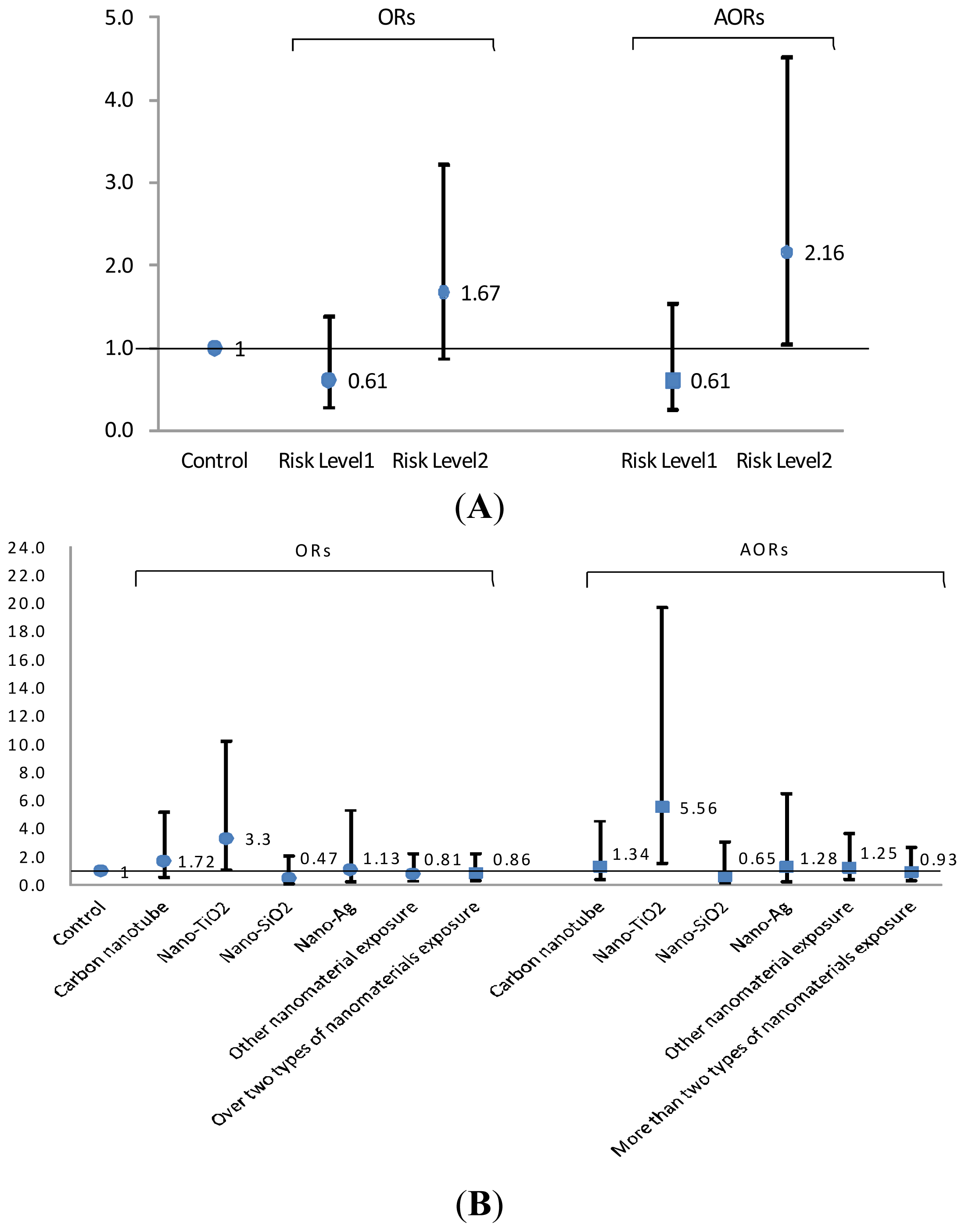
2.3. Risk Levels in FENO Values
3. Discussion
3.1. Inflammation and NOS2 Expression
3.2. Different Nanomaterials (NM) Exposure and NOS Expression
3.3. Determinants of Exhaled Nitric Oxide Levels (FENO)
3.4. NF-κB in Exhaled Breath Condensate
3.5. Strengths and Limitations
4. Subjects and Methods
4.1. Study Subjects and Data Collection
4.2. Fractional Exhaled Nitric Oxide (FENO) and Pulmonary Function Measurement
4.3. Exhaled Breath Condensate (EBC) Collection
4.4. Nuclear Factor Kappa B (NF-κB) Assay
4.5. Exposure Assessment
4.6. Statistical Analysis
5. Conclusions
Supplementary Information
ijms-15-00878-s001.pdfAcknowledgments
Conflicts of Interest
References
- Schmidt, C.W. Nanotechnology-related environment, health, and safety research: Examining the national strategy. Environ. Health Perspect 2009, 117, A158–A161. [Google Scholar]
- Stebounova, L.V.; Morgan, H.; Grassian, V.H.; Brenner, S. Health and safety implications of occupational exposure to engineered nanomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 2012, 4, 310–321. [Google Scholar]
- Maynard, A.D.; Aitken, R.J.; Butz, T.; Colvin, V.; Donaldson, K.; Oberdorster, G.; Philbert, M.A.; Ryan, J.; Seaton, A.; Stone, V.; et al. Safe handling of nanotechnology. Nature 2006, 444, 267–269. [Google Scholar]
- Peters, A.; Ruckerl, R.; Cyrys, J. Lessons from air pollution epidemiology for studies of engineered nanomaterials. J. Occup. Environ. Med 2011, 53, S8–S13. [Google Scholar]
- Eisen, E.A.; Costello, S.; Chevrier, J.; Picciotto, S. Epidemiologic challenges for studies of occupational exposure to engineered nanoparticles; a commentary. J. Occup. Environ. Med 2011, 53, S57–S61. [Google Scholar]
- Donaldson, K.; Stone, V.; Tran, C.L.; Kreyling, W.; Borm, P.J. Nanotoxicology. Occup. Environ. Med 2004, 61, 727–728. [Google Scholar]
- Nanoparticle Task Force ACOEM. Nanotechnology and health. J. Occup. Environ. Med 2011, 53, 687–689.
- Moller, W.; Felten, K.; Meyer, G.; Meyer, P.; Seitz, J.; Kreyling, W.G. Corrections in dose assessment of 99mTc radiolabeled aerosol particles targeted to central human airways using planar gamma camera imaging. J. Aerosol. Med. Pulm. Drug Deliv 2009, 22, 45–54. [Google Scholar]
- Oberdorster, G.; Oberdorster, E.; Oberdorster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect 2005, 113, 823–839. [Google Scholar]
- Brown, D.M.; Wilson, M.R.; MacNee, W.; Stone, V.; Donaldson, K. Size-dependent proinflammatory effects of ultrafine polystyrene particles: A role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol. Appl. Pharmacol 2001, 175, 191–199. [Google Scholar]
- Oberdorster, G. Pulmonary effects of inhaled ultrafine particles. Int. Arch. Occup. Environ. Health 2001, 74, 1–8. [Google Scholar]
- ATS Workshop Proceedings: Exhaled nitric oxide and nitric oxide oxidative metabolism in exhaled breath condensate: Executive summary. Am. J. Respir. Crit. Care Med 2006, 173, 811–813.
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.C.; Plummer, A.L.; Taylor, D.R. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med 2011, 184, 602–615. [Google Scholar]
- Eckel, S.P.; Berhane, K.; Salam, M.T.; Rappaport, E.B.; Linn, W.S.; Bastain, T.M.; Zhang, Y.; Lurmann, F.; Avol, E.L.; Gilliland, F.D. Residential traffic-related pollution exposures and exhaled nitric oxide in the children’s health study. Environ. Health Perspect 2011, 119, 1472–1477. [Google Scholar]
- Berhane, K.; Zhang, Y.; Linn, W.S.; Rappaport, E.B.; Bastain, T.M.; Salam, M.T.; Islam, T.; Lurmann, F.; Gilliland, F.D. The effect of ambient air pollution on exhaled nitric oxide in the Children’s Health Study. Eur. Respir. J 2011, 37, 1029–1036. [Google Scholar]
- Knol, A.B.; de Hartog, J.J.; Boogaard, H.; Slottje, P.; van der Sluijs, J.P.; Lebret, E.; Cassee, F.R.; Wardekker, J.A.; Ayres, J.G.; Borm, P.J.; et al. Expert elicitation on ultrafine particles: Likelihood of health effects and causal pathways. Part. Fibre Toxicol 2009, 6, 19. [Google Scholar]
- Barnes, P.J.; Dweik, R.A.; Gelb, A.F.; Gibson, P.G.; George, S.C.; Grasemann, H.; Pavord, I.D.; Ratjen, F.; Silkoff, P.E.; Taylor, D.R.; et al. Exhaled nitric oxide in pulmonary diseases: A comprehensive review. Chest 2010, 138, 682–692. [Google Scholar]
- Lane, C.; Knight, D.; Burgess, S.; Franklin, P.; Horak, F.; Legg, J.; Moeller, A.; Stick, S. Epithelial inducible nitric oxide synthase activity is the major determinant of nitric oxide concentration in exhaled breath. Thorax 2004, 59, 757–760. [Google Scholar]
- Hu, R.; Gong, X.; Duan, Y.; Li, N.; Che, Y.; Cui, Y.; Zhou, M.; Liu, C.; Wang, H.; Hong, F. Neurotoxicological effects and the impairment of spatial recognition memory in mice caused by exposure to TiO2 nanoparticles. Biomaterials 2010, 31, 8043–8050. [Google Scholar]
- Mikkelsen, L.; Sheykhzade, M.; Jensen, K.A.; Saber, A.T.; Jacobsen, N.R.; Vogel, U.; Wallin, H.; Loft, S.; Moller, P. Modest effect on plaque progression and vasodilatory function in atherosclerosis-prone mice exposed to nanosized TiO(2). Part. Fibre Toxicol 2011, 8, 32. [Google Scholar]
- Nurkiewicz, T.R.; Porter, D.W.; Hubbs, A.F.; Stone, S.; Chen, B.T.; Frazer, D.G.; Boegehold, M.A.; Castranova, V. Pulmonary nanoparticle exposure disrupts systemic microvascular nitric oxide signaling. Toxicol. Sci 2009, 110, 191–203. [Google Scholar]
- LeBlanc, A.J.; Moseley, A.M.; Chen, B.T.; Frazer, D.; Castranova, V.; Nurkiewicz, T.R. Nanoparticle inhalation impairs coronary microvascular reactivity via a local reactive oxygen species-dependent mechanism. Cardiovasc. Toxicol 2010, 10, 27–36. [Google Scholar]
- LeBlanc, A.J.; Cumpston, J.L.; Chen, B.T.; Frazer, D.; Castranova, V.; Nurkiewicz, T.R. Nanoparticle inhalation impairs endothelium-dependent vasodilation in subepicardial arterioles. J. Toxic. Environ. Health A 2009, 72, 1576–1584. [Google Scholar]
- Rosas-Hernandez, H.; Jimenez-Badillo, S.; Martinez-Cuevas, P.P.; Gracia-Espino, E.; Terrones, H.; Terrones, M.; Hussain, S.M.; Ali, S.F.; Gonzalez, C. Effects of 45-nm silver nanoparticles on coronary endothelial cells and isolated rat aortic rings. Toxicol. Lett 2009, 191, 305–313. [Google Scholar]
- Coccini, T.; Roda, E.; Barni, S.; Signorini, C.; Manzo, L. Long-lasting oxidative pulmonary insult in rat after intratracheal instillation of silica nanoparticles doped with cadmium. Toxicology 2012, 302, 203–211. [Google Scholar]
- Zhu, M.T.; Wang, B.; Wang, Y.; Yuan, L.; Wang, H.J.; Wang, M.; Ouyang, H.; Chai, Z.F.; Feng, W.Y.; Zhao, Y.L. Endothelial dysfunction and inflammation induced by iron oxide nanoparticle exposure: Risk factors for early atherosclerosis. Toxicol. Lett 2011, 203, 162–171. [Google Scholar]
- Weiner, C.P.; Knowles, R.G.; Moncada, S. Induction of nitric oxide synthases early in pregnancy. Am. J. Obstet. Gynecol 1994, 171, 838–843. [Google Scholar]
- Liu, H.C.; Hsu, J.Y.; Cheng, Y.W.; Chou, M.C. Exhaled nitric oxide in a Taiwanese population: Age and lung function as predicting factors. J. Formos. Med. Assoc 2009, 108, 772–777. [Google Scholar]
- Balint, B.; Donnelly, L.E.; Hanazawa, T.; Kharitonov, S.A.; Barnes, P.J. Increased nitric oxide metabolites in exhaled breath condensate after exposure to tobacco smoke. Thorax 2001, 56, 456–461. [Google Scholar]
- Olin, A.C.; Rosengren, A.; Thelle, D.S.; Lissner, L.; Bake, B.; Toren, K. Height, age, and atopy are associated with fraction of exhaled nitric oxide in a large adult general population sample. Chest 2006, 130, 1319–1325. [Google Scholar]
- Hoyt, J.C.; Robbins, R.A.; Habib, M.; Springall, D.R.; Buttery, L.D.; Polak, J.M.; Barnes, P.J. Cigarette smoke decreases inducible nitric oxide synthase in lung epithelial cells. Exp. Lung Res 2003, 29, 17–28. [Google Scholar]
- Wei, X.M.; Kim, H.S.; Kumar, R.K.; Heywood, G.J.; Hunt, J.E.; McNeil, H.P.; Thomas, P.S. Effects of cigarette smoke on degranulation and NO production by mast cells and epithelial cells. Respir. Res 2005, 6, 108. [Google Scholar]
- Su, Y.; Han, W.; Giraldo, C.; de Li, Y.; Block, E.R. Effect of cigarette smoke extract on nitric oxide synthase in pulmonary artery endothelial cells. Am. J. Respir. Cell Mol. Biol 1998, 19, 819–825. [Google Scholar]
- Robbins, R.A.; Millatmal, T.; Lassi, K.; Rennard, S.; Daughton, D. Smoking cessation is associated with an increase in exhaled nitric oxide. Chest 1997, 112, 313–318. [Google Scholar]
- Hogman, M.; Holmkvist, T.; Walinder, R.; Merilainen, P.; Ludviksdottir, D.; Hakansson, L.; Hedenstrom, H. Increased nitric oxide elimination from the airways after smoking cessation. Clin. Sci 2002, 103, 15–19. [Google Scholar]
- ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am. J. Respir. Crit. Care Med 2005, 171, 912–930.
- Gabriele, C.; Pijnenburg, M.W.; Monti, F.; Hop, W.; Bakker, M.E.; de Jongste, J.C. The effect of spirometry and exercise on exhaled nitric oxide in asthmatic children. Pediatr. Allergy Immunol 2005, 16, 243–247. [Google Scholar]
- Persson, M.G.; Wiklund, N.P.; Gustafsson, L.E. Endogenous nitric oxide in single exhalations and the change during exercise. Am. Rev. Respir. Dis 1993, 148, 1210–1214. [Google Scholar]
- Xie, Q.W.; Kashiwabara, Y.; Nathan, C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J. Biol. Chem 1994, 269, 4705–4708. [Google Scholar]
- Kharitonov, S.A.; Barnes, P.J. Exhaled markers of pulmonary disease. Am. J. Respir. Crit. Care Med 2001, 163, 1693–1722. [Google Scholar]
- Riediker, M.; Schubauer-Berigan, M.K.; Brouwer, D.H.; Nelissen, I.; Koppen, G.; Frijns, E.; Clark, K.A.; Hoeck, J.; Liou, S.H.; Ho, S.F.; et al. A road map toward a globally harmonized approach for occupational health surveillance and epidemiology in nanomaterial workers. J. Occup. Environ. Med 2012, 54, 1214–1223. [Google Scholar]
- Brouwer, D. Exposure to manufactured nanoparticles in different workplaces. Toxicology 2010, 269, 120–127. [Google Scholar]
- Brouwer, D.; Berges, M.; Virji, M.A.; Fransman, W.; Bello, D.; Hodson, L.; Gabriel, S.; Tielemans, E. Harmonization of measurement strategies for exposure to manufactured nano-objects; report of a workshop. Ann. Occup. Hyg 2012, 56, 1–9. [Google Scholar]
- Dahm, M.M.; Evans, D.E.; Schubauer-Berigan, M.K.; Birch, M.E.; Fernback, J.E. Occupational exposure assessment in carbon nanotube and nanofiber primary and secondary manufacturers. Ann. Occup. Hyg 2012, 56, 542–556. [Google Scholar]
- Kuhlbusch, T.A.; Asbach, C.; Fissan, H.; Gohler, D.; Stintz, M. Nanoparticle exposure at nanotechnology workplaces: A review. Part. Fibre Toxicol 2011, 8, 22. [Google Scholar]
- Borm, P.J.; Robbins, D.; Haubold, S.; Kuhlbusch, T.; Fissan, H.; Donaldson, K.; Schins, R.; Stone, V.; Kreyling, W.; Lademann, J.; et al. The potential risks of nanomaterials: A review carried out for ECETOC. Part. Fibre Toxicol 2006, 3, 11. [Google Scholar]
- Paik, S.Y.; Zalk, D.M.; Swuste, P. Application of a pilot control banding tool for risk level assessment and control of nanoparticle exposures. Ann. Occup. Hyg 2008, 52, 419–428. [Google Scholar]
- Brouwer, D.H. Control banding approaches for nanomaterials. Ann. Occup. Hyg 2012, 56, 506–514. [Google Scholar]
- Sambrook, J.; Russell, D.W. Assay for luciferase in extracts of Mammalian cells. CSH Protoc 2006. [Google Scholar] [CrossRef]
- Schulte, P.A.; Schubauer-Berigan, M.K.; Mayweather, C.; Geraci, C.L.; Zumwalde, R.; McKernan, J.L. Issues in the development of epidemiologic studies of workers exposed to engineered nanoparticles. J. Occup. Environ. Med 2009, 51, 323–335. [Google Scholar]
- Laney, A.S.; McCauley, L.A.; Schubauer-Berigan, M.K. Workshop summary: Epidemiologic design strategies for studies of nanomaterial workers. J. Occup. Environ. Med 2011, 53, S87–S90. [Google Scholar]
- Methner, M.; Hodson, L.; Dames, A.; Geraci, C. Nanoparticle Emission Assessment Technique (NEAT) for the identification and measurement of potential inhalation exposure to engineered nanomaterials—Part B: Results from 12 field studies. J. Occup.Environ. Hyg 2010, 7, 163–176. [Google Scholar]
- Demou, E.; Stark, W.J.; Hellweg, S. Particle emission and exposure during nanoparticle synthesis in research laboratories. Ann. Occup. Hyg 2009, 53, 829–838. [Google Scholar]
- Demou, E.; Peter, P.; Hellweg, S. Exposure to manufactured nanostructured particles in an industrial pilot plant. Ann. Occup. Hyg 2008, 52, 695–706. [Google Scholar]
- Dahm, M.M.; Evans, D.E.; Schubauer-Berigan, M.K.; Birch, M.E.; Deddens, J.A. Occupational exposure assessment in carbon nanotube and nanofiber primary and secondary manufacturers: Mobile direct-reading sampling. Ann. Occup. Hyg 2013, 57, 328–344. [Google Scholar]
- Zalk, D.M.; Paik, S.Y.; Swuste, P. Evaluating the control banding nanotool: A qualitative risk assessment method for controlling nanoparticle exposures. J. Nanopart. Res 2009, 11, 1685–1704. [Google Scholar]
- Van Duuren-Stuurman, B.; Vink, S.R.; Verbist, K.J.; Heussen, H.G.; Brouwer, D.H.; Kroese, D.E.; van Niftrik, M.F.; Tielemans, E.; Fransman, W. Stoffenmanager Nano version 1.0: A web-based tool for risk prioritization of airborne manufactured nano objects. Ann. Occup. Hyg 2012, 56, 525–541. [Google Scholar]
- Ostiguy, C.; Riediker, M.; Triolet, J.; Troisfontaines, P.; Vernez, D. Development of a Specific Control Banding Tool for Nanomaterials; French Agency for Food, Environmental and Occupational Health and Safety: Maisons-Alfort, France, 2010. [Google Scholar]
- Riediker, M.; Ostiguy, C.; Triolet, J.; Troisfontaine, P.; Vernez, D.; Bourdel, G.; Thieriet, N.; Cadène, A. Development of a control banding tool for nanomaterials. J. Nanomater 2012, 2012. [Google Scholar] [CrossRef]
- Höck, J.; Epprecht, T.; Furrer, E.; Hofmann, H.; Höhner, K.; Krug, H.; Lorenz, C.; Limbach, L.; Gehr, P.; Nowack, B.; et al. Precautionary Matrix for Synthetic Nanomaterials; Federal Office of Public Health FOPH: Berne, Switzerland, 2011. [Google Scholar]
- National Institute for Occupational Safety and Health, Education and Information Division; American Industrial Hygiene Association, Risk Assessment, Committee, Qualitative Risk Characterization and Management of Occupational Hazards: Control Banding (CB)—Literature Review and Critical Analysis; National Institute for Occupational Health and Safety: Cincinnati, OH, USA, 2009.
- CB Nanotool 2.0. Available online: http://www.controlbanding.net/Services.html (accessed on 16 March 2010).
- Smith, A.D.; Cowan, J.O.; Brassett, K.P.; Herbison, G.P.; Taylor, D.R. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N. Engl. J. Med 2005, 352, 2163–2173. [Google Scholar]
- Dweik, R.A.; Sorkness, R.L.; Wenzel, S.; Hammel, J.; Curran-Everett, D.; Comhair, S.A.; Bleecker, E.; Busse, W.; Calhoun, W.J.; Castro, M.; et al. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am. J. Respir. Crit. Care Med 2010, 181, 1033–1041. [Google Scholar]
- Bora, M.; Alpaydin, A.O.; Yorgancioglu, A.; Akkas, G.; Isisag, A.; Coskun, A.S.; Celik, P. Does asthma control as assessed by the asthma control test reflect airway inflammation? Multidiscip. Respir. Med 2011, 6, 291–298. [Google Scholar]
| Variables | All subjects (n = 437) | |||
|---|---|---|---|---|
| Exhaled nitric oxide (ppb) | p-Value a | |||
| n | Mean | (SD) | ||
| Age | 0.650 | |||
| ≤40 years | 317 | 20.7 | (17.8) | |
| >40 years | 120 | 20.2 | (15.5) | |
| Gender | 0.011 | |||
| Male | 300 | 21.7 | (18.0) | |
| Female | 137 | 18.0 | (15.0) | |
| Ethnic groups | 0.084 | |||
| Hoklo | 341 | 19.8 | (15.9) | |
| Hakka | 55 | 26.6 | (25.6) | |
| Others | 39 | 19.4 | (12.1) | |
| Education | 0.253 | |||
| ≤Senior high and vocational school | 68 | 16.5 | (8.6) | |
| University and College | 206 | 21.3 | (18.5) | |
| ≥Graduate School | 159 | 21.4 | (17.9) | |
| Cigarette smoking | 0.011 | |||
| Current smokers | 66 | 15.5 | (10.6) | |
| Never smokers | 368 | 21.4 | (17.9) | |
| Alcohol use | 0.998 | |||
| Yes | 40 | 19.0 | (12.2) | |
| No | 396 | 20.8 | (17.6) | |
| Exercise before tests | 0.008 | |||
| Yes | 24 | 13.3 | (8.5) | |
| No | 410 | 21.0 | (17.5) | |
| Risk levels | 0.040 | |||
| Control | 196 | 20.5 | (19.1) | |
| Risk level 1 | 126 | 18.1 | (12.2) | |
| Risk level 2 | 115 | 23.3 | (18.2) | |
| Control and nanomaterial exposed groups | 0.068 | |||
| Control | 196 | 20.5 | (19.1) | |
| Carbon nanotube | 57 | 24.1 | (18.7) | |
| Nano-TiO2 | 17 | 28.0 | (21.5) | |
| Nano-SiO2 | 36 | 17.9 | (10.8) | |
| Nano-Ag | 16 | 20.8 | (11.3) | |
| Other NM exposure | 54 | 16.5 | (10.6) | |
| More than two types of NM exposure | 61 | 20.3 | (16.3) | |
| Disease History | ||||
| Chronic bronchitis | 0.139 | |||
| Yes | 23 | 25.8 | (20.1) | |
| No | 412 | 20.3 | (17.0) | |
| Asthma | <0.001 | |||
| Yes | 9 | 58.7 | (37.8) | |
| No | 425 | 19.8 | (15.6) | |
| Allergic rhinitis | <0.001 | |||
| Yes | 80 | 27.1 | (22.8) | |
| No | 355 | 19.2 | (15.4) | |
| Atopic dermatitis | 0.257 | |||
| Yes | 27 | 24.2 | (15.6) | |
| No | 409 | 20.4 | (17.3) | |
| Hypertension | 0.731 | |||
| Yes | 31 | 19.1 | (10.3) | |
| No | 402 | 20.7 | (17.7) | |
| LnFENO | Age (years) | Height (cm) | Weight (kg) | LnNF-κB (EBC) | |
|---|---|---|---|---|---|
| Age (years) | 0.033 | 1 | |||
| Height (cm) | 0.098 * | −0.231 ** | 1 | ||
| Weight (kg) | 0.105 * | −0.083 | 0.631 ** | 1 | |
| LnNF-κB (EBC) | 0.188 ** | 0.031 | 0.141 ** | 0.126 ** | 1 |
| FEV1.0% | 0.028 | −0.240 ** | −0.100 * | −0.207 ** | −0.006 |
| FVC (%) | −0.048 | 0.000 | 0.141 ** | 0.117 * | −0.058 |
| MMF (%) | −0.021 | −0.052 | −0.086 | −0.107 * | −0.059 |
| PEFR (%) | 0.098 * | 0.048 | −0.022 | 0.065 | −0.048 |
| FEF25 (%) | 0.056 | −0.005 | 0.021 | 0.082 | −0.048 |
| FEF50 (%) | −0.035 | −0.073 | −0.075 | −0.037 | −0.069 |
| FEF75 (%) | −0.036 | −0.149 ** | 0.014 | −0.138 ** | −0.046 |
| FEV1/FVC | 0.012 | 0.285 ** | −0.215 ** | −0.168 ** | −0.061 |
| Mode1 (Enter) | Mode2 (Stepwise) | |||||
|---|---|---|---|---|---|---|
| β | SE | p-Value | β | SE | p-Value | |
| Risk levels | ||||||
| RL1 vs. control | −0.050 | 0.076 | 0.506 | −0.056 | 0.075 | 0.459 |
| RL2 vs. control | 0.178 | 0.078 | 0.023 | 0.170 | 0.077 | 0.028 |
| Age (years) | 0.006 | 0.004 | 0.143 | |||
| Gender (Male vs. Female) | 0.118 | 0.102 | 0.250 | 0.198 | 0.071 | 0.006 |
| Height (cm) | 0.006 | 0.006 | 0.327 | |||
| Weight (kg) | 0.001 | 0.003 | 0.836 | |||
| Cigarette smoking (Yes vs. No) | −0.237 | 0.093 | 0.011 | −0.233 | 0.093 | 0.012 |
| Exercise before tests (Yes vs. No) | −0.296 | 0.139 | 0.034 | −0.295 | 0.138 | 0.034 |
| Asthma (Yes vs. No) | 1.020 | 0.236 | <0.001 | 1.042 | 0.222 | <0.001 |
| Allergic rhinitis (Yes vs. No) | 0.289 | 0.081 | <0.001 | 0.289 | 0.080 | <0.001 |
| PEFR (%) | 0.006 | 0.002 | 0.001 | 0.006 | 0.002 | 0.001 |
| LnNF-κB (EBC) | 0.204 | 0.069 | 0.003 | 0.213 | 0.068 | 0.002 |
| Mode1 (Enter) | Mode2 (Stepwise) | |||||
|---|---|---|---|---|---|---|
| β | SE | p-Value | β | SE | p-Value | |
| Nanomaterials exposure | ||||||
| Carbon nanotube vs. control | 0.045 | 0.124 | 0.715 | 0.030 | 0.122 | 0.807 |
| Nano-TiO2 vs. control | 0.351 | 0.166 | 0.035 | 0.334 | 0.165 | 0.044 |
| Nano-SiO2 vs. control | 0.007 | 0.120 | 0.956 | 0.022 | 0.119 | 0.857 |
| Nano-Ag vs. control | 0.153 | 0.170 | 0.367 | 0.127 | 0.169 | 0.452 |
| Other NM exposure vs. control | −0.039 | 0.100 | 0.695 | −0.049 | 0.100 | 0.623 |
| More than two types of NM exposure vs. control | 0.051 | 0.098 | 0.600 | 0.052 | 0.097 | 0.591 |
| Age (years) | 0.006 | 0.004 | 0.131 | |||
| Gender (Male vs. Female) | 0.099 | 0.104 | 0.342 | 0.182 | 0.073 | 0.013 |
| Height (cm) | 0.005 | 0.006 | 0.425 | |||
| Weight (kg) | 0.002 | 0.003 | 0.625 | |||
| Cigarette smoking (Yes vs. No) | −0.222 | 0.094 | 0.018 | −0.219 | 0.093 | 0.019 |
| Exercise before tests (Yes vs. No) | −0.275 | 0.141 | 0.051 | −0.274 | 0.140 | 0.051 |
| Asthma (Yes vs. No) | 1.008 | 0.239 | <0.001 | 1.053 | 0.225 | <0.001 |
| Allergic rhinitis (Yes vs. No) | 0.289 | 0.082 | <0.001 | 0.290 | 0.081 | <0.001 |
| PEFR (%) | 0.006 | 0.002 | 0.002 | 0.006 | 0.002 | 0.001 |
| LnNF-κB (EBC) | 0.185 | 0.070 | 0.009 | 0.196 | 0.069 | 0.005 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, W.-T.; Liao, H.-Y.; Chung, Y.-T.; Li, W.-F.; Tsou, T.-C.; Li, L.-A.; Lin, M.-H.; Ho, J.-J.; Wu, T.-N.; Liou, S.-H. Effect of Nanoparticles Exposure on Fractional Exhaled Nitric Oxide (FENO) in Workers Exposed to Nanomaterials. Int. J. Mol. Sci. 2014, 15, 878-894. https://doi.org/10.3390/ijms15010878
Wu W-T, Liao H-Y, Chung Y-T, Li W-F, Tsou T-C, Li L-A, Lin M-H, Ho J-J, Wu T-N, Liou S-H. Effect of Nanoparticles Exposure on Fractional Exhaled Nitric Oxide (FENO) in Workers Exposed to Nanomaterials. International Journal of Molecular Sciences. 2014; 15(1):878-894. https://doi.org/10.3390/ijms15010878
Chicago/Turabian StyleWu, Wei-Te, Hui-Yi Liao, Yu-Teh Chung, Wan-Fen Li, Tsui-Chun Tsou, Lih-Ann Li, Ming-Hsiu Lin, Jiune-Jye Ho, Trong-Neng Wu, and Saou-Hsing Liou. 2014. "Effect of Nanoparticles Exposure on Fractional Exhaled Nitric Oxide (FENO) in Workers Exposed to Nanomaterials" International Journal of Molecular Sciences 15, no. 1: 878-894. https://doi.org/10.3390/ijms15010878




