Angiogenesis-Related Pathways in the Pathogenesis of Ovarian Cancer
Abstract
:1. Ovarian Cancer: Pathogenesis and Clinical Aspects
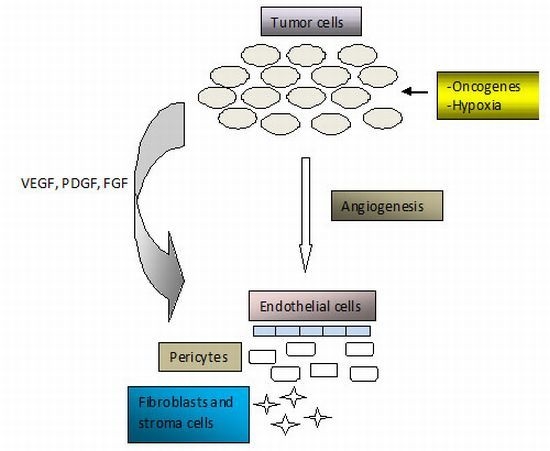
2. Angiogenesis in Cancer Pathogenesis
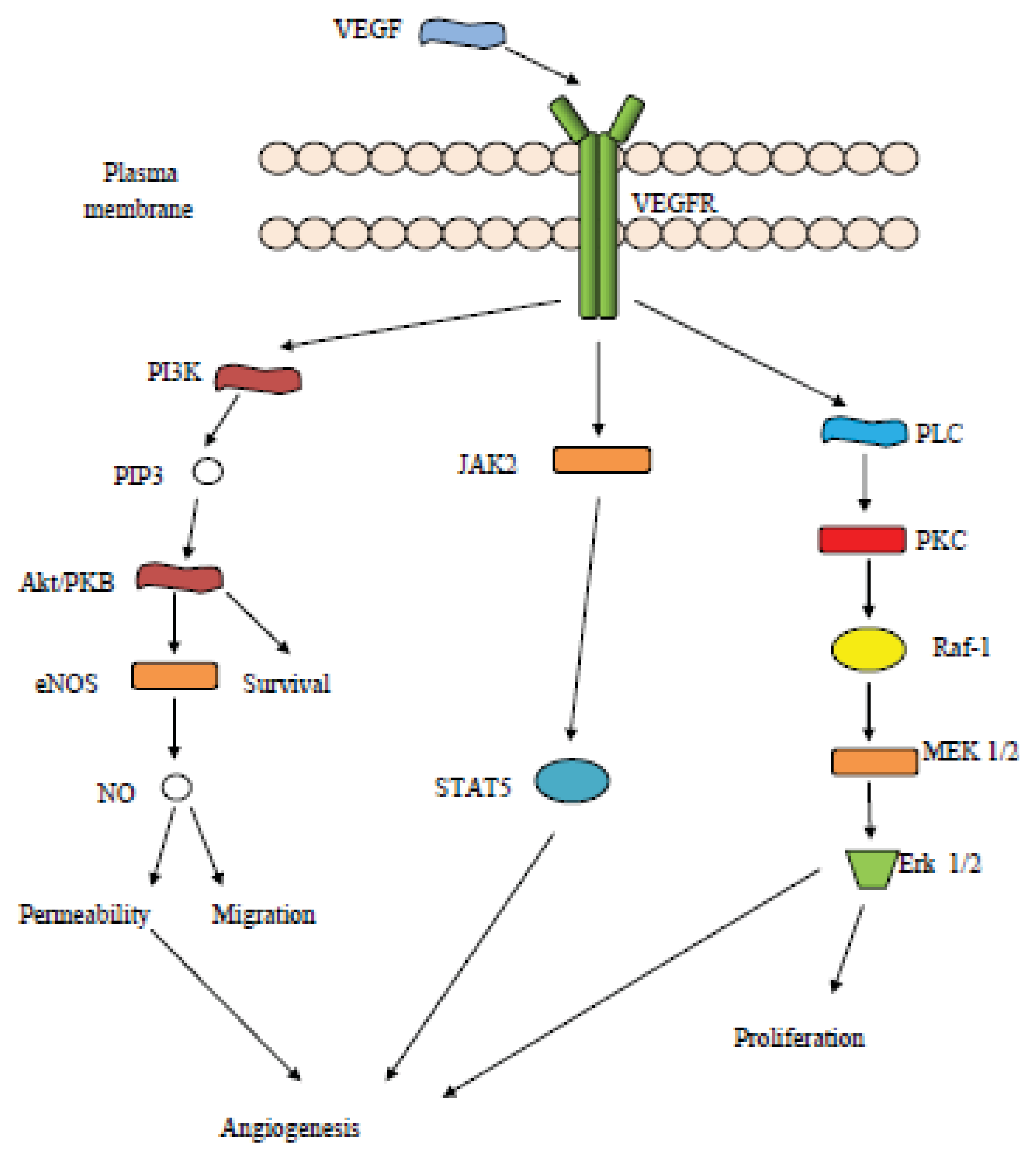
3. Vascular Endothelial Growth Factor (VEGF) Related Pathways
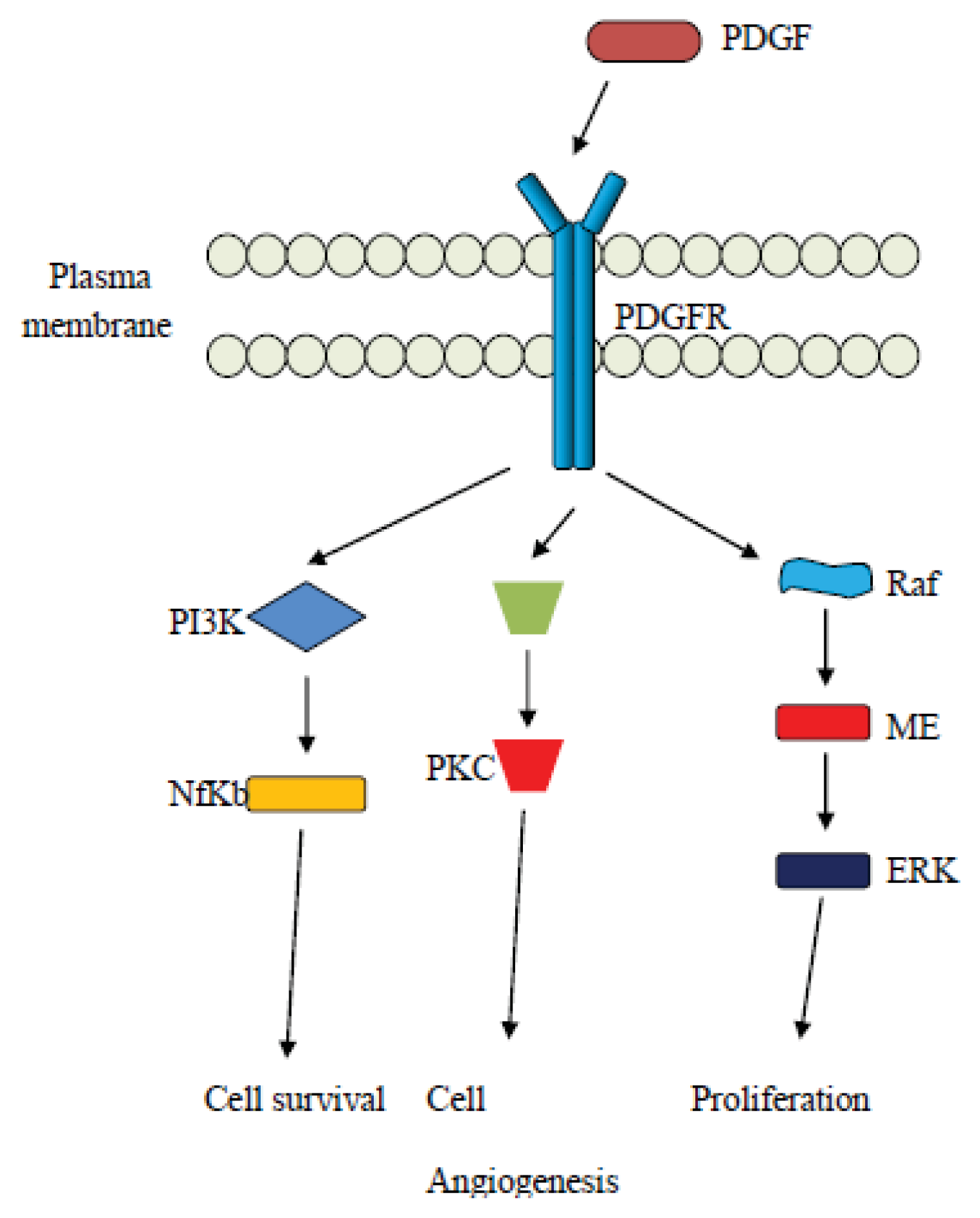
4. The Platelet Derived Growth Factor (PDGF) Pathway
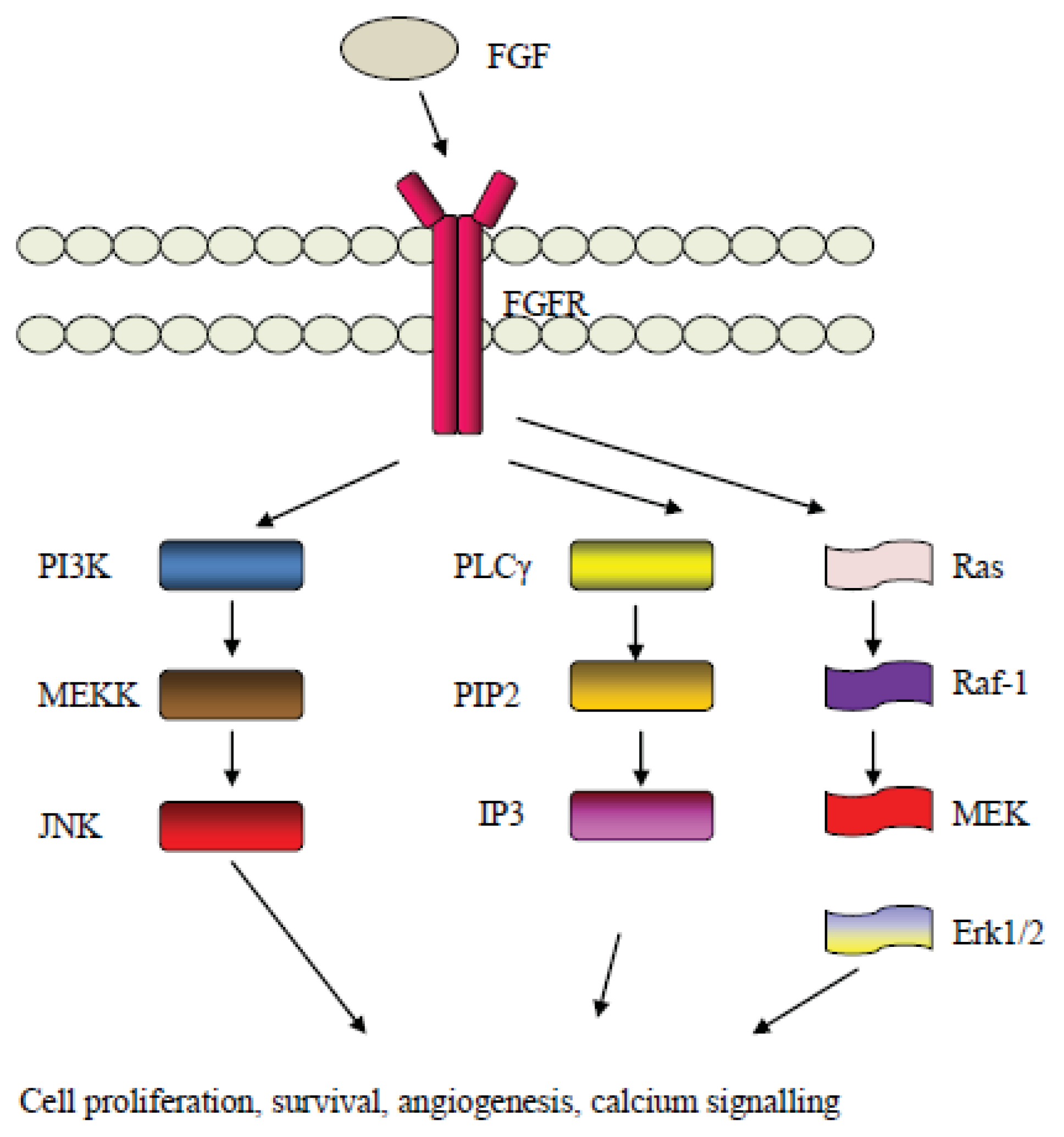
5. The Fibroblast Growth Factor (FGF) Pathway
6. The Angiopoietin Pathway and the Tie2 Receptor
7. Targeting the Angiogenesis Related Pathways for Ovarian Cancer Treatment
8. Conclusions
Conflict of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer Statistics, 2012. CA Cancer J. Clin 2012, 62, 10–29. [Google Scholar]
- Yabroff, K.R.; Lamont, E.B.; Mariotto, A.; Warren, J.L.; Topor, M.; Meekins, A.; Brown, M.L. Cost of care for elderly cancer patients in the United States. J. Natl. Cancer Inst 2008, 100, 630–641. [Google Scholar]
- Gilks, C.B.; Prat, J. Ovarian carcinoma pathology and genetics: Recent advances. Hum. Pathol 2009, 40, 1213–1223. [Google Scholar]
- Kurman, R.J.; Shih, I.M. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am. J. Surg. Pathol 2010, 34, 433–443. [Google Scholar]
- Vaughan, S.; Coward, J.I.; Bast, R.C., Jr; Berchuck, A.; Berek, J.S.; Brenton, J.D.; Coukos, G.; Crum, C.C.; Drapkin, R.; Etemadmoghadam, D.; et al. Rethinking ovarian cancer: Recommendations for improving outcomes. Nat. Rev. Cancer 2011, 11, 719–725. [Google Scholar]
- Liliac, L.; Amalinei, C.; Balan, R.; Grigoras, A.; Caruntu, I.D. Ovarian cancer: Insights into genetics and pathogeny. Histol. Histopathol 2012, 27, 707–719. [Google Scholar]
- Cheng, W.; Liu, J.; Yoshida, H.; Rosen, D.; Naora, H. Lineage infidelity of epithelial ovarian cancers is controlled by HOX genes that specify regional identity in the reproductive tract. Nat. Med 2005, 11, 531–537. [Google Scholar]
- Lauchlan, S.C. The secondary Müllerian system. Obstet. Gynecol. Surv 1972, 27, 133–146. [Google Scholar]
- Lauchlan, S.C. The secondary müllerian system revisited. Int. J. Gynecol. Pathol 1994, 13, 73–79. [Google Scholar]
- Lamb, J.D.; Garcia, R.L.; Goff, B.A.; Paley, P.J.; Swisher, E.M. Predictors of occult neoplasia in women undergoing risk-reducing salpingo-oophorectomy. Am. J. Obstet. Gynecol 2006, 194, 1702–1709. [Google Scholar]
- Piek, J.M.; van Diest, P.J.; Zweemer, R.P.; Jansen, J.W.; Poort-Keesom, R.J.; Menko, F.H.; Gille, J.J.; Jongsma, A.P.; Pals, G.; Kenemans, P.; et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J. Pathol 2001, 195, 451–456. [Google Scholar]
- Finch, A.; Shaw, P.; Rosen, B.; Murphy, J.; Narod, S.A.; Colgan, T.J. Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol. Oncol 2006, 100, 58–64. [Google Scholar]
- Callahan, M.J.; Crum, C.P.; Medeiros, F.; Kindelberger, D.W.; Elvin, J.A.; Garber, J.E.; Feltmate, C.M.; Berkowitz, R.S.; Muto, M.G. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J. Clin. Oncol 2007, 25, 3985–3990. [Google Scholar]
- Hermsen, B.B.; van Diest, P.J.; Berkhof, J.; Menko, F.H.; Gille, J.J.; Piek, J.M.; Meijer, S.; Winters, H.A.; Kenemans, P.; Mensdorff-Pouilly, S.V.; et al. Low prevalence of (pre) malignant lesions in the breast and high prevalence in the ovary and Fallopian tube in women at hereditary high risk of breast and ovarian cancer. Int. J. Cancer 2006, 119, 1412–1418. [Google Scholar]
- Kindelberger, D.W.; Lee, Y.; Miron, A.; Hirsch, M.S.; Feltmate, C.; Medeiros, F.; Callahan, M.J.; Garner, E.O.; Gordon, R.W.; Birch, C.; et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am. J. Surg. Pathol 2007, 31, 161–169. [Google Scholar]
- Shih, I.M.; Kurman, R.J. Ovarian tumorigenesis: A proposed model based on morphological and molecular genetic analysis. Am. J. Pathol 2004, 164, 1511–1518. [Google Scholar]
- Diaz-Padilla, I.; Malpica, A.L.; Minig, L.; Chiva, L.M.; Gershenson, D.M.; Gonzalez-Martin, A. Ovarian low-grade serous carcinoma: A comprehensive update. Gynecol. Oncol 2012, 126, 279–285. [Google Scholar]
- Singer, G.; Oldt, R., III; Cohen, Y.; Wang, B.G.; Sidransky, D.; Kurman, R.J.; Shih, I.M. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J. Natl. Cancer Inst. 2003, 95, 484–486. [Google Scholar]
- Wang, S.E.; Narasanna, A.; Perez-Torres, M.; Xiang, B.; Wu, F.Y.; Yang, S.; Carpenter, G.; Gazdar, A.F.; Muthuswamy, S.K.; Arteaga, C.L. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell 2006, 10, 25–38. [Google Scholar]
- Campbell, I.G.; Russell, S.E.; Choong, D.Y.; Montgomery, K.G.; Ciavarella, M.L.; Hooi, C.S.; Cristiano, B.E.; Pearson, R.B.; Phillips, W.A. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res 2004, 64, 7678–7681. [Google Scholar]
- Jones, S.; Wang, T.L.; Shih, I.M.; Mao, T.L.; Nakayama, K.; Roden, R.; Glas, R.; Slamon, D.; Diaz, L.A., Jr; Vogelstein, B.; Papadopoulos, N.; et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science 2010, 330, 228–231. [Google Scholar]
- Ahmed, A.A.; Etemadmoghadam, D.; Temple, J.; Lynch, A.G.; Riad, M.; Sharma, R.; Stewart, C.; Fereday, S.; Caldas, C.; Defazio, A.; et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J. Pathol 2010, 221, 49–56. [Google Scholar]
- Efeyan, A.; Serrano, M. p53: Guardian of the genome and policeman of the oncogenes. Cell Cycle 2007, 6, 1006–1010. [Google Scholar]
- Lane, D.P. Cancer. p53, guardian of the genome. Nature 1992, 358, 15–16. [Google Scholar]
- Kuo, K.T.; Guan, B.; Feng, Y.; Mao, T.L.; Chen, X.; Jinawath, N.; Wang, Y.; Kurman, R.J.; Shih, I.M.; Wang, T.L. Analysis of DNA copy number alterations in ovarian serous tumors identifies new molecular genetic changes in low-grade and high-grade carcinomas. Cancer Res 2009, 69, 4036–4042. [Google Scholar]
- Nakayama, K.; Nakayama, N.; Jinawath, N.; Salani, R.; Kurman, R.J.; Shih, I.M.; Wang, T.L. Amplicon profiles in ovarian serous carcinomas. Int. J. Cancer 2007, 120, 2613–2617. [Google Scholar]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. ICON7 Investigators. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med 2011, 365, 2484–2496. [Google Scholar]
- Itamochi, H.; Kigawa, J. Clinical trials and future potential of targeted therapy for ovarian cancer. Int. J. Clin. Oncol 2012, 17, 430–440. [Google Scholar]
- Aghajanian, C.; Blank, S.V.; Goff, B.A.; Judson, P.L.; Teneriello, M.G.; Husain, A.; Sovak, M.A.; Yi, J.; Nycum, L.R. OCEANS: A randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J. Clin. Oncol 2012, 30, 2039–2045. [Google Scholar]
- Hall, A. The role of angiogenesis in cancer. Comp. Clin. Path 2005, 13, 95–99. [Google Scholar]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med 1971, 285, 1182–1186. [Google Scholar]
- Weidner, N.; Folkman, J.; Pozza, F.; Bevilacqua, P.; Allred, E.N.; Moore, D.H.; Meli, S.; Gasparini, G. Tumor angiogenesis: A new significant and independent prognostic indicator in early-stage breast carcinoma. J. Natl. Cancer Inst 1992, 84, 1875–1887. [Google Scholar]
- Smith-McCune, K.K.; Weidner, N. Demonstration and characterization of the angiogenic properties of cervical dysplasia. Cancer Res 1994, 54, 800–804. [Google Scholar]
- Shibuya, M.; Luo, J.C.; Toyoda, M.; Yamaguchi, S. Involvement of VEGF and its receptors in ascites tumor formation. Cancer Chemother. Pharmacol 1999, 43, 72–77. [Google Scholar]
- Sherer, D.M.; Eliakim, R.; Abulafia, O. The role of angiogenesis in the accumulation of peritoneal fluid in benign conditions and the development of malignant ascites in the female. Gynecol. Obstet. Invest 2000, 50, 217–224. [Google Scholar]
- Bergers, E.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar]
- Bergers, G.; Hanahan, D.; Coussens, L.M. Angiogenesis and apoptosis are cellular parameters of neoplastic progression in transgenic mouse models of tumorigenesis. Int. J. Dev. Biol 1998, 42, 995–1002. [Google Scholar]
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar]
- Ferrara, N. VEGF and the quest for tumour angiogenesis factors. Nat. Rev. Cancer 2002, 2, 795–803. [Google Scholar]
- Hicklin, D.J.; Ellis, L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol 2005, 23, 1011–1027. [Google Scholar]
- Dvorak, H.F. Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol 2002, 20, 4368–4380. [Google Scholar]
- Gasparini, G.; Brooks, P.C.; Biganzoli, E.; Vermeulen, P.B.; Bonoldi, E.; Dirix, L.Y.; Ranieri, G.; Miceli, R.; Cheresh, D.A. Vascular integrin alpha(v)beta: A new prognostic indicator in breast cancer. Clin. Cancer Res 1998, 4, 2625–2634. [Google Scholar]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol 2002, 29, 15–18. [Google Scholar]
- Ranieri, G.; Coviello, M.; Chiriatti, A.; Stea, B.; Montemuro, S.; Quaranta, M.; Dittami, R.; Paradiso, A. Vascular endothelial growth factor concentrations in gastrointestinal cancer patients and healthy controls. Oncol. Rep 2004, 11, 435–439. [Google Scholar]
- Ranieri, G.; Coviello, M.; Patruno, R.; Valerio, P.; Martino, D.; Milella, P.; Catalano, V.; Scotto, F.; De Ceglia, A.; Quaranta, M.; et al. Vascular endothelial growth factor concentrations in the plasma-activated platelets rich (P-ARP) of healthy controls and colorectal cancer patients. Oncol. Rep 2004, 12, 817–820. [Google Scholar]
- Jayne, D.G.; Perry, S.L.; Morrison, E.; Farmery, S.M.; Guillou, P.J. Activated mesothelial cells produce heparin-binding growth factors: Implications for tumour metastases. Br. J. Cancer 2000, 82, 1233–1238. [Google Scholar]
- Sako, A.; Kitayama, J.; Yamaguchi, H.; Kaisaki, S.; Suzuki, H.; Fukatsu, K.; Fujii, S.; Nagawa, H. Vascular endothelial growth factor synthesis by human omental mesothelial cells is augmented by fibroblast growth factor-2: Possible role of mesothelial cell on the development of peritoneal metastasis. J. Surg. Res 2003, 115, 113–120. [Google Scholar]
- Stadlmann, S.; Amberger, A.; Pollheimer, J.; Gastl, G.; Offner, F.A.; Margreiter, R.; Zeimet, A.G. Ovarian carcinoma cells and IL-1beta-activated human peritoneal mesothelial cells are possible sources of vascular endothelial growth factor in inflammatory and malignant peritoneal effusions. Gynecol. Oncol 2005, 97, 784–789. [Google Scholar]
- Gerber, S.A.; Rybalko, V.Y.; Bigelow, C.E.; Lugade, A.A.; Foster, T.H.; Frelinger, J.G.; Lord, E.M. Preferential attachment of peritoneal tumor metastases to omental immune aggregates and possible role of a unique vascular microenvironment in metastatic survival and growth. Am. J. Pathol 2006, 169, 1739–1752. [Google Scholar]
- Naora, H.; Montell, D.J. Ovarian cancer metastasis: Integrating insights from disparate model organisms. Nat. Rev. Cancer 2005, 5, 355– 366. [Google Scholar]
- Nagy, J.A.; Masse, E.M.; Herzberg, K.T.; Meyers, M.S.; Yeo, K.T.; Yeo, T.K.; Sioussat, T.M.; Dvorak, H.F. Pathogenesis of ascites tumor growth: Vascular permeability factor, vascular hyperpermeability, and ascites fluid accumulation. Cancer Res 1995, 55, 360–368. [Google Scholar]
- Luo, J.C.; Yamaguchi, S.; Shinkai, A.; Shitara, K.; Shibuya, M. Significant expression of vascular endothelial growth Factor/vascular permeability factor in mouse ascites tumors. Cancer Res 1998, 58, 2652–2660. [Google Scholar]
- Liang, Y.; Brekken, R.A.; Hyder, S.M. Vascular endothelial growth factor induces proliferation of breast cancer cells and inhibits the anti-proliferative activity of anti-hormones. Endocr. Relat. Cancer 2006, 13, 905–919. [Google Scholar]
- Masood, R.; Cai, J.; Zheng, T.; Smith, D.L.; Hinton, D.R.; Gill, P.S. Vascular endothelial growth factor (VEGF) is an autocrine growth factor for VEGF receptor-positive human tumors. Blood 2001, 98, 1904–1913. [Google Scholar]
- Frumovitz, M.; Sood, A.K. Vascular endothelial growth factor (VEGF) pathway as a therapeutic target in gynecologic malignancies. Gynecol. Oncol 2007, 104, 768–778. [Google Scholar]
- Spannuth, W.A.; Sood, A.K.; Coleman, R.L. Angiogenesis as a strategic target for ovarian cancer therapy. Nat. Clin. Pract 2008, 4, 194–204. [Google Scholar]
- Han, E.S.; Wakabayashi, M.; Leong, L. Angiogenesis inhibitors in the treatment of epithelial ovarian cancer. Curr. Treat. Options Oncol 2013, 14, 22–33. [Google Scholar]
- Duncan, W.C.; Nio-Kobayashi, J. Targeting angiogenesis in the pathological ovary. Reprod. Fertil. Dev 2013, 25, 362–371. [Google Scholar]
- Shibuya, M.; Claesson-Welsh, L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp. Cell Res 2006, 312, 549–560. [Google Scholar]
- Leung, D.W.; Cachianes, G.; Kuang, W.J.; Goeddel, D.V.; Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989, 246, 1306–1309. [Google Scholar]
- Ferrara, N.; Henzel, W.J. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophys. Res. Commun 1989, 161, 851–858. [Google Scholar]
- Roskoski, R., Jr. Vascular endothelial growth factor (VEGF) signaling in tumor progression. Crit. Rev. Oncol. Hematol. 2007, 62, 179–213. [Google Scholar]
- Hoff, P.M.; Machado, K.K. Role of angiogenesis in the pathogenesis of cancer. Cancer Treat Rev 2012, 38, 825–833. [Google Scholar]
- Ferrara, N. Vascular endothelial growth factor. Eur. J. Cancer 1996, 32A, 2413–2422. [Google Scholar]
- Taggarshe, D.; Lobocki, C.; Silberberg, B.; McKendrick, A.; Mittal, V.K. Clinicopathological significance of the expression of estrogen receptor-beta and vascular endothelial growth factor-A in colorectal cancer. Am. Surg 2012, 78, 1376–1382. [Google Scholar]
- Soffietti, R.; Trevisan, E.; Bertero, L.; Bosa, C.; Ruda, R. Anti-angiogenic approaches to malignant gliomas. Curr. Cancer Drug Targets 2012, 12, 279–288. [Google Scholar]
- de Vries, C.; Escobedo, J.A.; Ueno, H.; Houck, K.; Ferrara, N.; Williams, L.T. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 1992, 255, 989–991. [Google Scholar]
- Jakeman, L.B.; Winer, J.; Bennett, G.L.; Altar, C.A.; Ferrara, N. Binding sites for vascular endothelial growth factor are localized on endothelial cells in adult rat tissues. J. Clin. Invest 1992, 89, 244–253. [Google Scholar]
- Ellis, L.M.; Hicklin, D.J. VEGF-targeted therapy: Mechanisms of anti-tumour activity. Nat. Rev. Cancer 2008, 8, 579–591. [Google Scholar]
- Joukov, V.; Pajusola, K.; Kaipainen, A.; Chilov, D.; Lahtinen, I.; Kukk, E.; Saksela, O.; Kalkkinen, N.; Alitalo, K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 1996, 15, 290–298. [Google Scholar]
- Al-Rawi, M.A.; Jiang, W.G. Lymphangiogenesis and cancer metastasis. Front. Biosci 2011, 16, 723–739. [Google Scholar]
- Spiliotaki, M.; Markomanolaki, H.; Mela, M.; Mavroudis, D.; Georgoulias, V.; Agelaki, S. Targeting the insulin-like growth factor I receptor inhibits proliferation and VEGF production of non-small cell lung cancer cells and enhances paclitaxel-mediated anti-tumor effect. Lung Cancer 2011, 73, 158–165. [Google Scholar]
- Salgado, R.; Benoy, I.; Weytjens, R.; Van Bockstaele, D.; Van Marck, E.; Huget, P.; Hoylaerts, M.; Vermeulen, P.; Dirix, L.Y. Arterio-venous gradients of IL-6, plasma and serum VEGF and D-dimers in human cancer. Br. J. Cancer 2002, 87, 1437–1444. [Google Scholar]
- White, F.C.; Benehacene, A.; Scheele, J.S.; Kamps, M. VEGF mRNA is stabilized by ras and tyrosine kinase oncogenes, as well as by UV radiation—Evidence for divergent stabilization pathways. Growth Factors 1997, 14, 199–212. [Google Scholar]
- Burger, R.A. Overview of anti-angiogenic agents in development for ovarian cancer. Gynecol. Oncol 2011, 121, 230–238. [Google Scholar]
- Millauer, B.; Wizigmann-Voos, S.; Schnurch, H.; Martinez, R.; Moller, N.P.; Risau, W.; Ullrich, A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 1993, 72, 835–846. [Google Scholar]
- Youssoufian, H.; Hicklin, D.J.; Rowinsky, E.K. Review: Monoclonal antibodies to the vascular endothelial growth factor receptor-2 in cancer therapy. Clin. Cancer Res 2007, 13, 5544–5548. [Google Scholar]
- Carmeliet, P.; Moons, L.; Luttun, A.; Vincenti, V.; Compernolle, V.; De Mol, M.; Wu, Y.; Bono, F.; Devy, L.; Beck, H.; et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat. Med 2001, 7, 575–583. [Google Scholar]
- Yang, S.; Toy, K.; Ingle, G.; Ingle, G.; Zlot, C.; Williams, P.M.; Fuh, G.; Li, B.; de Vos, A.; Gerritsen, M.E. Vascular endothelial growth factor-induced genes in human umbilical vein endothelial cells: Relative roles of KDR and Flt-1 receptors. Arterioscler. Thromb. Vasc. Biol 2002, 22, 1797–1803. [Google Scholar]
- Kukk, E.; Lymboussaki, A.; Taira, S.; Kaipainen, A.; Jeltsch, M.; Joukov, V.; Alitalo, K. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development 1996, 122, 3829–3837. [Google Scholar]
- Zhang, L.; Zhou, F.; Han, W.; Shen, B.; Luo, J.; Shibuya, M.; He, Y. VEGFR-3 ligand-binding and kinase activity are required for lymphangiogenesis but not for angiogenesis. Cell Res 2010, 20, 1319–1331. [Google Scholar]
- Banerjee, S.; Kaye, S. The role of targeted therapy in ovarian cancer. Eur. J. Cancer 2011, 47, 116–130. [Google Scholar]
- Sato, S.; Itamochi, H. Bevacizumab and ovarian cancer. Curr. Opin. Obstet. Gynecol 2012, 24, 8–13. [Google Scholar]
- Bamberger, E.S.; Perrett, C.W. Angiogenesis in epithelian ovarian cancer. Mol. Pathol 2002, 55, 348–359. [Google Scholar]
- Numnum, T.M.; Rocconi, R.P.; Whitworth, J.; Barnes, M.N. The use of bevacizumab to palliate symptomatic ascites in patients with refractory ovarian carcinoma. Gynecol. Oncol 2006, 102, 425–428. [Google Scholar]
- Teoh, D.; Secord, A.A. Antiangiogenic therapies in epithelial ovarian cancer. Cancer Control 2011, 18, 31–43. [Google Scholar]
- Burger, R.A.; Sill, M.W.; Monk, B.J.; Greer, B.E.; Sorosky, J.I. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol 2007, 25, 5165–5171. [Google Scholar]
- Zhang, L.; Yang, N.; Katsaros, D.; Huang, W.; Park, J.W.; Fracchioli, S.; Vezzani, C.; de la Longrais, I.A.R.; Yao, W.; Rubin, S.C.; et al. The oncogene phosphatidylinositol 3′-kinase catalytic subunit alpha promotes angiogenesis via vascular endothelial growth factor in ovarian carcinoma. Cancer Res 2003, 63, 4225–4231. [Google Scholar]
- Xu, L.; Pathak, P.S.; Fukumura, D. Hypoxia-induced activation of p38 mitogen-activated protein kinase and phosphatidylinositol 3′-kinase signaling pathways contributes to expression of interleukin 8 in human ovarian carcinoma cells. Clin. Cancer Res 2004, 10, 701–707. [Google Scholar]
- Chen, H.; Ye, D.; Xie, X.; Chen, B.; Lu, W. VEGF, VEGFRs expressions and activated STATs in ovarian epithelial carcinoma. Gynecol. Oncol 2004, 94, 630–635. [Google Scholar]
- Bermudez, Y.; Yang, H.; Saunders, B.O.; Cheng, J.Q.; Nicosia, S.V.; Kruk, P.A. VEGF- and LPA-induced telomerase in human ovarian cancer cells is Sp1-dependent. Gynecol. Oncol 2007, 106, 526–537. [Google Scholar]
- Weis, S.; Cui, J.; Barnes, L.; Cheresh, D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J. Cell Biol 2004, 167, 223–229. [Google Scholar]
- Mukhopadhyay, D.; Nagy, J.A.; Manseau, E.J.; Dvorak, H.F. Vascular permeability factor/vascular endothelial growth factor-mediated signaling in mouse mesentery vascular endothelium. Cancer Res 1998, 58, 1278–1284. [Google Scholar]
- Oikawa, T.; Onozawa, C.; Sakaguchi, M.; Morita, I.; Murota, S. Three isoforms of platelet-derived growth factors all have the capability to induce angiogenesis in vivo. Biol. Pharm. Bull 1994, 17, 1686–1688. [Google Scholar]
- Lu, C.; Thaker, P.H.; Lin, Y.G.; Spannuth, W.; Landen, C.N.; Merritt, W.M.; Jennings, N.B.; Langley, R.R.; Gershenson, D.M.; Yancopoulos, G.D.; et al. Impact of vessel maturation on antiangiogenic therapy in ovarian cancer. Am. J. Obstet. Gynecol 2008, 198. [Google Scholar] [CrossRef]
- Erber, R.; Thurner, A.; Katsen, A.D.; Groth, G.; Kerger, H.; Hammes, H.P.; Menger, M.D.; Ullrich, A.; Vajkoczy, P. Combined inhibition of VEGF and PDGF signalling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell surviving mechanisms. FASEB J 2004, 18, 338–340. [Google Scholar]
- Lassus, H.; Sihto, H.; Leminen, A.; Nordling, S.; Joensuu, H.; Nupponen, N.N.; Butzow, R. Genetic alterations and protein expression of KIT and PDGFRA in serous ovarian carcinoma. Br. J. Cancer 2004, 91, 2048–2055. [Google Scholar]
- Fredriksson, L.; Li, H.; Fieber, C.; Li, X.; Eriksson, U. Tissue plasminogen activator is a potent activator of PDGF-C.C. EMBO J 2004, 23, 3793–3802. [Google Scholar]
- Kazlauskas, A.; Cooper, J.A. Autophosphorylation of the PDGF receptor in the kinase insert region regulates interactions with cell proteins. Cell 1989, 58, 1121–1133. [Google Scholar]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 2008, 22, 1276–1312. [Google Scholar]
- Board, R.; Jayson, G.C. Platelet-derived growth factor receptor (PDGFR): A target for anticancer therapeutics. Drug Resist. Updat 2005, 8, 75–83. [Google Scholar]
- Valius, M.; Kazlauskas, A. Phospholipase C-gamma 1 and phosphatidylinositol 3 kinase are the downstream mediators of the PDGF receptor’s mitogenic signal. Cell 1993, 73, 321–334. [Google Scholar]
- Coughlin, S.R.; Escobedo, J.A.; Williams, L.T. Role of phosphatidylinositol kinase in PDGF receptor signal transduction. Science 1989, 243, 1191–1194. [Google Scholar]
- Heldin, C.H.; Ostman, A.; Ronnstrand, L. Signal transduction via platelet-derived growth factor receptors. Biochim. Biophys. Acta 1998, 1378, 79–113. [Google Scholar]
- Apte, S.M.; Bucana, C.D.; Killion, J.J.; Gershenson, D.; Fidler, I.J. Expression of platelet-derived growth factor and activated receptor in clinical specimens of epithelial ovarian cancer and ovarian carcinoma cell lines. Gynecol. Oncol 2004, 93, 78–86. [Google Scholar]
- Matei, D.; Graeber, T.G.; Baldwin, R.L.; Karlan, B.Y.; Rao, J.; Chang, D.D. Gene expression in epithelial ovarian carcinoma. Oncogene 2002, 21, 6289–6298. [Google Scholar]
- Apte, S.M.; Fan, D.; Killion, J.J.; Fidler, I.J. Targeting the platelet-derived growth factor receptor in antivascular therapy for human ovarian carcinoma. Clin. Cancer Res 2004, 10, 897–908. [Google Scholar]
- Matei, D.; Emerson, R.E.; Lai, Y.C.; Baldridge, L.A.; Rao, J.; Yiannoutsos, C.; Donner, D.D. Autocrine activation of PDGFRalpha promotes the progression of ovarian cancer. Oncogene 2006, 25, 2060–2069. [Google Scholar]
- Pietras, K.; Pahler, J.; Bergers, G.; Hanahan, D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med 2008, 5. [Google Scholar] [CrossRef]
- Uren, A.; Yu, J.C.; Gholami, N.S.; Pierce, J.H.; Heidaran, M.A. The alpha PDGFR tyrosine kinase mediates locomotion of two different cell types through chemotaxis and chemokinesis. Biochem. Biophys. Res. Commun 1994, 204, 628–634. [Google Scholar]
- Pietras, K.; Sjöblom, T.; Rubin, K.; Heldin, C.H.; Ostman, A. PDGF receptors as cancer drug targets. Cancer Cell 2003, 3, 439–443. [Google Scholar]
- Colvin, J.S.; Bohne, B.A.; Harding, G.W.; McEwen, D.G.; Ornitz, D.M. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor. Nat. Genet 1996, 12, 390–397. [Google Scholar]
- Chaffer, C.L.; Dopheide, B.; Savagner, P.; Thompson, E.W.; Williams, E.D. Aberrant fibroblast growth factor receptor signaling in bladder and other cancers. Differentiation 2007, 75, 831–842. [Google Scholar]
- Alshenawy, H.A. Prognostic significance of vascular endothelial growth factor, basic fibroblastic growth factor, and microvessel density and their relation to cell proliferation in B-cell non-Hodgkin’s lymphoma. Ann. Diagn. Pathol 2010, 14, 321–327. [Google Scholar]
- Itoh, N.; Ornitz, D.M. Evolution of the Fgf and Fgfr gene families. Trends Genet 2004, 20, 565–569. [Google Scholar]
- Turner, N.; Grose, R. Fibroblast growth factor signaling: From development to cancer. Nat. Rev. Cancer 2010, 10, 116–129. [Google Scholar]
- Korc, M.; Friesel, R.E. The role of fibroblast growth factors in tumor growth. Curr. Cancer Drug Targets 2009, 9, 639–651. [Google Scholar]
- Lappi, D.A. Tumor targeting through fibroblast growth factor receptors. Semin. Cancer Biol 1995, 6, 279–288. [Google Scholar]
- Zhang, Y.; Gorry, M.C.; Post, J.C.; Ehrlich, G.D. Genomic organization of the human fibroblast growth factor receptor 2 (FGFR2) gene and comparative analysis of the human FGFR gene family. Gene 1999, 230, 69–79. [Google Scholar]
- Byron, S.A.; Gartside, M.G.; Wellens, C.L.; Goodfellow, P.J.; Birrer, M.J.; Campbell, I.G.; Pollock, P.M. FGFR2 mutations are rare across histologic subtypes of ovarian cancer. Gynecol. Oncol 2010, 117, 125–129. [Google Scholar]
- Carstens, R.P.; McKeehan, W.L.; Garcia-Blanco, M.A. An intronic sequence element mediates both activation and repression of rat fibroblast growth factor receptor 2 pre-mRNA splicing. Mol. Cell Biol 1998, 18, 2205–2217. [Google Scholar]
- Steele, I.A.; Edmondson, R.J.; Bulmer, J.N.; Bolger, B.S.; Leung, H.Y.; Davies, B.R. Induction of FGF receptor 2-IIIb expression and response to its ligands in epithelial ovarian cancer. Oncogene 2001, 20, 5878–5887. [Google Scholar]
- Yoneda, J.; Kuniyasu, H.; Crispens, M.A.; Price, J.E.; Bucana, C.D.; Fidler, I.J. Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J. Natl. Cancer Inst 1998, 90, 447–454. [Google Scholar]
- Barton, D.P.; Cai, A.; Wendt, K.; Young, M.; Gamero, A.; de Cesare, S. Angiogenic protein expression in advanced epithelial ovarian cancer. Clin. Cancer Res 1997, 3, 1579–1586. [Google Scholar]
- Madsen, C.V.; Steffensen, K.D.; Olsen, D.A.; Waldstrøm, M.; Søgaard, C.H.; Brandslund, I.; Jakobsen, A. Serum platelet-derived growth factor and fibroblast growth factor in patients with benign and malignant ovarian tumors. Anticancer Res 2012, 32, 3817–3825. [Google Scholar]
- Steele, I.A.; Edmondson, R.J.; Leung, H.Y.; Davies, B.R. Ligands to FGF receptor 2-IIIb induce proliferation, motility, protection from cell death and cytoskeletal rearrangements in epithelial ovarian cancer cell lines. Growth Factors 2006, 24, 45–53. [Google Scholar]
- Tebben, P.J.; Kalli, K.R.; Cliby, W.A.; Hartmann, L.C.; Grande, J.P.; Singh, R.J.; Kumar, R. Elevated fibroblast growth factor 23 in women with malignant ovarian tumors. Mayo Clin. Proc 2005, 80, 745–751. [Google Scholar]
- Jouanneau, J.; Moens, G.; Montesano, R.; Thiery, J.P. FGF-1 but not FGF-4 secreted by carcinoma cells promotes in vitro and in vivo angiogenesis and rapid tumor proliferation. Growth Factors 1995, 12, 37–47. [Google Scholar]
- Seghezzi, G.; Patel, S.; Ren, C.J.; Gualandris, A.; Pintucci, C.; Robbins, E.S.; Shapiro, R.L.; Galloway, A.C.; Rifkin, D.B.; Mignatti, P. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: An autocrine mechanism contributing to angiogenesis. J. Cell Biol 1998, 141, 1659–1673. [Google Scholar]
- Katoh, M.; Katoh, M. FGF signaling network in the gastrointestinal tract. Int. J. Oncol 2006, 29, 163–168. [Google Scholar]
- Akai, J.; Halley, P.A.; Storey, K.G. FGF-dependent Notch signaling maintains the spinal cord stem zone. Genes Dev 2005, 19, 2877–2887. [Google Scholar]
- Reiss, Y. Angiopoietins. Recent Results Cancer Res. 2010, 180, 3–13. [Google Scholar]
- Falcon, B.L.; Hashizume, H.; Koumoutsakos, P.; Chou, J.; Bready, J.V.; Coxon, A.; Oliner, J.D.; McDonald, D.M. Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. Am. J. Pathol 2009, 175, 2159–2170. [Google Scholar]
- Papapetropoulos, A.; Fulton, D.; Mahboubi, K.; Kalb, R.G.; O’Connor, D.S.; Li, F.; Altieri, D.C.; Sessa, W.C. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J. Biol. Chem 2000, 275, 9102–9105. [Google Scholar]
- Petrillo, M.; Scambia, G.; Ferrandina, G. Novel targets for VEGF-independent anti-angiogenic drugs. Expert Opin. Investig. Drugs 2012, 21, 451–472. [Google Scholar]
- Yuan, H.T.; Khankin, E.V.; Karumanchi, S.A.; Parikh, S.M. Angiopoietin 2 is a partial agonist/antagonist of tie2 signaling in the endothelium. Mol. Cell Biol 2009, 29, 2011–2022. [Google Scholar]
- Zhang, L.; Yang, N.; Park, J.W.; Katsaros, D.; Fracchioli, S.; Cao, G.; O’Brien-Jenkins, A.; Randall, T.C.; Rubin, S.C.; Coukos, G. Tumor-derived vascular endothelial growth factor up-regulates angiopoietin-2 in host endothelium and destabilizes host vasculature, supporting angiogenesis in ovarian cancer. Cancer Res 2003, 63, 3403–3412. [Google Scholar]
- Oliner, J.; Min, H.; Leal, J.; Yu, D.; Rao, S.; You, E.; Tang, X.; Kim, H.; Meyer, S.; Han, S.J.; et al. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell 2004, 6, 507–516. [Google Scholar]
- Thomas, M.; Felcht, M.; Kruse, K.; Kretschmer, S.; Deppermann, C.; Biesdorf, A.; Rohr, K.; Benest, A.V.; Fiedler, U.; Augustin, H.G. Angiopoietin-2 stimulation of endothelial cells induces alphavbeta3 integrin internalization and degradation. J. Biol. Chem 2010, 285, 23842–23849. [Google Scholar]
- Sánchez-Muñoz, A.; Mendiola, C.; Pérez-Ruiz, E.; Rodríguez-Sánchez, C.A.; Jurado, J.M.; Alonso-Carrión, L.; Ghanem, I.; de Velasco, G.; Quero-Blanco, C.; Alba, E. Bevacizumab plus low-dose metronomic oral cyclophosphamide in heavily pretreated patients with recurrent ovarian cancer. Oncology 2010, 79, 98–104. [Google Scholar]
- McGonigle, K.F.; Muntz, H.G.; Vuky, J.; Paley, P.J.; Veljovich, D.S.; Greer, B.E.; Goff, B.A.; Gray, H.J.; Malpass, T.W. Combined weekly topotecan and biweekly bevacizumab in women with platinum-resistant ovarian, peritoneal, or fallopian tube cancer: Results of a phase 2 study. Cancer 2011, 117, 3731–3740. [Google Scholar]
- Kudoh, K.; Takano, M.; Kouta, H.; Kikuchi, R.; Kita, T.; Miyamoto, M.; Watanabe, A.; Kato, M.; Goto, T.; Kikuchi, Y. Effects of bevacizumab and pegylated liposomal doxorubicin for the patients with recurrent or refractory ovarian cancers. Gynecol. Oncol 2011, 122, 233–237. [Google Scholar]
- Sorbe, B.; Graflund, M.; Horvath, G.; Swahn, M.; Boman, K.; Bangshöj, R.; Lood, M.; Malmström, H. Phase II study of docetaxel weekly in combination with carboplatin every 3 weeks as first-line chemotherapy in stage IIB to stage IV epithelial ovarian cancer. Int. J. Gynecol. Cancer 2012, 22, 47–53. [Google Scholar]
- Agheli, A.; Park, S.C.; Huang, C.J.; Wang, J.C. Apparent beneficial effects by nab-paclitaxel in the treatment of refractory metastatic ovarian carcinoma. Anticancer Drugs 2009, 20, 525–526. [Google Scholar]
- Mesiano, S.; Ferrara, N.; Jaffe, R.B. Role of vascular endothelial growth factor in ovarian cancer: Inhibition of ascites formation by immunoneutralization. Am. J. Pathol 1998, 153, 1249–1256. [Google Scholar]
- Hu, L.; Hofmann, J.; Zaloudek, C.; Ferrara, N.; Hamilton, T.; Jaffe, R.B. Vascular endothelial growth factor immunoneutralization plus Paclitaxel markedly reduces tumor burden and ascites in athymic mouse model of ovarian cancer. Am. J. Pathol 2002, 161, 1917–1924. [Google Scholar]
- Mabuchi, S.; Terai, Y.; Morishige, K.; Tanabe-Kimura, A.; Sasaki, H.; Kanemura, M.; Tsunetoh, S.; Tanaka, Y.; Sakata, M.; Burger, R.A.; et al. Maintenance treatment with bevacizumab prolongs survival in an in vivo ovarian cancer model. Clin. Cancer Res 2008, 14, 7781–7789. [Google Scholar]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Gynecologic Oncology Group. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med 2011, 365, 2473–2483. [Google Scholar]
- Poveda, A.M.; Selle, F.; Hilpert, F.; Reuss, A.; Pasic, A.; Savarese, A.; Vergote, I.B.; Witteveen, P.; Bamias, A.; Bollag, D.; Pujade-Lauraine, E. Weekly paclitaxel (PAC), pegylated liposomal doxorubicin (PLD), or topotecan (TOP) + bevacizumab (BEV) in platinum (PT)-resistant recurrent ovarian cancer (OC). Analysis by chemotherapy (CT) cohort in the GCIG AURELIA randomized phase III trial. Ann. Oncol 2012, 23. [Google Scholar] [CrossRef]
- Sharma, T.; Dhingra, R.; Singh, S.; Sharma, S.; Tomar, P.; Malhotra, M.; Bhardwaj, T.R. Aflibercept: A novel VEGF targeted agent to explore the future perspectives of anti-angiogenic therapy for the treatment of multiple tumors. Mini Rev. Med. Chem 2013, 13, 530–540. [Google Scholar]
- Troiani, T.; Martinelli, E.; Orditura, M.; De Vita, F.; Ciardiello, F.; Morgillo, F. Beyond bevacizumab: New anti-VEGF strategies in colorectal cancer. Expert Opin. Investig. Drugs 2012, 21, 949–959. [Google Scholar]
- Byrne, A.T.; Ross, L.; Holash, J.; Nakanishi, M.; Hu, L.; Hofmann, J.I.; Yancopoulos, G.D.; Jaffe, R.B. Vascular endothelial growth factor-trap decreases tumor burden, inhibits ascites, and causes dramatic vascular remodeling in an ovarian cancer model. Clin. Can. Res 2003, 9, 5721–5728. [Google Scholar]
- Baffert, F.; Le, T.; Sennino, B.; Thurston, G.; Kuo, C.J.; Hu-Lowe, D.; McDonald, D.M. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am. J. Physiol. Heart Circ. Physiol 2006, 290, 547–559. [Google Scholar]
- Freyer, G.; Isambert, N.; You, B.; Zanetta, S.; Falandry, C.; Favier, L.; Trillet-Lenoir, V.; Assadourian, S.; Soussan-Lazard, K.; Ziti-Ljajic, S.; et al. Phase I dose-escalation study of aflibercept in combination with docetaxel and cisplatin in patients with advanced solid tumours. Br. J. Cancer 2012, 107, 598–603. [Google Scholar]
- Coleman, R.L.; Duska, L.R.; Ramirez, P.T.; Heymach, J.V.; Kamat, A.A.; Modesitt, S.C.; Schmeler, K.M.; Iyer, R.B.; Garcia, M.E.; Miller, D.L.; et al. Phase 1–2. study of docetaxel plus aflibercept in patients with recurrent ovarian, primary peritoneal, or fallopian tube cancer. Lancet Oncol 2011, 12, 1109–1117. [Google Scholar]
- Roth, G.J.; Heckel, A.; Colbatzky, F.; Handschuh, S.; Kley, J.; Lehmann-Lintz, T.; Lotz, R.; Tontsch-Grunt, U.; Walter, R.; Hilberg, F. Design, synthesis, and evaluation of indolinones as triple angiokinase inhibitors and the discovery of a highly specific 6-methoxycarbonyl-substituted indolinone (BIBF 1120). J. Med. Chem 2009, 52, 4466–4480. [Google Scholar]
- Hilberg, F.; Roth, G.J.; Krssak, M.; Kautschitsch, S.; Sommergruber, W.; Tontsch-Grunt, U.; Garin-Chesa, P.; Bader, G.; Zoephel, A.; Quant, J.; et al. BIBF 1120: Triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res 2008, 68, 4774–4782. [Google Scholar]
- Du Bois, A.; Huober, J.; Stopfer, P.; Pfisterer, J.; Wimberger, P.; Loibl, S.; Reichardt, V.L.; Harter, P. A phase I open-label dose-escalation study of oral BIBF 1120 combined with standard paclitaxel and carboplatin in patients with advanced gynecological malignancies. Ann. Oncol 2010, 21, 370–375. [Google Scholar]
- Friedlander, M.; Hancock, K.C.; Rischin, D.; Messing, M.J.; Stringer, C.A.; Matthys, G.M.; Ma, B.; Hodge, J.P.; Lager, J.J. A Phase II, open-label study evaluating pazopanib in patients with recurrent ovarian cancer. Gynecol. Oncol 2010, 119, 32–37. [Google Scholar]
- Hamberg, P.; Verweij, J.; Sleijfer, S. (Pre-)Clinical pharmacology and activity of pazopanib, a novel multikinase angiogenesis inhibitor. Oncologist 2010, 15, 539–547. [Google Scholar]
- Matulonis, U.A.; Berlin, S.; Ivy, P.; Tyburski, K.; Krasner, C.; Zarwan, C.; Berkenblit, A.; Campos, S.; Horowitz, N.; Cannistra, S.A.; et al. Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J. Clin. Oncol 2009, 27, 5601–5606. [Google Scholar]
- Sahade, M.; Caparelli, F.; Hoff, P.M. Cediranib: A VEGF receptor tyrosine kinase inhibitor. Future Oncol 2012, 8, 775–781. [Google Scholar]
- Wedge, S.R.; Kendrew, J.; Hennequin, L.F.; Valentine, P.J.; Barry, S.T.; Brave, S.R.; Smith, N.R.; James, N.H.; Dukes, M.; Curwen, J.O.; et al. AZD2171: A highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res 2005, 65, 4389–4400. [Google Scholar]
- Matei, D.; Sill, M.W.; Lankes, H.A.; DeGeest, K.; Bristow, R.E.; Mutch, D.; Yamada, S.D.; Cohn, D.; Calvert, V.; Farley, J.; et al. Activity of sorafenib in recurrent ovarian cancer and primary peritoneal carcinomatosis: A gynecologic oncology group trial. J. Clin. Oncol 2011, 29, 69–75. [Google Scholar]
- Matsumura, N.; Mandai, M.; Okamoto, T.; Yamaguchi, K.; Yamamura, S.; Oura, T.; Baba, T.; Hamanishi, J.; Kang, H.S.; Matsui, S.; et al. Sorafenib efficacy in ovarian clear cell carcinoma revealed by transcriptome profiling. Cancer Sci 2010, 101, 2658–2663. [Google Scholar]
- Bauerschlag, D.O.; Schem, C.; Tiwari, S.; Egberts, J.H.; Weigel, M.T.; Kalthoff, H.; Jonat, W.; Maass, N.; Meinhold-Heerlein, I. Sunitinib (SU11248) inhibits growth of human ovarian cancer in xenografted mice. Anticancer Res 2010, 30, 3355–3360. [Google Scholar]
- Campos, S.M.; Penson, R.T.; Matulonis, U.; Horowitz, N.S.; Whalen, C.; Pereira, L.; Tyburski, K.; Roche, M.; Szymonifka, J.; Berlin, S. A phase II trial of Sunitinib malate in recurrent and refractory ovarian, fallopian tube and peritoneal carcinoma. Gynecol. Oncol 2013, 128, 215–220. [Google Scholar]
- Karlan, B.Y.; Oza, A.M.; Richardson, G.E.; Provencher, D.M.; Hansen, V.L.; Buck, M.; Chambers, S.K.; Ghatage, P.; Pippitt, C.H., Jr.; Brown, J.V., III; et al. Randomized, double-blind, placebo-controlled phase II study of AMG 386 combined with weekly paclitaxel in patients with recurrent ovarian cancer. J. Clin. Oncol 2012, 30, 362–371. [Google Scholar]
- Polverino, A.; Coxon, A.; Starnes, C.; Diaz, Z.; DeMelfi, T.; Wang, L.; Bready, J.; Estrada, J.; Cattley, R.; Kaufman, S.; et al. AMG 706, an oral, multikinase inhibitor that selectively targets vascular endothelial growth factor, platelet-derived growth factor, and kit receptors, potently inhibits angiogenesis and induces regression in tumor xenografts. Cancer Res 2006, 66, 8715–8721. [Google Scholar]
- Teoh, D.; Secord, A.A. Antiangiogenic agents in combination with chemotherapy for the treatment of epithelial ovarian cancer. Int. J. Gynecol. Cancer 2012, 22, 348–359. [Google Scholar]
- Pasquier, E.; Carré, M.; Pourroy, B.; Camoin, L.; Rebaï, O.; Briand, C.; Braguer, D. Antiangiogenic activity of paclitaxel is associated with its cytostatic effect, mediated by the initiation but not completion of a mitochondrial apoptotic signaling pathway. Mol. Cancer Ther 2004, 3, 1301–1310. [Google Scholar]
- Merchan, J.R.; Jayaram, D.R.; Supko, J.G.; He, X.; Bubley, G.J.; Sukhatme, V.P. Increased endothelial uptake of paclitaxel as a potential mechanism for its antiangiogenic effects: Potentiation by Cox-2 inhibition. Int. J. Cancer 2005, 113, 490–498. [Google Scholar]
- Jubb, A.M.; Hurwitz, H.I.; Bai, W.; Holmgren, E.B.; Tobin, P.; Guerrero, A.S.; Kabbinavar, F.; Holden, S.N.; Novotny, W.F.; Frantz, G.D.; et al. Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J. Clin. Oncol 2006, 24, 217–227. [Google Scholar]
- Dowlati, A.; Gray, R.; Johnson, D.H.; Schiller, J.H.; Brahmer, J.; Sandler, A.B. Prospective correlative assessment of biomarkers in E4599 randomized phase II/III trial of carboplatin and paclitaxel ± bevacizumab in advanced non-small cell lung cancer (NSCLC). J. Clin. Oncol 2006, 24, 7027. [Google Scholar]
- Horowitz, N.S.; Penson, R.T.; Duda, D.G.; di Tomaso, E.; Boucher, Y.; Ancukiewicz, M.; Cohen, K.S.; Berlin, S.; Krasner, C.N.; Moses, M.A.; et al. Safety, Efficacy, and Biomarker Exploration in a Phase II Study of Bevacizumab, Oxaliplatin, and Gemcitabine in Recurrent Müllerian Carcinoma. Clin. Ovarian Cancer Other Gynecol. Malig 2011, 4, 26–33. [Google Scholar]
- Duda, D.G.; Willett, C.G.; Ancukiewicz, M.; di Tomaso, E.; Shah, M.; Czito, B.G.; Bentley, R.; Poleski, M.; Lauwers, G.Y.; Carroll, M.; et al. Plasma soluble VEGFR-1 is a potential dual biomarker of response and toxicity for bevacizumab with chemoradiation in locally advanced rectal cancer. Oncologist 2010, 15, 577–583. [Google Scholar]
- Sorensen, A.G.; Batchelor, T.T.; Zhang, W.T.; Chen, P.J.; Yeo, P.; Wang, M.; Jennings, D.; Wen, P.Y.; Lahdenranta, J.; Ancukiewicz, M.; et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res 2009, 69, 5296–5300. [Google Scholar]
- Zhu, A.X.; Sahani, D.V.; Duda, D.G.; di Tomaso, E.; Ancukiewicz, M.; Catalano, O.A.; Sindhwani, V.; Blaszkowsky, L.S.; Yoon, S.S.; Lahdenranta, J.; et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: A phase II study. J. Clin. Oncol 2009, 27, 3027–3035. [Google Scholar]
- Motzer, R.J.; Bukowski, R.M. Targeted therapy for metastatic renal cell carcinoma. J. Clin. Oncol 2006, 24, 5601–5608. [Google Scholar]
- Grepin, R.; Guyot, M.; Jacquin, M.; Durivault, J.; Chamorey, E.; Sudaka, A.; Serdjebi, C.; Lacarelle, B.; Scoazec, J.Y.; Negrier, S.; et al. Acceleration of clear cell renal cell carcinoma growth in mice following bevacizumab/Avastin treatment: The role of CXCL cytokines. Oncogene 2012, 31, 1683–1694. [Google Scholar]
- Ebos, J.M.; Lee, C.R.; Cruz-Munoz, W.; Bjarnason, G.A.; Christensen, J.G.; Kerbel, R.S. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 2009, 15, 232–239. [Google Scholar]
- Grunewald, M.; Avraham, I.; Dor, Y.; Bachar-Lustig, E.; Itin, A.; Jung, S.; Chimenti, S.; Landsman, L.; Abramovitch, R.; Keshet, E. VEGF-induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell 2006, 124, 175–189. [Google Scholar]
- Favaro, E.; Amadori, A.; Indraccolo, S. Cellular interactions in the vascular niche: Implications in the regulation of tumor dormancy. Acta Pathol. Microbiol. Immunol. Scand. 2008, 116, 648–659. [Google Scholar]

© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gavalas, N.G.; Liontos, M.; Trachana, S.-P.; Bagratuni, T.; Arapinis, C.; Liacos, C.; Dimopoulos, M.A.; Bamias, A. Angiogenesis-Related Pathways in the Pathogenesis of Ovarian Cancer. Int. J. Mol. Sci. 2013, 14, 15885-15909. https://doi.org/10.3390/ijms140815885
Gavalas NG, Liontos M, Trachana S-P, Bagratuni T, Arapinis C, Liacos C, Dimopoulos MA, Bamias A. Angiogenesis-Related Pathways in the Pathogenesis of Ovarian Cancer. International Journal of Molecular Sciences. 2013; 14(8):15885-15909. https://doi.org/10.3390/ijms140815885
Chicago/Turabian StyleGavalas, Nikos G., Michalis Liontos, Sofia-Paraskevi Trachana, Tina Bagratuni, Calliope Arapinis, Christine Liacos, Meletios A. Dimopoulos, and Aristotle Bamias. 2013. "Angiogenesis-Related Pathways in the Pathogenesis of Ovarian Cancer" International Journal of Molecular Sciences 14, no. 8: 15885-15909. https://doi.org/10.3390/ijms140815885
APA StyleGavalas, N. G., Liontos, M., Trachana, S.-P., Bagratuni, T., Arapinis, C., Liacos, C., Dimopoulos, M. A., & Bamias, A. (2013). Angiogenesis-Related Pathways in the Pathogenesis of Ovarian Cancer. International Journal of Molecular Sciences, 14(8), 15885-15909. https://doi.org/10.3390/ijms140815885