Identification of the High Molecular Weight Isoform of Phostensin
Abstract
:1. Introduction
2. Results and Discussion
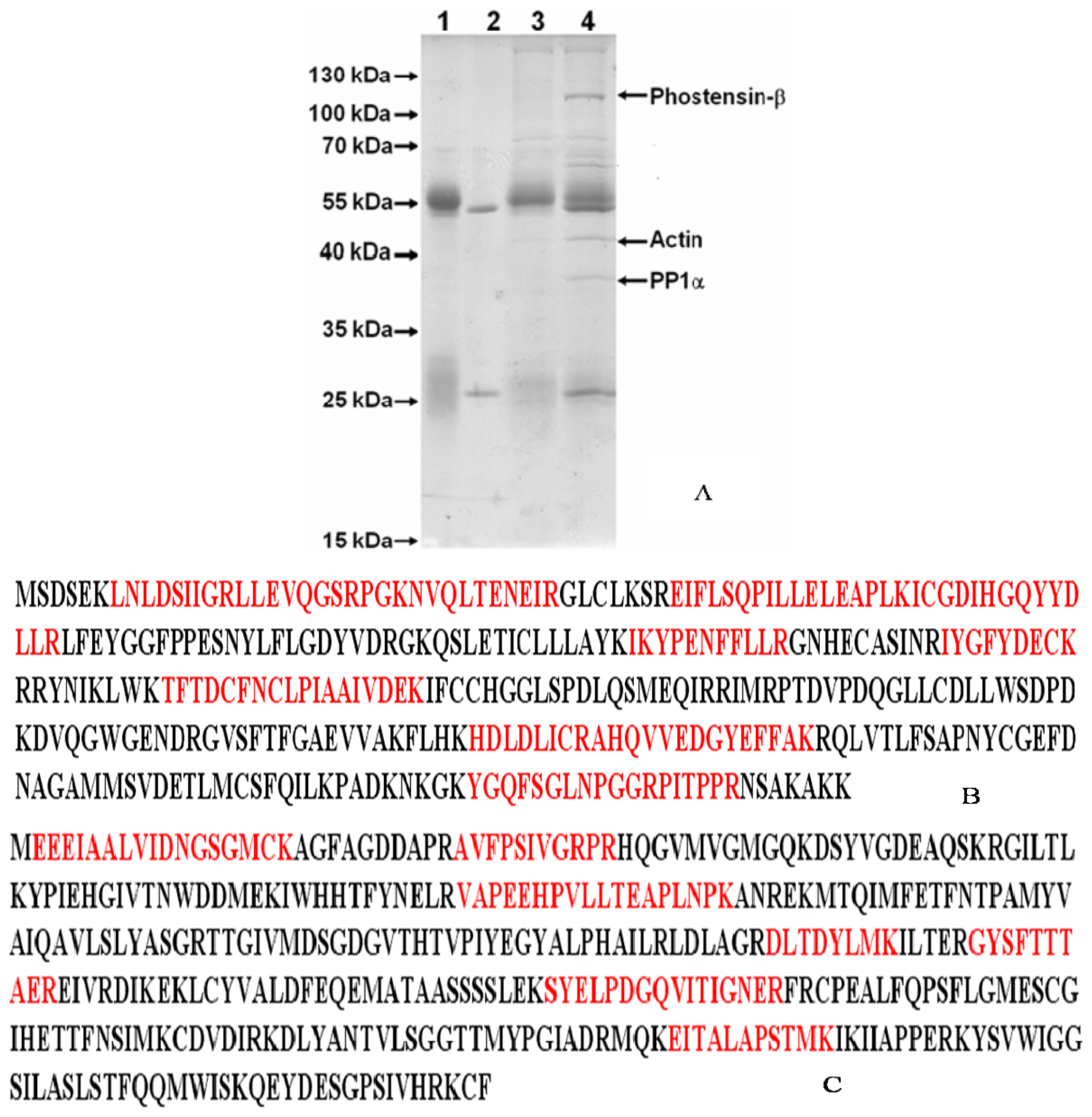
2.1. Identification of the High Molecular Weight of Phostensin
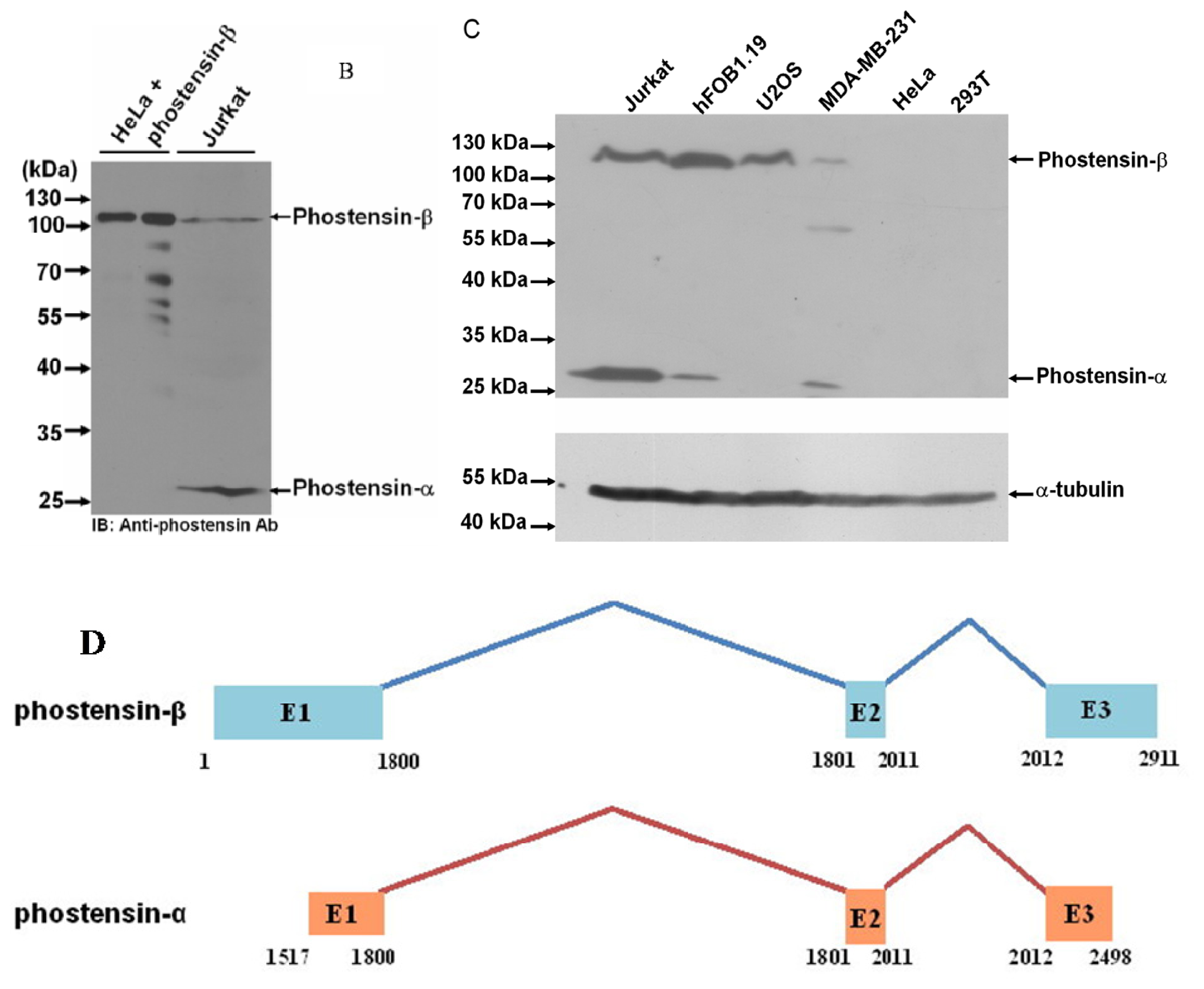
2.2. Phostensin-α Is not a Degraded Form of Phostensin-β
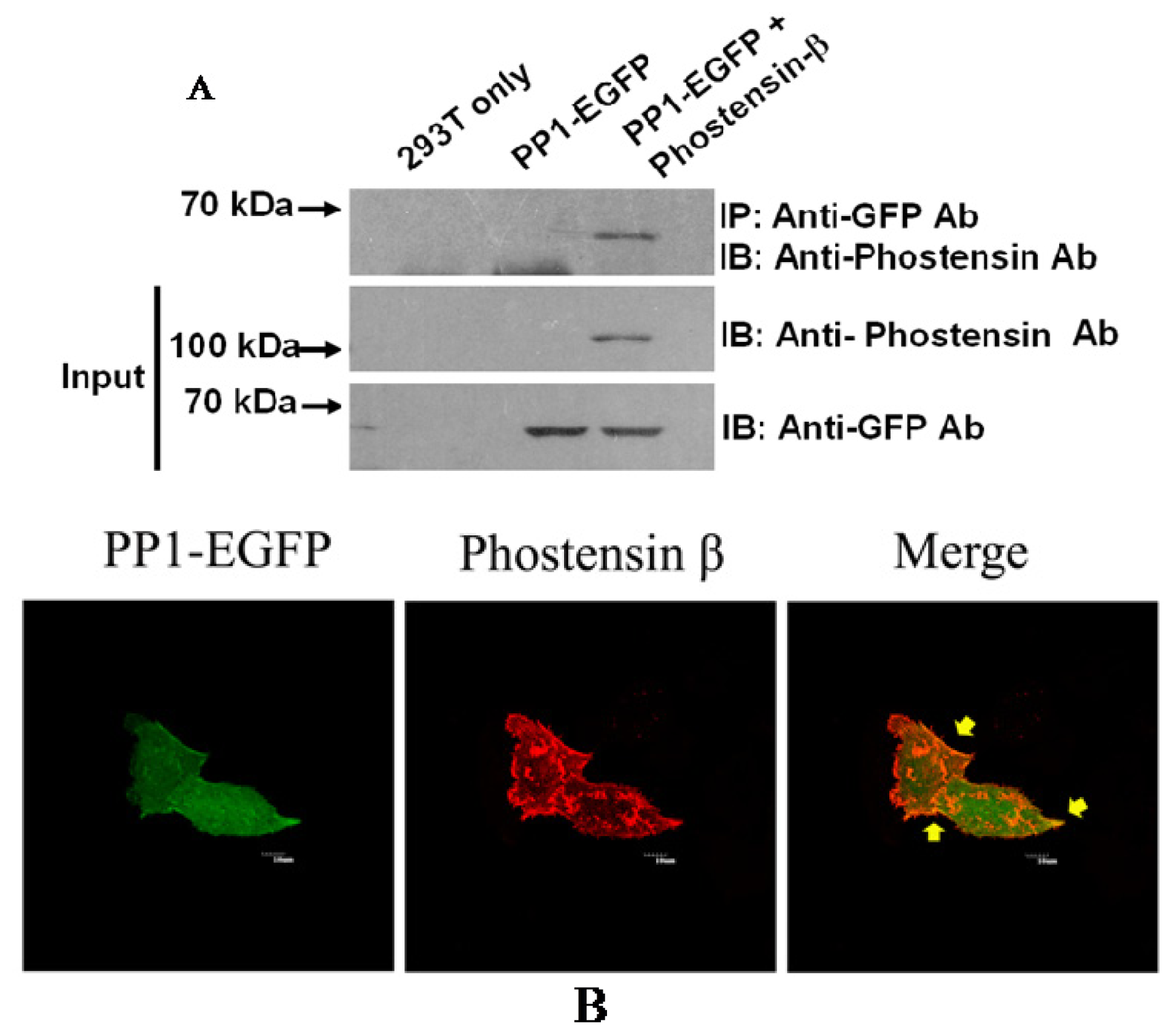
2.3. Phostensin-β Binds to PP1 and F-Actin
3. Experimental Section
3.1. Materials
3.2. Identification of Phostensin-β
3.3. Cell Culture and Transfection
3.4. Chase the Degradation Process of Phostensin
3.5. Immunoprecipitation
3.6. Immunocytochemistry
4. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsYSL and HLH contributed to method design, experiments and data analysis. WTL contributed to data analysis. THL and HBH contributed to method design, data analysis and manuscript writing.
References
- Kao, S.C.; Chen, C.Y.; Wang, S.L.; Yang, J.J.; Hung, W.C.; Chen, Y.C.; Lai, N.S.; Liu, H.T.; Huang, H.L.; Chen, H.C.; et al. Identification of phostensin, a PP1 F-actin cytoskeleton targeting subunit. Biochem. Biophys. Res. Commun 2007, 356, 594–598. [Google Scholar]
- Nagase, T.; Kikuno, R.; Ohara, O. Prediction of the coding sequences of unidentified human genes. XXII. The complete sequences of 50 new cDNA clones which code for large proteins. DNA Res 2001, 8, 319–327. [Google Scholar]
- Shigenari, A.; Ando, A.; Renard, C.; Chardon, P.; Shiina, T.; Kulski, J.K.; Yasue, H.; Inoko, H. Nucleotide sequencing analysis of the swine 433-kb genomic segment located between the non-classical and classical SLA class I gene clusters. Immunogenetics 2004, 55, 695–705. [Google Scholar]
- Lin, Y.S.; Huang, K.Y.; Wang, T.F.; Huang, H.L.; Yu, H.C.; Yen, J.Y.; Hung, S.H.; Liu, S.Q.; Lai, N.S.; Huang, H.B. Immunolocalization of phostensin in lymphatic cells and tissues. J. Histochem. Cytochem 2011, 59, 741–749. [Google Scholar]
- Lai, N.S.; Wang, T.F.; Wang, S.L.; Chen, C.Y.; Yen, J.Y.; Huang, H.L.; Li, C.; Huang, K.Y.; Liu, S.Q.; Lin, T.H.; et al. Phostensin caps to the pointed end of actin filaments and modulates actin dynamics. Biochem. Biophys. Res. Commun 2009, 387, 676–681. [Google Scholar]
- Wang, T.F.; Lai, N.S.; Huang, K.Y.; Huang, H.L.; Lu, M.C.; Lin, Y.S.; Chen, C.Y.; Liu, S.Q.; Lin, T.H.; Huang, H.B. Identification and characterization of the actin-binding motif of phostensin. Int. J. Mol. Sci 2012, 13, 15967–15982. [Google Scholar]
- Su, Y.A.; Yang, J.; Tao, L.; Nguyen, H.; He, P. Undetectable and decreased expression of KIAA1949 (Phostensin) encoded on chromosome 6p21.33 in human breast cancers revealed by transcriptome analysis. J. Cancer 2010, 1, 38–50. [Google Scholar]
- Negrini, M.; Sabbioni, S.; Possati, L.; Rattan, S.; Corallini, A.; Barbanti-Brodano, G.; Croce, C.M. Suppression of tumorigenicity of breast cancer cells by microcell-mediated chromosome transfer: Studies on chromosomes 6 and 11. Cancer Res 1994, 54, 1331–1336. [Google Scholar]
- Theile, M.; Seitz, S.; Arnold, W.; Jandrig, B.; Frege, R.; Schlag, P.M.; Haensch, W.; Guski, H.; Winzer, K.J.; Barrett, J.C.; et al. A defined chromosome 6q fragment (at D6S310) harbors a putative tumor suppressor gene for breast cancer. Oncogene 1996, 13, 677–685. [Google Scholar]
- Trent, J.M.; Stanbridge, E.J.; McBride, H.L.; Meese, E.U.; Casey, G.; Araujo, D.E.; Witkowski, C.M.; Nagle, R.B. Tumorigenicity in human melanoma cell lines controlled by introduction of human chromosome 6. Science 1990, 247, 568–571. [Google Scholar]
- Cohen, P. The structure and regulation of protein phosphatases. Annu. Rev. Biochem 453–508.
- Cohen, P.T.W. Protein phosphatase 1—Targeted in many directions. J. Cell Sci 2002, 115, 241–256. [Google Scholar]
- Shenolikar, S. Protein serine/threonine phosphatases—New avenues for cell regulation. Annu. Rev. Cell Biol 1994, 10, 55–86. [Google Scholar]



© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lin, Y.-S.; Huang, H.-L.; Liu, W.-T.; Lin, T.-H.; Huang, H.-B. Identification of the High Molecular Weight Isoform of Phostensin. Int. J. Mol. Sci. 2014, 15, 1068-1079. https://doi.org/10.3390/ijms15011068
Lin Y-S, Huang H-L, Liu W-T, Lin T-H, Huang H-B. Identification of the High Molecular Weight Isoform of Phostensin. International Journal of Molecular Sciences. 2014; 15(1):1068-1079. https://doi.org/10.3390/ijms15011068
Chicago/Turabian StyleLin, Yu-Shan, Hsien-Lu Huang, Wei-Ting Liu, Ta-Hsien Lin, and Hsien-Bin Huang. 2014. "Identification of the High Molecular Weight Isoform of Phostensin" International Journal of Molecular Sciences 15, no. 1: 1068-1079. https://doi.org/10.3390/ijms15011068



