Magnetic Nanosystem for Cancer Therapy Using Oncocalyxone A, an Antitomour Secondary Metabolite Isolated from a Brazilian Plant
Abstract
:1. Introduction
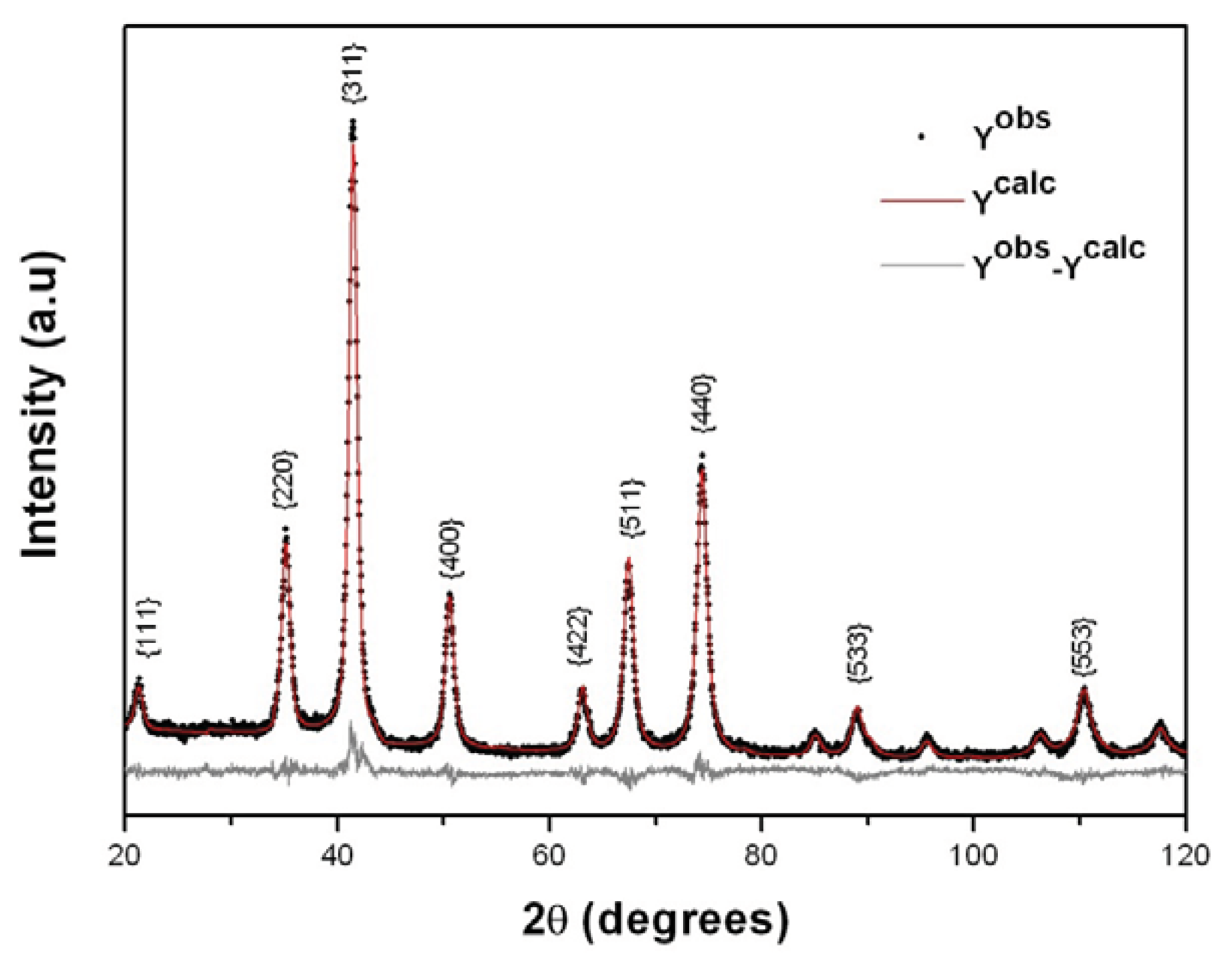
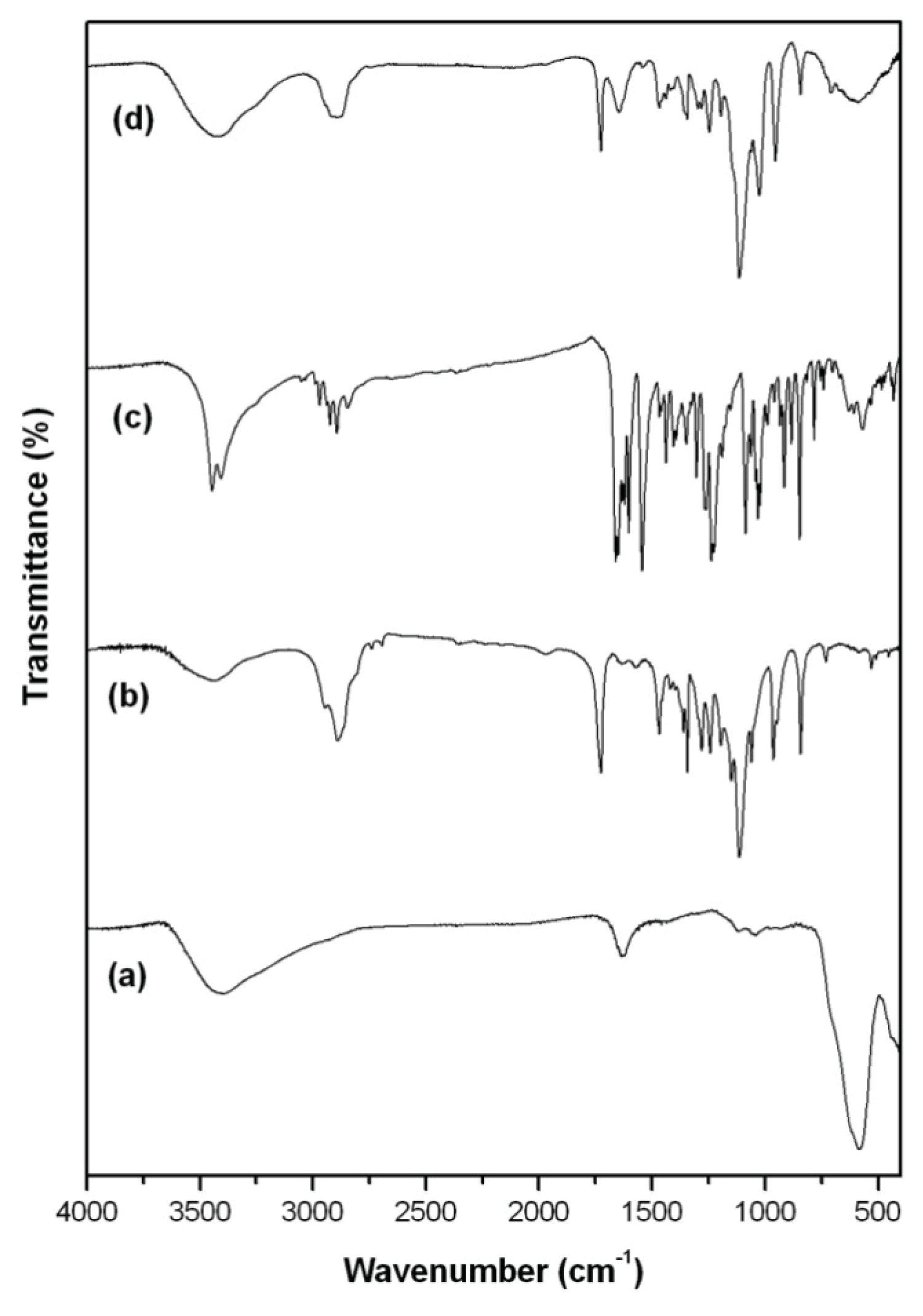
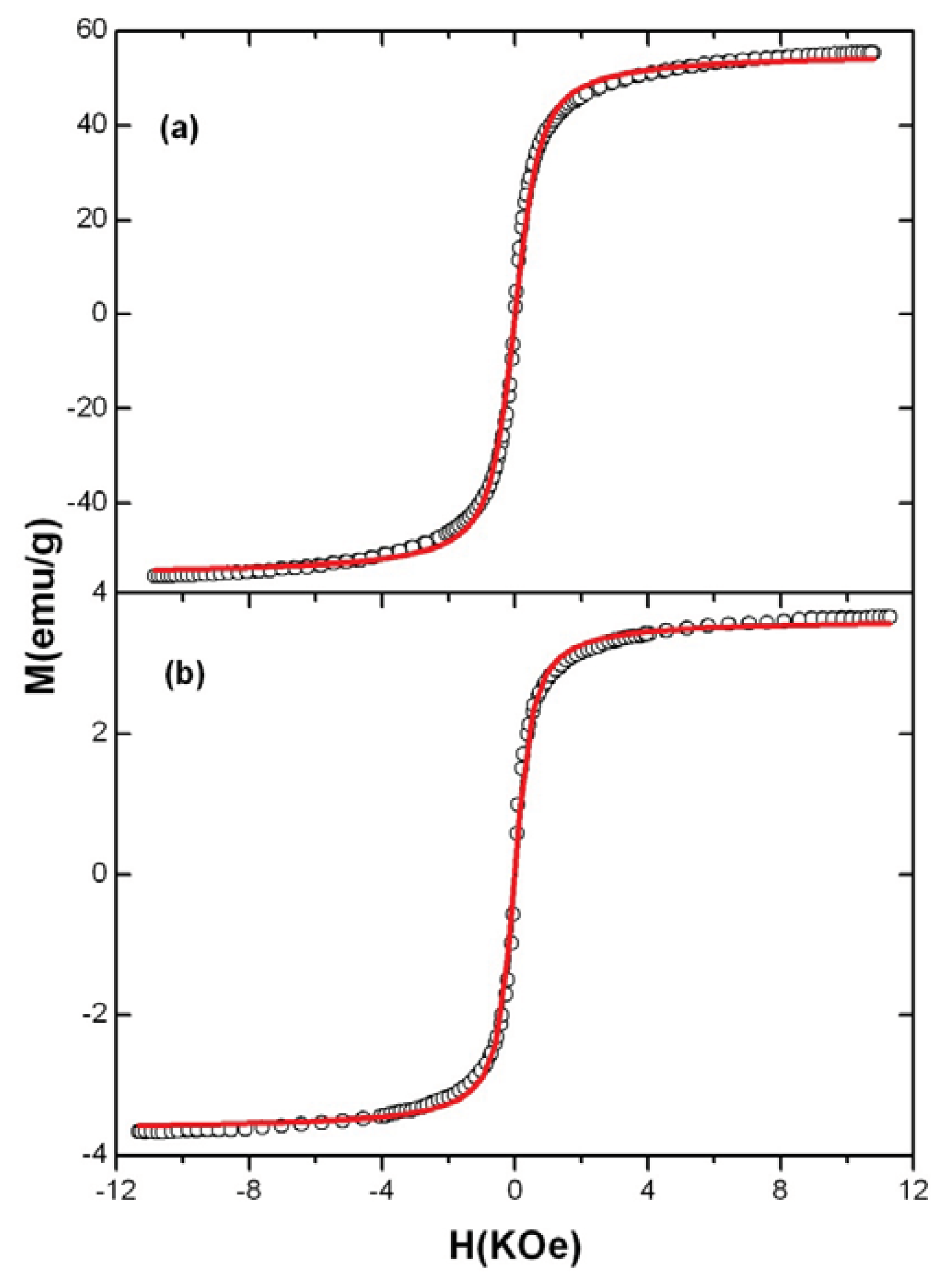
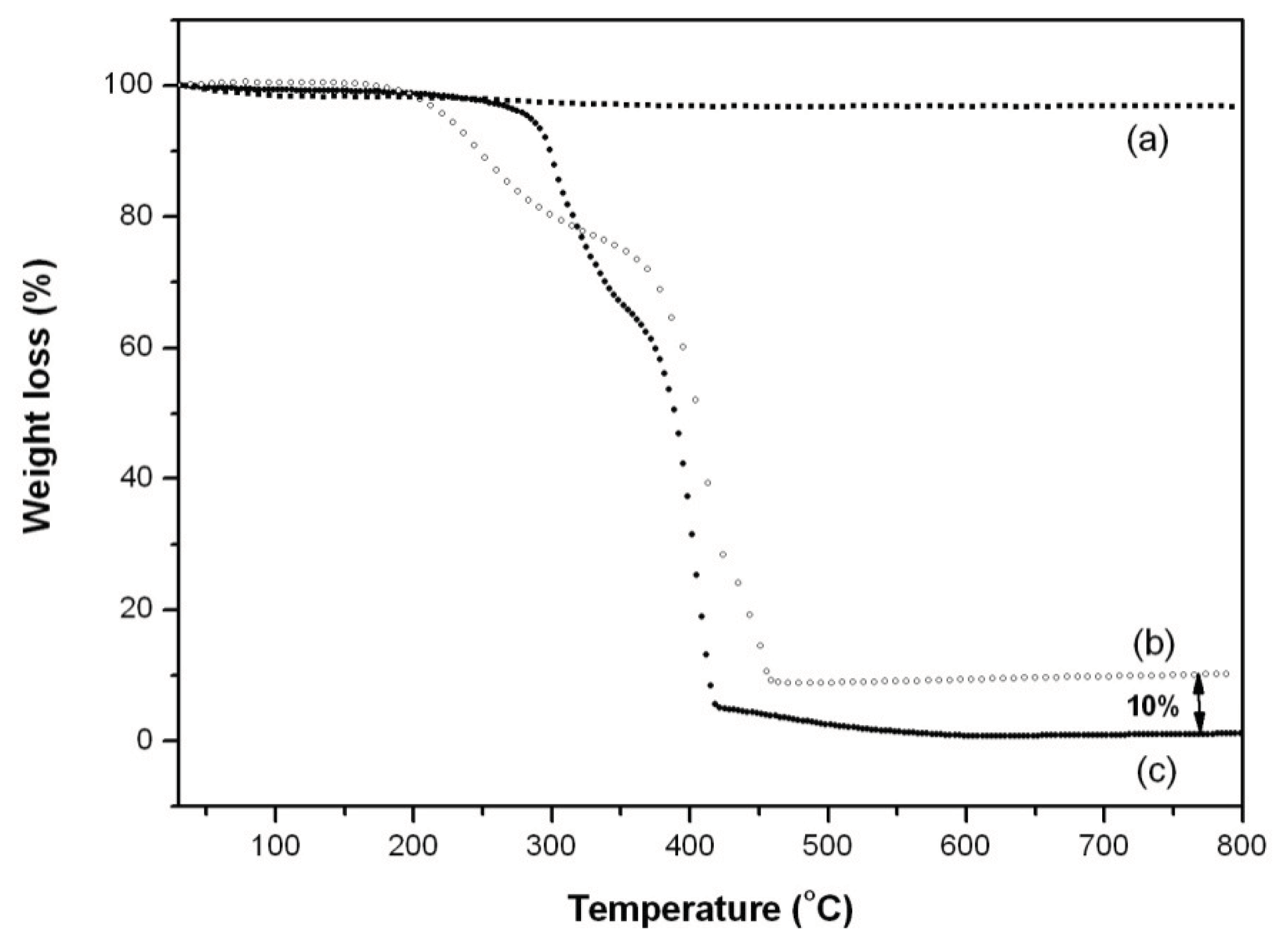
2. Results and Discussion
3. Material and Methods
3.1. Materials
3.2. Oleic Acid-Coated Fe3O4 (OA-Fe3O4)
3.3. Synthesis of OA-Fe3O4 Coated with Onco A-E114Cl20 (MO-20)
3.4. Oncocalyxone Releasing from Copolymer E114Cl20
3.5. Characterization
4. Conclusions
Acknowledgements
Conflicts of Interest
References
- Pessoa, O.D.L.; de Lemos, T.L.G.; Silveira, E.R.; Braz-Filho, R. Novel cordiachromes isolated from Auxemma oncocalyx. Nat. Prod. Lett 1993, 2, 145–150. [Google Scholar]
- Ferreira, M.A.D.; Nunes, O.D.R.H.; Fentenele, J.B.; Pessoa, O.D.L.; de Lemos, T.L.G.; Viana, G.S.B. Analgesic and anti-inflammatory activities of a fraction rich in oncocalyxone A isolated from Auxemma oncocalyx. Phytomedicine 2004, 11, 315–322. [Google Scholar]
- Ferreira, M.A.D.; Nascimento, N.R.F.; Sousa, C.M.; Pessoa, O.D.L.; de Lemos, T.L.G.; Ventura, J.S.; Schattner, M.; Chudzinski-Travassi, A.M. Oncocalyxone A inhibits human platelet aggregation by increasing cGMP and by binding to GP Ibα glycoprotein. Br. J. Pharmacol 2008, 154, 1216–1224. [Google Scholar]
- Pessoa, O.D.L.; de Lemos, T.L.G.; de Carvalho, M.G.; Braz-Filho, R. Cordiachoromes from Auxemma oncocalyx. Phytochemistry 1995, 40, 1777–1786. [Google Scholar]
- Marques, W.B.; dos Santos, H.S.; Pessoa, O.D.L.; Braz-Filho, R.; de Lemos, T.L.G. Anthracene derivatives from Auxemma oncocalyx. Phytochemistry 2000, 55, 793–797. [Google Scholar]
- Costa, C.O.C.; Souza, A.A.; Luz, R.C.S.; de Lemos, T.L.G.; Pessoa, O.D.L.; Kubota, L.T.; Goulart, M.O.F. Eletrochemical determination of Oncocalyxone A using an iron-phthalocyanine/iron-porphirin modified glassy carbon electrode. J. Braz. Chem. Soc 2008, 19, 697–703. [Google Scholar]
- Alexiou, C.; Schmid, R.J.; Jurgons, R.; Kremer, M.; Wanner, G.; Bergemann, C.; Huengens, E.; Nawroth, T.; Arnold, W.; Parak, F.G. Targeting cancer cells: Magnetic nanoparticles as drug carriers. Eur. Biophys. J 2006, 35, 446–450. [Google Scholar]
- Jain, T.P.; Morales, M.A.; Sahoo, S.K.; Pelecki, D.L.L.; Labhasetwar, V. Iron oxide nanoparticles for sustained delivery of anticancer agents. Mol. Pharmaceut 2005, 2, 194–205. [Google Scholar]
- Voltairas, P.A.; Fotiadis, D.I.; Michalis, L.K. Hydrodynamics of magnetic drug targeting. J. Biomech 2002, 35, 813–821. [Google Scholar]
- Hu, F.X.; Neoh, K.G.; Kang, E.T. Synthesis and in vitro anti-cancer evaluation of tamoxifen-loaded magnetite/PLLA composite nanoparticles. Biomaterials 2006, 27, 5725–5733. [Google Scholar]
- Giri, J.; Pradhan, P.; Somani, V.; Chelawat, H.; Chhatre, S.; Banerjee, R.; Bahadur, D. Synthesis and characterizations of water-based ferrofluids of substituted ferrites [Fe1−xBxFe2O4, B = Mn, Co (x = 0–1)] for biomedical applications. J. Mag. Mag. Mater 2008, 320, 724–730. [Google Scholar]
- Shubayev, V.I.; Pisanic, T.R., II; Jin, S. Magnetic nanoparticles for theragnostics. Adv. Drug Deliver. Ver. 2009, 61, 467–477. [Google Scholar]
- Barreto, A.C.H.; Santiago, V.R.; Mazzetto, S.E.; Denardin, J.C.; Lavín, R.; Mele, G.; Ribeiro, M.E.N.P.; Vieira, I.G.P.; Gonçalves, T.; Ricardo, N.M.P.S.; et al. Magnetic nanoparticles for a new drug delivery system to control quercetin for cancer chemotherapy. J. Nanopart. Res 2011, 13, 6545–6553. [Google Scholar]
- Boyer, C.; Whittaker, M.R.; Bulmus, V.; Liu, J.; Davis, T.P. The design and utility of polymer-stabilized iron-oxide nanoparticles for nanomedicine applications. NPG Asia Mater 2010, 2, 23–30. [Google Scholar]
- Doraiswamy, P.M.; Finefrock, A.E. Metals in our minds: Therapeutic implications for neurodegenerative disorders. Lanc. Neur 2004, 3, 431–434. [Google Scholar]
- Gupta, A.K.; Curtis, A.S.G. Surface modified superparamagnetic nanoparticles for drug delivery: Interaction studies with human fibroblasts in culture. J. Mater. Sci. Mater. Med 2004, 15, 493–496. [Google Scholar]
- Wang, Y.; Ng, W.Y.; Chen, Y.; Shuter, B.; Yi, J.; Ding, J.; Wang, S.C.; Feng, S.S. Formulation of superparamagnetic iron oxides by nanoparticles of biodegradable polymers for magnetic resonance imaging. Adv. Funct. Mater 2008, 18, 308–318. [Google Scholar]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Ver 2008, 108, 2064–2110. [Google Scholar]
- Cavalcante, I.M. Micelas de Copolímeros em Bloco para Administração de Fármacos Hidrofóbicos: Caraterização, Solubilização e Liberação. Ph.D. Thesis, Federal University of Ceará, Fortaleza, Brazil, 2009. [Google Scholar]
- Pessoa, C.; Silveira, E.R.; de Lemos, T.L.G.; Wetmore, L.A.; Moraes, M.O.; Leyva, A. Antiproliferative effects of compounds derived from plants of Northeast Brazil. Phytother. Res 2000, 14, 187–191. [Google Scholar]
- Leyva, A.; Pessoa, C.; Boogaerdt, F.; Sokaroski, R.; de Lemos, T.L.G.; Wetmore, L.A.; Huruta, R.R.; Moraes, M.O. Oncocalyxones A and C, 1,4-anthracenediones from Auxemma oncocalyx: Comparison with anticancer 1,9-anthracenediones. Anticancer Res 2000, 20, 1029–1031. [Google Scholar]
- Costa-Lotufo, L.V.; Ferreira, M.A.D.; de Lemos, T.L.G.; Pessoa, O.D.L.; Viana, G.S.B.; Cunha, G.M.A. Toxicity to sea urchin egg development of the quinone fraction obtained from Auxemma oncoalyx. Braz. J. Med. Biol. Res 2002, 35, 927–930. [Google Scholar]
- Braga, T.P.; Vasconcelos, I.F.; Sasaki, J.M.; Fabris, J.D.; Oliveira, D.Q.L.; Valentini, A. Magnetic composites base on hybrid spheres of aluminum oxide and superparamagnetic nanoparticles of iron oxides. J. Mag. Mag. Mater 2010, 322, 633–637. [Google Scholar]
- Kumari, A.; Yadav, S.K.; Pakade, Y.B.; Singh, B.; Yadav, S.C. Development of biodegradable nanoparticles for delivery of quercetin. Colloids Surf. B 2010, 80, 184–192. [Google Scholar]
- Hrdina, A.; Lai, E.; Li, C.; Sadi, B.; Kramer, G. A comparative study of magnetic transferability of superparamagnetic nanoparticles. J. Mag. Mag. Mater 2010, 322, 2622–2627. [Google Scholar]
- Slavov, L.; Abrashev, M.V.; Merodiiska, T.; Gelev, Ch.; Vandenberghe, R.E.; Markova-Deneva, I.; Nedkov, I. Raman spectroscopy investigation of magnetite nanoparticles in ferrofluids. J. Mag. Mag. Mater. 2010, 322, 1904–1911. [Google Scholar]
- Chinnasamy, C.N.; Narayanasamy, A.; Ponpandian, N. Mixed spinel structure in nanocrystalline NiFe2O4. Phys. Rev. B 2001, 63, 184108. [Google Scholar]
- Cao, H.; He, J.; Deng, L.; Gao, X. Fabrication of cyclodextrin-functionalized superparamagnetic Fe3O4/amino-silane core-shell nanoparticles via layer-by-layer method. Appl. Surf. Sci 2009, 255, 7974–7980. [Google Scholar]
- Ying, X.Y.; Du, Y.Z.; Hong, L.H.; Yuan, H.; Hu, F.Q. Magnetic lipid nanoparticles loading doxorubicin for intracellular delivery: Preparation and characteristics. J. Mag. Mag. Mater 2011, 323, 1088–1093. [Google Scholar]
- Wei, Y.; Han, B.; Hu, X.; Lin, Y.; Wang, X.; Deng, X. Synthesis of Fe3O4 nanoparticles and their magnetic properties. Procedia Eng 2012, 27, 632–637. [Google Scholar]
- Ilgin, P.; Avci, G.; Silan, C.; Ekici, S.; Aktas, N.; Ayyala, R.S.; John, V.T.; Sahiner, N. Colloidal drug carries from (sub)micron hyaluronic acid hydrogel particles with tunable properties for biomedical applications. Carbohydr. Polym 2010, 82, 997–1003. [Google Scholar]
- Higuchi, T. Rate of release of medicaments from ointment bases containing drugs in suspension. J. Pharm. Sci 1961, 50, 874–875. [Google Scholar]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm 1983, 15, 25–35. [Google Scholar]
- Manadas, R.; Pina, M.E.; Veiga, F. A dissolução in vitro na previsão da absorção oral de fármacos em formas farmacêuticas de liberação modificada. Braz. J. Pharm. Sci 2002, 38, 375–399. [Google Scholar]
- Lopes, C.M.; Lobo, J.M.S.; Costa, P. Formas farmacêuticas de liberação modificada: Polímeros hidrifílicos. Braz. J. Pharm. Sci 2005, 41, 143–154. [Google Scholar]
- Nappini, S.; Bombelli, F.B.; Bonini, M.; Nordèn, B.; Baglioni, P. Magnetoliposomes for controlled drug release in the presence of low frequency magnetic field. Soft. Matter 2010, 6, 154–162. [Google Scholar]
- Bedells, A.D.; Arafeh, R.M.; Yang, Z.; Attwood, D.; Heatley, F.; Padget, J.C.; Price, C.; Booth, C. Micellisation of diblock copoly(oxyethylene/oxybutylene) in aqueous solution. J. Chem. Soc. Faraday Trans 1993, 89, 1235–1242. [Google Scholar]
- Heatley, F.; Yu, G.E.; Sun, W.B.; Pywell, E.J.; Mobbs, R.H.; Booth, C. Triad sequence assignment of the 13C-NMR spectra of copolymers of ethylene oxide and 1,2-butylene oxide. Eur. Polym. J 1990, 26, 583–592. [Google Scholar]
- Barreto, A.C.H.; Maia, F.J.N.; Santiago, V.R.; Ribeiro, V.G.P.; Denardin, J.C.; Mele, G.; Carbone, L.; Lomonaco, D.; Mazzetto, S.E.; Fechine, P.B.A. Novel ferrofluids coated a renewable material obtained from cashew nut shell liquid. Microfluid Nanofluid 2012, 12, 677–686. [Google Scholar]



| Copolymer | Mn (g·mol−1) | wE | wh | Mw/Mn | cmc (g·dm−3) |
|---|---|---|---|---|---|
| E114CL20 | 7328 | 0.689 | 0.311 | 1.36 | 0.0089 |
| Model | Equation | Parameters | r2 | |
|---|---|---|---|---|
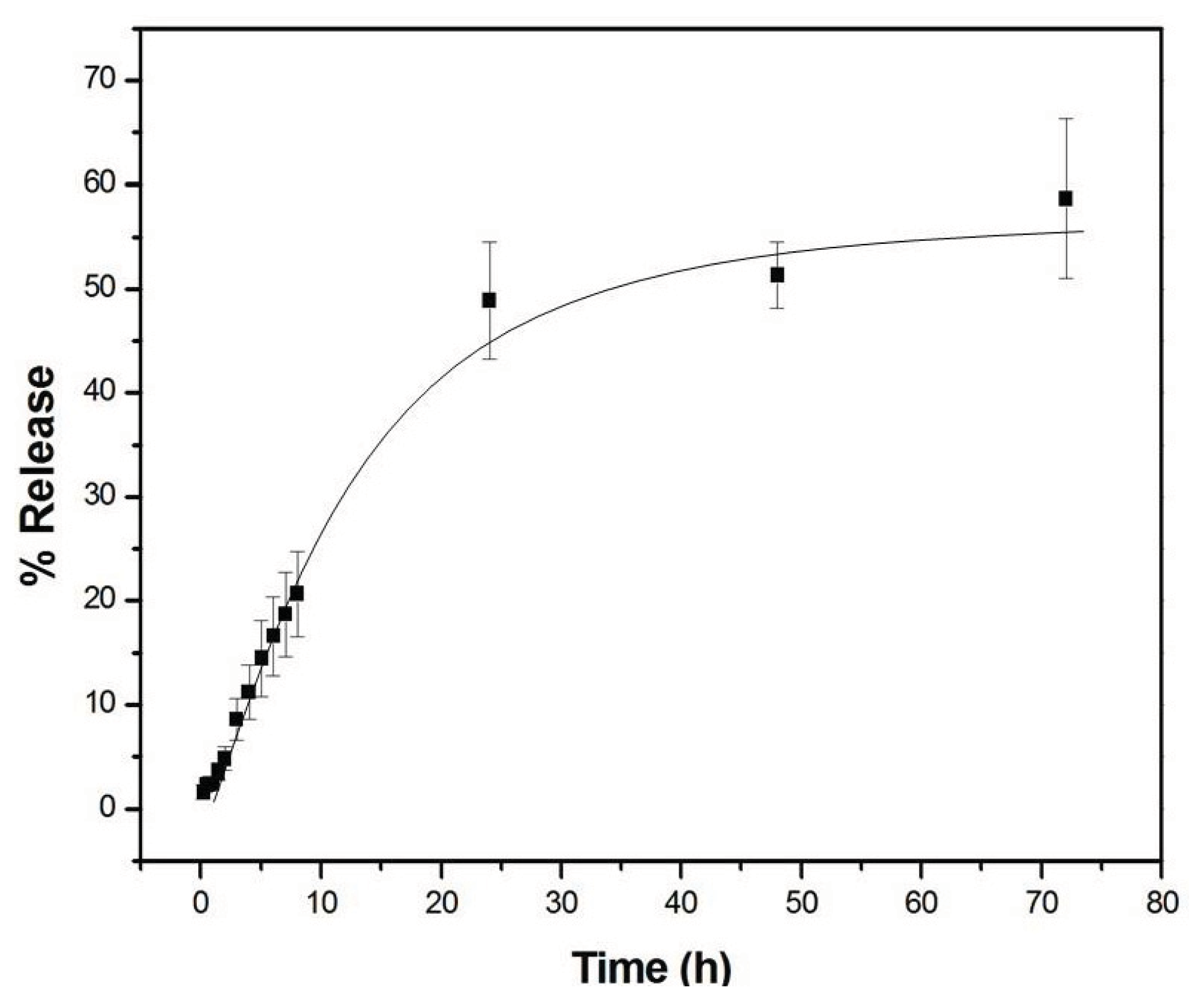
| Higuchi | KH | 0.9709 | ||
| 6.8541 ± 0.24 | ||||
| Korsmeyer-Peppas | K | n | 0.9831 | |
| 5.3243 ± 0.54 | 0.5717 ± 0.02 | |||
 | ||
|---|---|---|
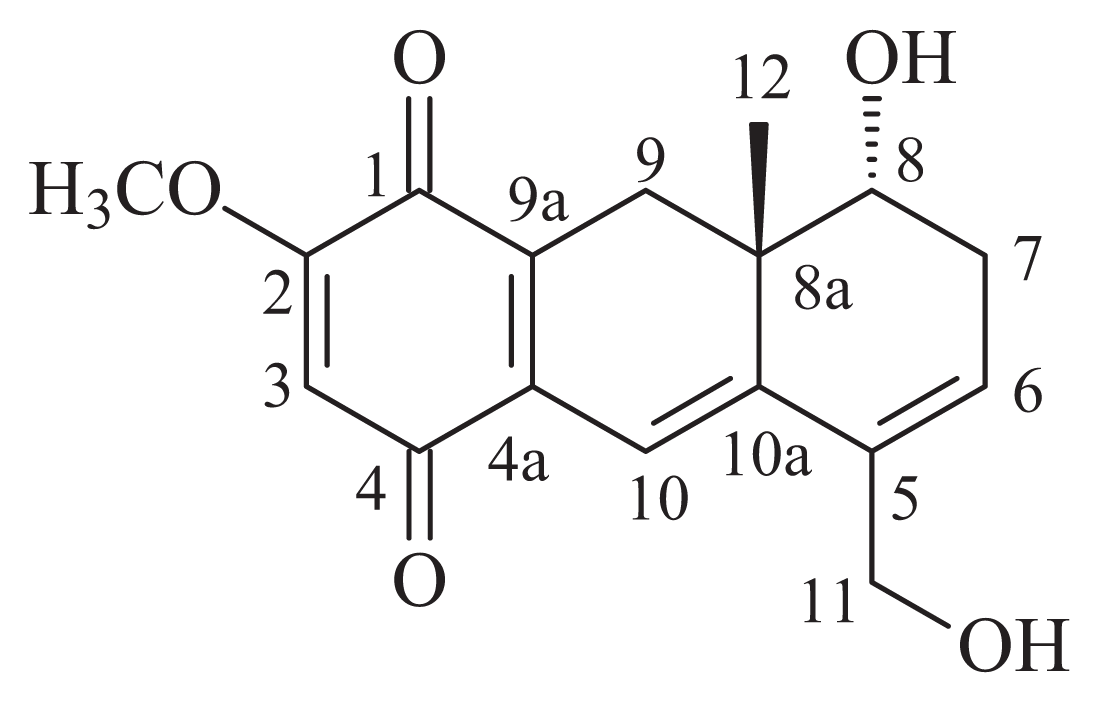
| Position | δC (ppm) | δH (ppm) |
| 1 | 180.7 | – |
| 2 | 159.3 | – |
| 3 | 105.9 | 6.00 (s) |
| 4 | 185.5 | – |
| 4a | 134.1 | – |
| 5 | 135.0 | – |
| 6 | 127.9 | 6.03 (br s) |
| 7 | 31.4 | 2.60 (d, J = 18.6 Hz) |
| 8 | 69.6 | 2.37 (dd, J = 18.6 Hz) |
| 8a | 38.8 | 3.56 (br s) |
| 9 | 28.7 | 2.93 (d, J = 18.2 Hz)/2.34 (d, J = 18.2 Hz) |
| 9a | 132.5 | – |
| 10 | 111.3 | 6.49 (s) |
| 10a | 146.2 | – |
| 11 | 61.1 | 4.17 (br s) |
| 12 | 20.8 | 0.74 (s) |
| CH3O | 56.2 | 3.78 (s) |
| HO-8 | - | 4.93 (d, J = 3.7 Hz) |
| HO-11 | - | 4.87 (br s) |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Barreto, A.C.H.; Santiago, V.R.; Freire, R.M.; Mazzetto, S.E.; Denardin, J.C.; Mele, G.; Cavalcante, I.M.; Ribeiro, M.E.N.P.; Ricardo, N.M.P.S.; Gonçalves, T.; et al. Magnetic Nanosystem for Cancer Therapy Using Oncocalyxone A, an Antitomour Secondary Metabolite Isolated from a Brazilian Plant. Int. J. Mol. Sci. 2013, 14, 18269-18283. https://doi.org/10.3390/ijms140918269
Barreto ACH, Santiago VR, Freire RM, Mazzetto SE, Denardin JC, Mele G, Cavalcante IM, Ribeiro MENP, Ricardo NMPS, Gonçalves T, et al. Magnetic Nanosystem for Cancer Therapy Using Oncocalyxone A, an Antitomour Secondary Metabolite Isolated from a Brazilian Plant. International Journal of Molecular Sciences. 2013; 14(9):18269-18283. https://doi.org/10.3390/ijms140918269
Chicago/Turabian StyleBarreto, Antônio C. H., Vivian R. Santiago, Rafael M. Freire, Selma E. Mazzetto, Juliano C. Denardin, Giuseppe Mele, Igor M. Cavalcante, Maria E. N. P. Ribeiro, Nágila M. P. S. Ricardo, Tamara Gonçalves, and et al. 2013. "Magnetic Nanosystem for Cancer Therapy Using Oncocalyxone A, an Antitomour Secondary Metabolite Isolated from a Brazilian Plant" International Journal of Molecular Sciences 14, no. 9: 18269-18283. https://doi.org/10.3390/ijms140918269





