Delta Opioid Receptor and Its Peptide: A Receptor-Ligand Neuroprotection
Abstract
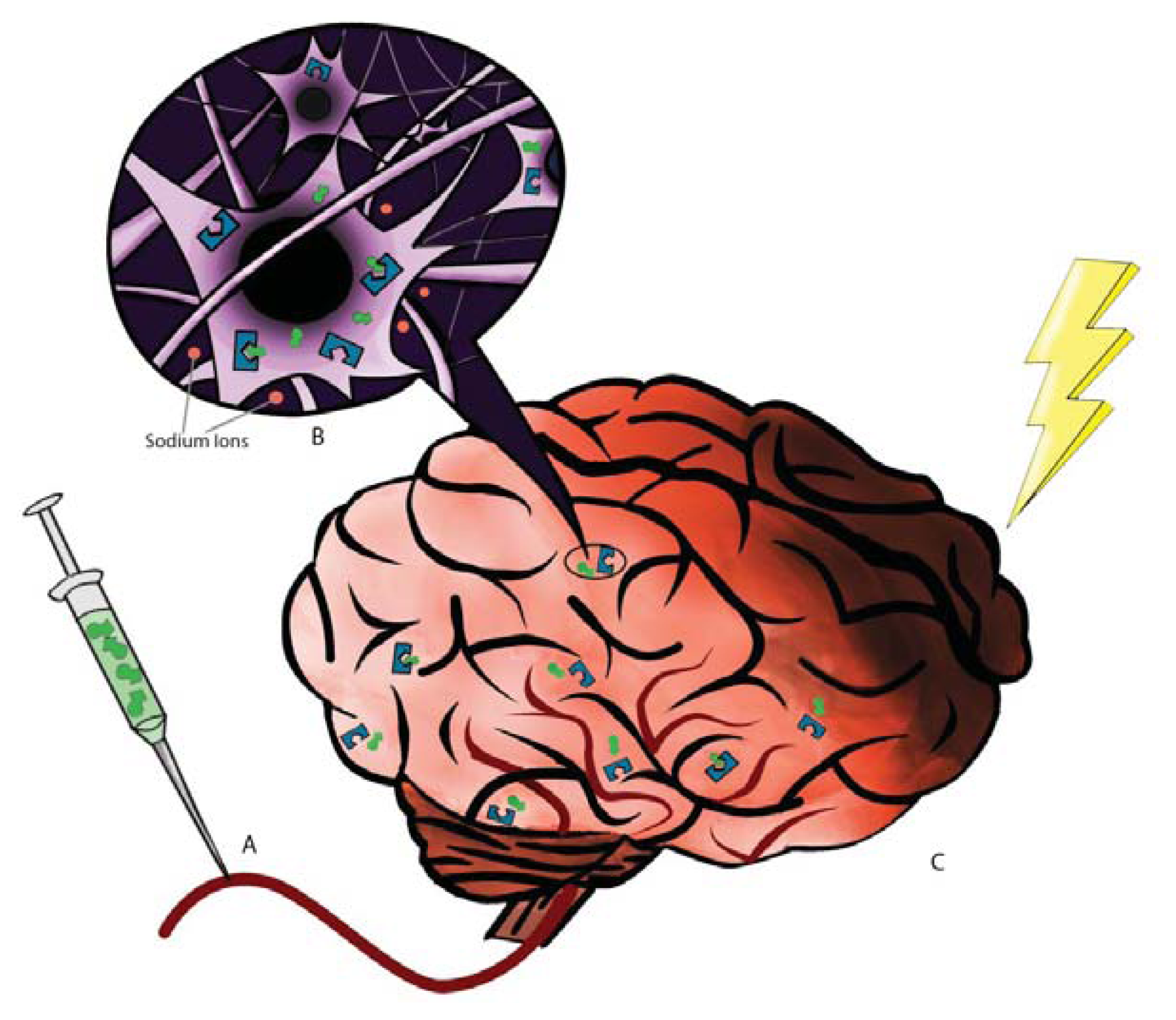
:1. Introduction
2. DOR: The Receptor and Neuroprotection
3. DADLE: The Ligand and Neuroprotection
4. Conclusions and Future Directions
Acknowledgments
Conflicts of Interest
References
- Feng, Y.; He, X.; Yang, Y.; Chao, D.; Lazarus, L.H.; Xia, Y. Current research on opioid receptor function. Curr. Drug Targets 2012, 13, 230–246. [Google Scholar]
- Lim, Y.J.; Zheng, S.; Zuo, Z. Morphine preconditions Purkinje cells against cell death under in vitro simulated ischemia-reperfusion conditions. Anesthesiology 2004, 100, 562–568. [Google Scholar]
- Hiller, J.M.; Fan, L.Q. Laminar distribution of the multiple opioid receptors in the human cerebral cortex. Neurochem. Res 1996, 21, 1333–1345. [Google Scholar]
- Mansour, A.; Khachaturian, H.; Lewis, M.E.; Akil, H.; Watson, S.J. Autoradiographic differentiation of mu, delta and kappa opioid in the rat forebrain and midbrain. J. Neurosci 1987, 7, 2445–2464. [Google Scholar]
- Xia, Y.; Haddad, G.G. Ontogeny and distribution of opioid receptors in the rat brainstem. Brain Res 1991, 549, 181–193. [Google Scholar]
- Xiang, H.; Hochman, D.W.; Saya, H.; Fujiwara, T.; Schwartzkroin, P.A.; Morrison, R.S. Evidence for p53-mediated modulation of neuronal viability. J. Neurosci 1996, 16, 6753–6765. [Google Scholar]
- Borlongan, C.V.; Wang, Y.; Su, T.P. Delta opioid peptide (D-Ala 2, D-Leu 5) enkephalin: Linking hibernation and neuroprotection. Front. Biosci 2004, 9, 3392–3398. [Google Scholar]
- Peart, J.N.; Gross, E.R.; Gross, G.J. Opioid-induced preconditioning: Recent advances and future perspectives. Vascul. Pharmacol 2005, 42, 211–218. [Google Scholar]
- D’Alecy, L.G. Delta-1 opioid agonist acutely increases hypoxic tolerance. J. Pharmacol. Exp. Ther 1994, 268, 683–688. [Google Scholar]
- Bofetiado, D.M.; Mayfield, K.P.; D’Alecy, L.G. Alkaloid d agonist BW 373U86 increases hypoxia tolerance. Anesth. Analg 1996, 82, 1237–1241. [Google Scholar]
- Xia, Y.; Haddad, G.G. Major difference in the expression of δ- and μ-opioid receptors between turtle and rat brain. J. Comp. Neurol 2001, 436, 202–210. [Google Scholar]
- Sick, T.J.; Rosenthal, M.; LaManna, J.C.; Lutz, P.L. Brain potassium ion homeostasis, anoxia, and metabolic inhibition in turtles and rats. Am. J. Physiol 1982, 243, R281–R288. [Google Scholar]
- Xia, Y.; Jiang, C.; Haddad, G.G. Oxidative and glycolytic pathways in rat (newborn, adult) and turtle: Role in anoxia. Am. J. Physiol 1992, 262, R595–R603. [Google Scholar]
- Zhang, J.H.; Xia, Y.; Haddad, G.G. Activation of δ-opioid receptors protects cortical neurons from glutamate excitotoxic injury. Soc. Neurosci. Abstr 1999, 28, 736. [Google Scholar]
- Zhang, J.H.; Haddad, G.G.; Xia, Y. δ-, but not μ- and κ-opioid receptor activation protects neocortical neurons from glutamate-induced excitotoxic injury. Brain Res 2000, 885, 143–153. [Google Scholar]
- Boutin, H.; Dauphin, F.; MacKenzie, E.T.; Jauzac, P. Differential time-course decreases in nonselective, mu-, delta-, and kappa-opioid receptors after focal cerebral ischemia in mice. Stroke 1999, 30, 1271–1277. [Google Scholar]
- Frerichs, K.U.; Hallenbeck, J.M. Hibernation in ground squirrels induces state and species-specific tolerance to hypoxia and aglycemia: An in vitro study in hippocampal slices. J. Cereb. Blood Flow Metab 1998, 18, 168–175. [Google Scholar]
- Kevelaitis, E.; Peynet, J.; Mouas, C.; Launay, J.M.; Menasche, P. Opening of potassium channels: The common cardioprotective link between preconditioning and natural hibernation? Circulation 1999, 99, 3079–3085. [Google Scholar]
- Mayfield, K.P.; Kozak, W.; Malvin, G.M.; Porreca, F. Hypoxia decreases opioid delta receptor expression in mouse brain. Neuroscience 1996, 72, 785–789. [Google Scholar]
- Sung, J.H.; Chao, D.M.; Xia, Y. Neuronal Responses to Hypoxia. In New Frontiers in Neurological Research; Wang, Y., Ed.; Research Signpost: Kerala, India, 2008; pp. 73–153. [Google Scholar]
- Kang, X.; Chao, D.; Gu, Q.; Ding, G.; Wang, Y.; Balboni, G.; Lazarus, L.H.; Xia, Y. δ-opioid receptors protect from anoxic disruption of Na+ homeostasis via Na+ channel regulation. Cell Mol. Life Sci 2009, 66, 3505–3516. [Google Scholar]
- Hansen, A.J. Effect of anoxia on ion distribution in the brain. Physiol. Rev 1985, 65, 101–148. [Google Scholar]
- Bickler, P.E. Clinical perspectives: Neuroprotection lessons from hypoxia-tolerant organisms. J. Exp. Biol 2004, 207, 3243–3249. [Google Scholar]
- Chao, D.; Xia, Y. Ionic storm in hypoxic/ischemic stress: Can opioid receptors subside it? Prog. Neurobiol 2010, 90, 439–470. [Google Scholar]
- Yu, S.P.; Yeh, C.H.; Sensi, S.L.; Gwag, B.J.; Canzoniero, L.M.; Farhangrazi, Z.S.; Ying, H.S.; Tian, M.; Dugan, L.L.; Choi, D.W. Mediation of neuronal apoptosis by enchancement of outward potassium current. Science 1997, 278, 114–117. [Google Scholar]
- Liu, D.; Slevin, J.R.; Lu, C.; Chan, S.L.; Hansson, M.; Elmer, E.; Mattson, M.P. Involvement of mitochondrial K+ release and cellular efflux in ischemic and apoptotic neuronal death. J. Neurochem 2003, 86, 966–979. [Google Scholar]
- Wei, L.; Yu, S.P.; Gottron, F.; Snider, B.J.; Zipfei, G.J.; Choi, D.W. Potassium channel blockers attenuate hypoxia- and ischemia-induced neuronal death in vitro and in vivo. Stroke 2003, 34, 1281–1286. [Google Scholar]
- Mongin, A.A. Disruption of ionic and cell volume homeostasis in cerebral ischemia: The perfectstorm. Pathophysiology 2007, 14, 183–193. [Google Scholar]
- Nistico, R.; Piccirilli, S.; Sebastianelli, L.; Nistico, G.; Bernardi, G.; Mercuri, N.B. The blockade of K+-ATP channels has neuroprotective effects in an in vitro model of brain ischemia. Int. Rev. Neurobiol 2007, 82, 383–395. [Google Scholar]
- Karki, P.; Seong, C.; Kim, J.E.; Hur, K.; Shin, S.Y.; Lee, J.S.; Cho, B.; Park, I.S. Intracellular K+ inhibits apoptosis by suppressing the Apaf-1 apoptosome formation and subsequent downstream pathways but not cytochrome c release. Cell Death Differ 2007, 14, 2068–2075. [Google Scholar]
- Chao, D.; Qian, H.; Ghassemi, F.; Chen, J.S.; Xia, Y. Transgenic over-expression of δ-opioid receptors protects the cortex from anoxic disruption of ionic homeostasis. Soc. Neurosci. Abstr. 2006. 87.19/MM68. [Google Scholar]
- Chao, D.; Bazzy-Asaad, A.; Balboni, G.; Xia, Y. δ-, but not μ-, opioid receptor stabilizes K+ homeostasis by reducing Ca2+ influx in the cortex during acute hypoxia. J. Cell Physiol 2007, 212, 60–67. [Google Scholar]
- Chao, D.; Donnelly, D.F.; Feng, Y.; Bazzy-Asaad, A.; Xia, Y. Cortical δ-opioid receptors potentiate K+ homeostasis during anoxia and oxygen-glucose deprivation. J. Cereb. Blood Flow Metab 2007, 27, 356–368. [Google Scholar]
- Chao, D.; Bazzy-Asaad, A.; Balboni, G.; Salvadori, S.; Xia, Y. Activation of DOR attenuates anoxic K+ derangement via inhibition of Na+ entry in mouse cortex. Cereb. Cortex 2008, 18, 2217–2227. [Google Scholar]
- Chao, D.; Balboni, G.; Lazarus, L.H.; Salvadori, S.; Xia, Y. Na+ mechanism of δ-opioid receptor induced protection from anoxic K+ leakage in the cortex. Cell Mol. Life Sci 2009, 66, 1105–1115. [Google Scholar]
- Ma, M.C.; Qian, H.; Ghassemi, F.; Zhao, P.; Xia, Y. Oxygen sensitive δ-opioid receptor-regulated survival and death signals: Novel insights into neuronal preconditioning and protection. J. Biol. Chem 2005, 280, 16208–16218. [Google Scholar]
- Feng, Y.; Chao, D.; He, X.; Yang, Y.; Kang, X.; Lazarus, L.H.; Xia, Y. A novel insight into neuroprotection against hypoxic/ischemic stress. Acta Physiol. Sin 2009, 61, 585–592. [Google Scholar]
- Peng, P.-H.H.; Huang, H.-S.S.; Lee, Y.-J.J.; Chen, Y.-S.S.; Ma, M.-C. Novel role for the delta-opioid receptor in hypoxic preconditioning in rat retinas. J. Neurochem 2009, 108, 741–754. [Google Scholar]
- Narita, M.; Kuzumaki, N.; Miyatake, M.; Sato, F.; Wachi, H.; Seyama, Y.; Suzuki, T. Role of delta-opioid receptor function in neurogenesis and neuroprotection. J. Neurochem 2006, 97, 1494–1505. [Google Scholar]
- Sun, K.; Su, D.S.; Wang, X.R. Delta opioid agonist [D-Ala2, D-Leu5] enkephalin (DADLE) reduced oxygen-glucose deprivation caused neuronal injury through the MAPK pathway. Brain Res 2009, 1292, 100–106. [Google Scholar]
- Dawe, A.R.; Spurrier, W.A. Hibernation induced in ground squirrels by blood transfusion. Science 1969, 163, 298–299. [Google Scholar]
- Bruce, D.S.; Cope, G.W.; Elam, T.R.; Ruit, S.K.; Oeltgen, P.R.; Su, T.-P. Opioids and hibernation. I. Effects of naloxone on bear HIT’s depression of guinea-pig ileum contractility and on induction of summer hibernation in the ground squirrel. Life Sci 1987, 41, 2107–2113. [Google Scholar]
- Oeltgen, P.R.; Nilekani, S.P.; Nuchols, P.A.; Spurrier, W.A.; Su, T.-P. Further studies on opioid and hibernation: Delta opioid receptor ligand selectively induced hibernation in summer-active ground squirrels. Life Sci 1988, 43, 1565–1574. [Google Scholar]
- Oeltgen, P.R.; Welborn, J.R.; Nuchols, P.A.; Spurrier, W.A.; Bruce, D.S.; Su, T.-P. Opioids and hibernation. II. Effects of kappa opioid U69593 on induction of hibernation in summer-active ground squirrels by “hibernation induction trigger” (HIT). Life Sci 1987, 41, 2115–2120. [Google Scholar]
- Tsao, L.I.; Ladenheim, B.; Andrews, A.; Chiueh, C.C.; Cadet, J.L.; Su, T.-P. Delta opioid peptide (D-Ala 2, D-Leu 5) enkephalin blocks the long-term loss of dopamine transporter induced by multiple administrations of methamphetamine: Involvement of opioid receptors and reactive oxygen species. J. Pharmacol. Exp. Ther 1998, 287, 322–331. [Google Scholar]
- Tsao, L.I.; Cadet, J.L.; Su, T.P. Reversal by [D-Ala2, D-Leu5]enkephalin of the dopamine transporter loss caused by methamphetamine. Eur. J. Pharmacol 1999, 372, 5–7. [Google Scholar]
- Hayashi, T.; Tsao, L.I.; Cadet, J.L.; Su, T.P. [D-Ala2, D-Leu5] enkephalin blocks the methamphetamine-induced c-fos mRNA increase in mouse striatum. Eur. J. Pharmacol. 1999, 366, 7–8. [Google Scholar]
- Borlongan, C.V.; Wu, J.N.; Su, T.-P.; Wang, Y. Delta Opioid Peptide (DADLE) Enhances Survival of Cultured Fetal Cells. In Problems of Drug Dependence, 1999: Proceedings of the 61st Annual Scientific Meeting; U.S. Department of Health and Human Services: Bethesda, MD, USA, 1999; p. 13. [Google Scholar]
- Borlongan, C.V.; Su, T.-P.; Wang, Y. Treatment with delta opioid peptide enhances in vitro and in vivo survival of rat dopaminergic neurons. Neuroreport 2000, 11, 923–926. [Google Scholar]
- Hayashi, T.; Tsao, L.I.; Su, T.P. Antiapoptotic and cytotoxic properties of delta opioid peptide [D-Ala2, D-Leu5] enkephalin in PC12 cells. Synapse 2002, 43, 86–94. [Google Scholar]
- Borlongan, C.V.; Oeltgen, P.R.; Su, T.-P.; Wang, Y. Delta opioid peptide (DADLE) protects against ischemia reperfusion damage in the striatum and cerebral cortex. Soc. Neurosci. Abstr 1999, 24, 979. [Google Scholar]
- Borlongan, C.V.; Hayashi, T.; Oeltgen, P.R.; Su, T.P.; Wang, Y. Hibernation like state induced by an opioid peptide protects against experimental stroke. BMC Biol 2009, 7, 31. [Google Scholar]
- Hayashi, T.; Sakai, K.; Sasaki, C.; Itoyama, Y.; Abe, K. Loss of bag-1 immunoreactivity in rat brain after transient middle cerebral artery occlusion. Brain Res 2000, 852, 496–500. [Google Scholar]
- Wang, Y.; Chang, C.F.; Morales, M.; Chou, J.; Chen, H.L.; Chiang, Y.H.; Lin, S.Z.; Cadet, J.L.; Deng, X.; Wang, J.Y.; et al. Bone morphogenetic protein-6 reduces ischemia-induced brain damage in rats. Stroke 2001, 32, 2170–2178. [Google Scholar]
- Borlongan, C.V.; Zhou, F.C.; Hayashi, T.; Su, T.P.; Hoffer, B.J.; Wang, Y. Involvement of GDNF in neuronal protection against 6- OHDA-induced parkinsonism following intracerebral transplantation of fetal kidney tissues in adult rats. Neurobiol. Dis 2001, 8, 636–646. [Google Scholar]
- Lin, L.F.; Doherty, D.H.; Lile, J.D.; Bektesh, S.; Collins, F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993, 260, 1130–1132. [Google Scholar]
- Wang, Y.; Lin, S.Z.; Chiou, A.L.; Williams, L.R.; Hoffer, B.J. Glial cell line derived neurotrophic factor protects against ischemia-induced injury in the cerebral cortex. J. Neurosci 1997, 17, 4341–4348. [Google Scholar]
- Kearns, C.M.; Cass, W.A.; Smoot, K.; Kryscio, R.; Gash, D.M. GDNF protection against 6-OHDA: Time dependence and requirement for protein synthesis. J. Neurosci 1997, 17, 7111–7118. [Google Scholar]
- Fox, C.M.; Gash, D.M.; Smoot, M.K.; Cass, W.A. Neuroprotective effects of GDNF against 6-OHDA in young and aged rats. Brain Res 2001, 896, 56–63. [Google Scholar]
- Tsai, S.Y.; Lee, C.T.; Hayashi, T.; Freed, W.J.; Su, T.P. Delta opioid peptide DADLE and naltrexone cause cell cycle arrest and differentiation in a CNS neural progenitor cell line. Synapse 2010, 64, 267–273. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Staples, M.; Acosta, S.; Tajiri, N.; Pabon, M.; Kaneko, Y.; Borlongan, C.V. Delta Opioid Receptor and Its Peptide: A Receptor-Ligand Neuroprotection. Int. J. Mol. Sci. 2013, 14, 17410-17419. https://doi.org/10.3390/ijms140917410
Staples M, Acosta S, Tajiri N, Pabon M, Kaneko Y, Borlongan CV. Delta Opioid Receptor and Its Peptide: A Receptor-Ligand Neuroprotection. International Journal of Molecular Sciences. 2013; 14(9):17410-17419. https://doi.org/10.3390/ijms140917410
Chicago/Turabian StyleStaples, Meaghan, Sandra Acosta, Naoki Tajiri, Mibel Pabon, Yuji Kaneko, and Cesar V. Borlongan. 2013. "Delta Opioid Receptor and Its Peptide: A Receptor-Ligand Neuroprotection" International Journal of Molecular Sciences 14, no. 9: 17410-17419. https://doi.org/10.3390/ijms140917410



