Disease-Causing Mutations in BEST1 Gene Are Associated with Altered Sorting of Bestrophin-1 Protein
Abstract
:1. Introduction
2. Results and Discussion
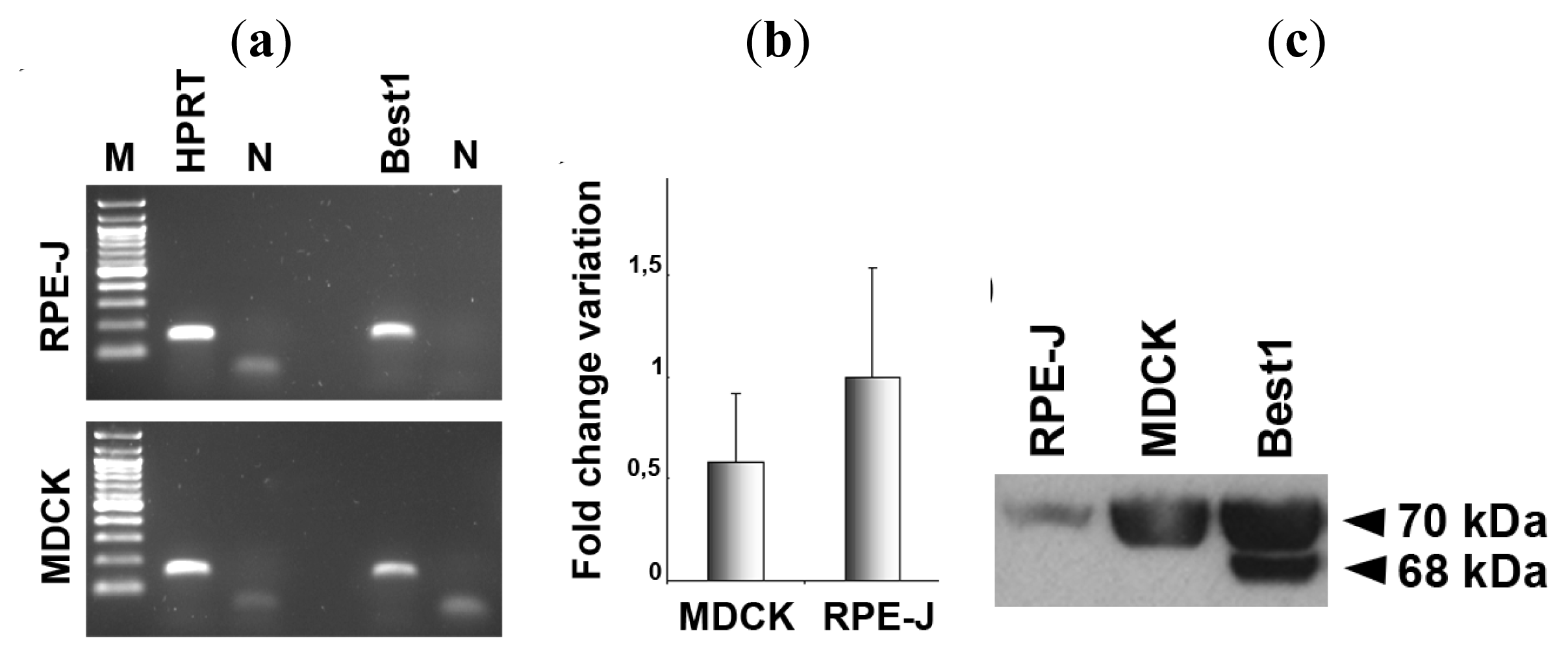
2.1. Expression of Bestrophin-1 in MDCK Cells
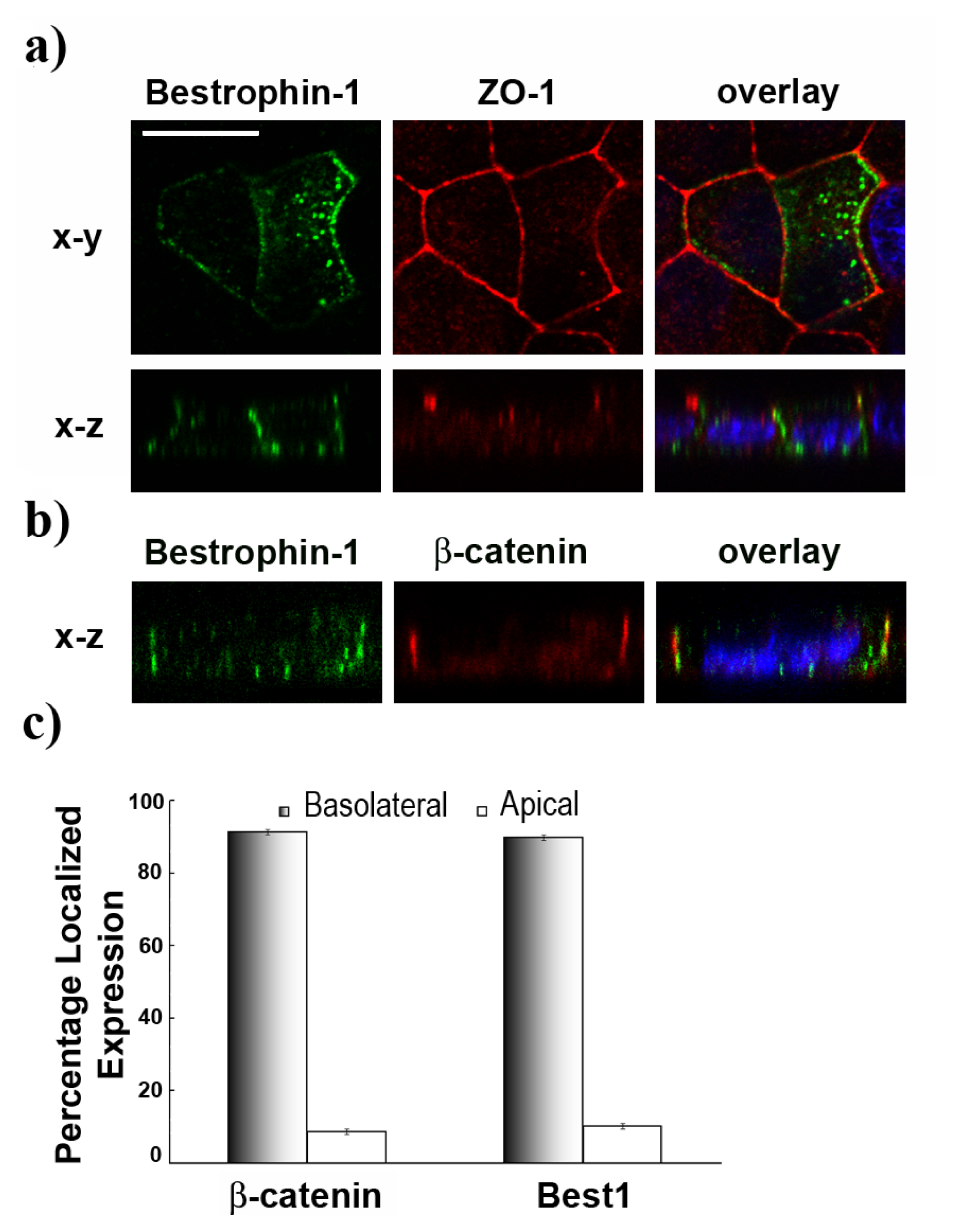
2.2. Basolateral Localization of Human Best1 in Polarized MDCK Cells
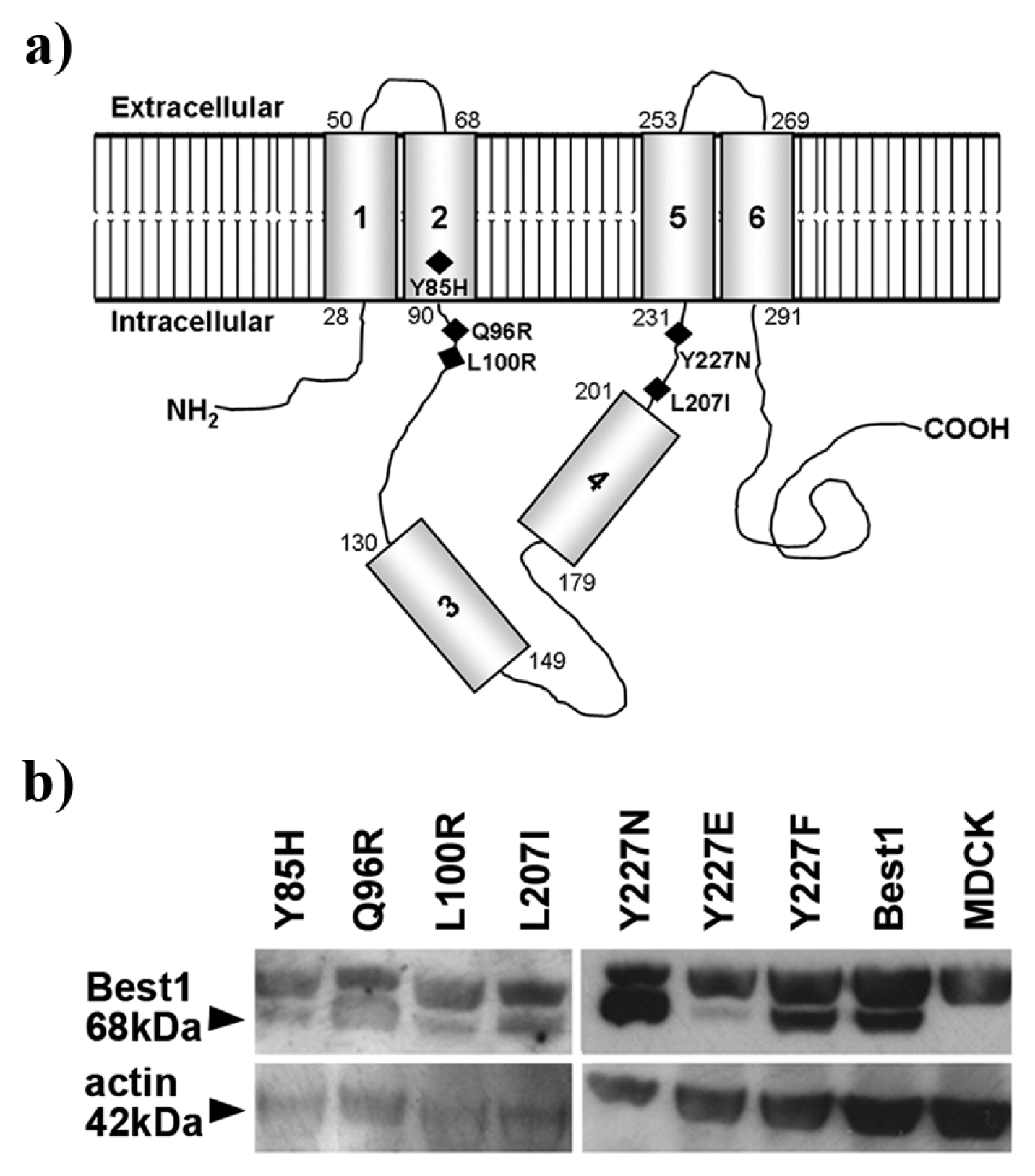
2.3. Best1 Mutants Are Synthesized by MDCK Cells
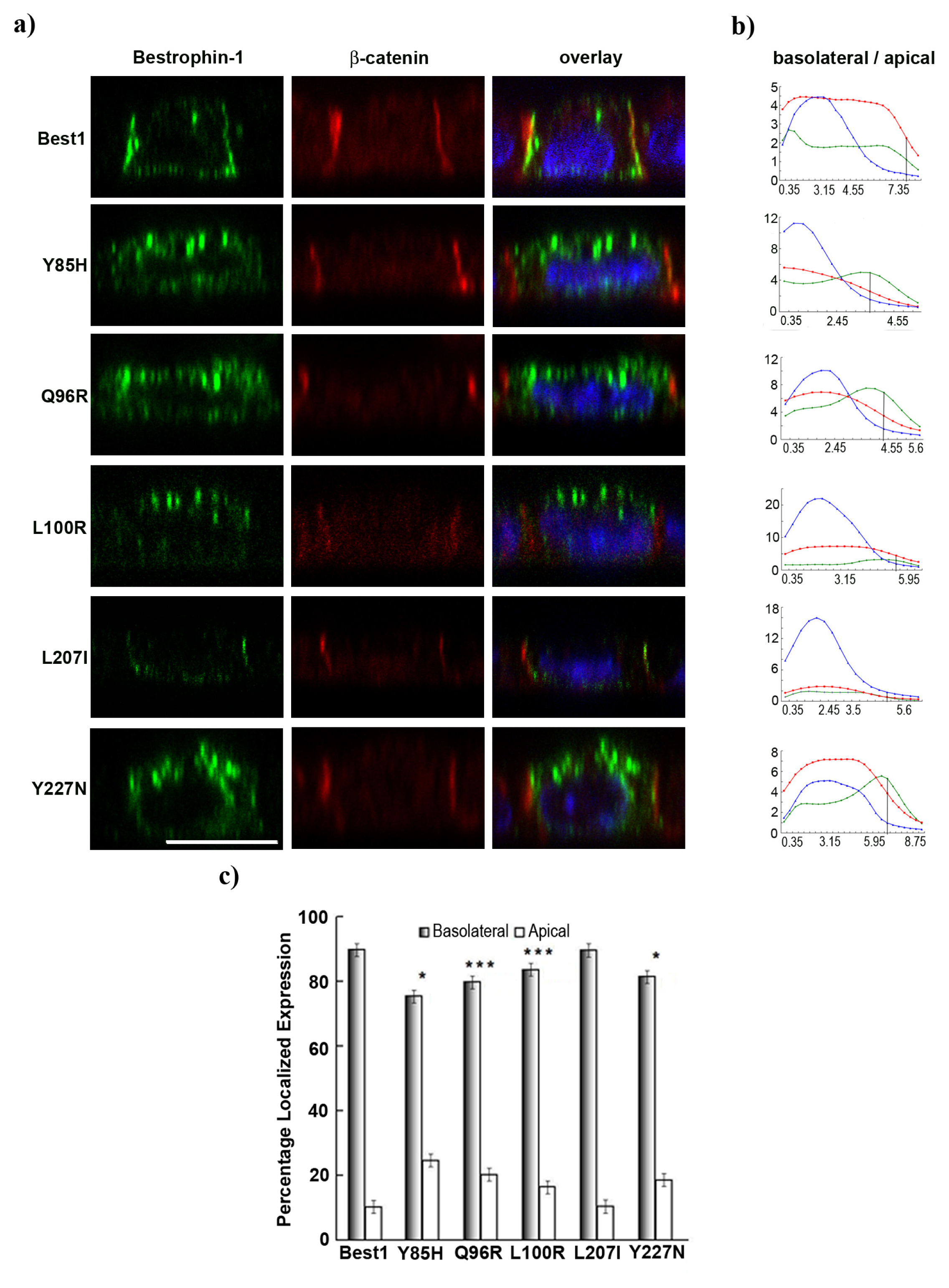
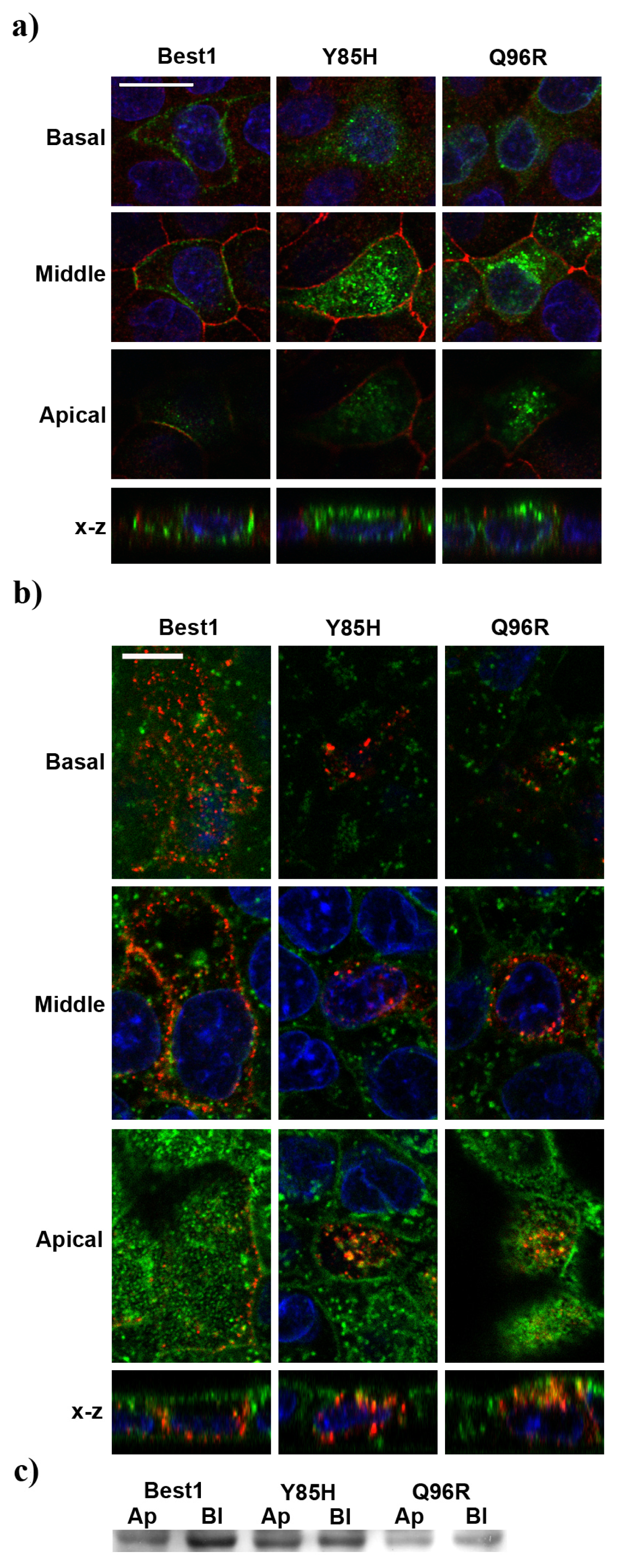
2.4. BVMD-Related Mutations Affect Best1 Basolateral Localization
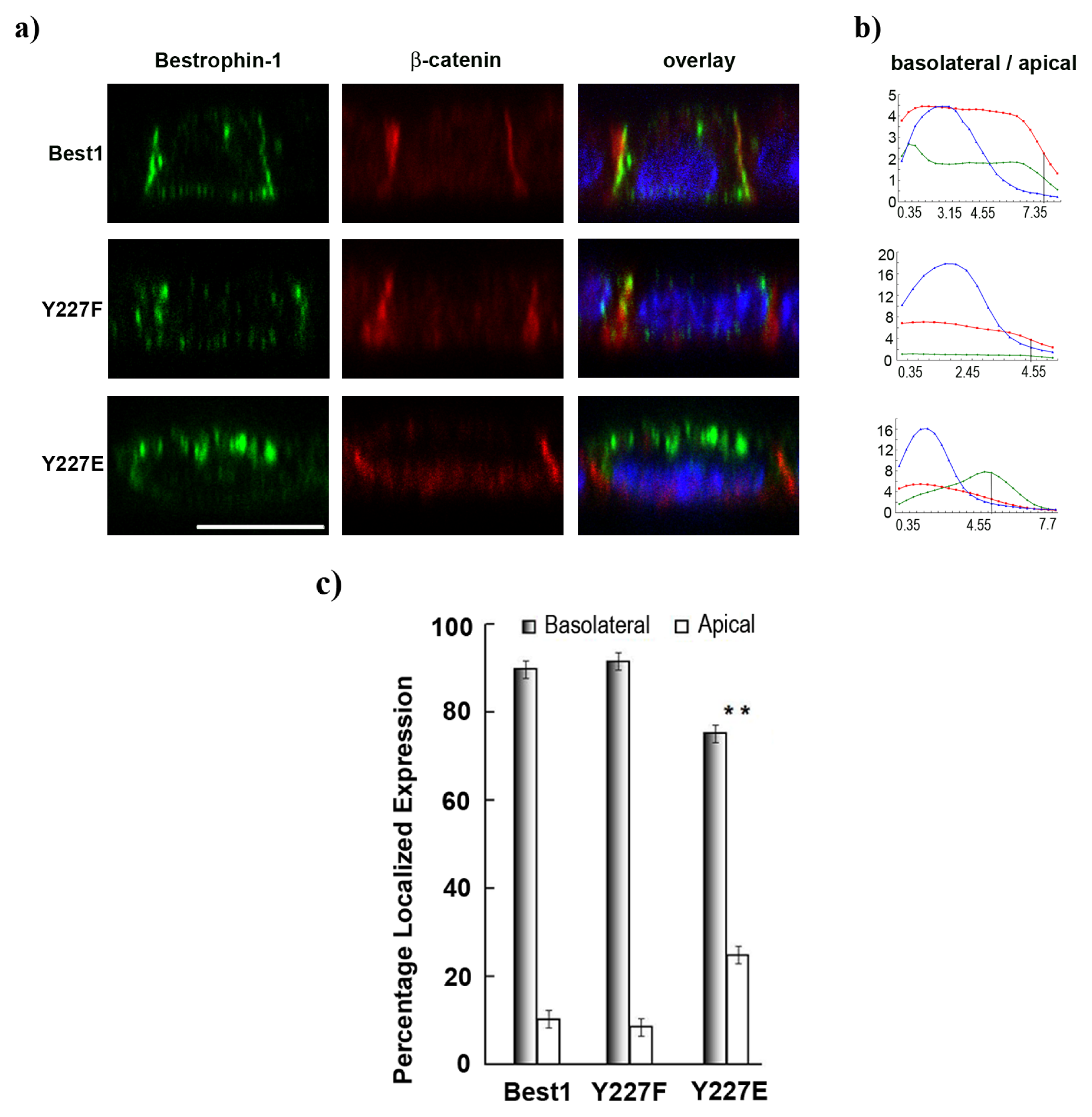
2.5. Pseudo-Phosphorylation of Tyrosine 227 Affects Best1 Basolateral Localization
3. Experimental Section
3.1. Materials
3.2. Expression Vector and Mutagenesis
3.3. Real-Time PCR Analysis
3.4. Cell Culture
3.5. Cell Lysis and Biotinylation Assay
3.6. Immunoprecipitation
3.7. Immunoblotting
3.8. Immunofluorescence Staining and Microscopy Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Boon, C.J.; Klevering, B.J.; Leroy, B.P.; Hoyng, C.B.; Keunen, J.E.; den Hollander, A.I. The spectrum of ocular phenotypes caused by mutations in the BEST1 gene. Prog. Retin. Eye Res 2009, 28, 187–205. [Google Scholar]
- Weingeist, T.A.; Kobrin, J.L.; Watzke, R.C. Histopathology of Best’s macular dystrophy. Arch. Ophthalmol 1982, 100, 1108–1114. [Google Scholar]
- Blodi, C.F.; Stone, E.M. Best’s vitelliform dystrophy. Ophthalmic Paediatr. Genet 1990, 11, 49–59. [Google Scholar]
- Weleber, R.G. Fast and slow oscillations of the electro-oculogram in Best’s macular dystrophy and retinitis pigmentosa. Arch. Ophthalmol 1989, 107, 530–537. [Google Scholar]
- Marquardt, A.; Stohr, H.; Passmore, L.A.; Kramer, F.; Rivera, A.; Weber, B.H. Mutations in a novel gene, VMD2, encoding a protein of unknown properties cause juvenile-onset vitelliform macular dystrophy (Best’s disease). Hum. Mol. Genet 1998, 7, 1517–1525. [Google Scholar]
- Petrukhin, K.; Koisti, M.J.; Bakall, B.; Li, W.; Xie, G.; Marknell, T.; Sandgren, O.; Forsman, K.; Holmgren, G.; Andreasson, S.; et al. Identification of the gene responsible for Best macular dystrophy. Nat. Genet 1998, 19, 241–247. [Google Scholar]
- Marmorstein, A.D.; Cross, H.E.; Peachey, N.S. Functional roles of bestrophins in ocular epithelia. Prog. Retin. Eye Res 2009, 28, 206–226. [Google Scholar]
- Davidson, A.E.; Millar, I.D.; Urquhart, J.E.; Burgess-Mullan, R.; Shweikh, Y.; Parry, N.; O’Sullivan, J.; Maher, G.J.; McKibbin, M.; Downes, S.M.; et al. Missense mutations in a retinal pigment epithelium protein, bestrophin-1, cause retinitis pigmentosa. Am. J. Hum. Genet 2009, 85, 581–592. [Google Scholar]
- Burgess, R.; Millar, I.D.; Leroy, B.P.; Urquhart, J.E.; Fearon, I.M.; De Baere, E.; Brown, P.D.; Robson, A.G.; Wright, G.A.; Kestelyn, P.; et al. Biallelic mutation of BEST1 causes a distinct retinopathy in humans. Am. J. Hum. Genet 2008, 82, 19–31. [Google Scholar]
- Moskova-Doumanova, V.; Pankov, R.; Lalchev, Z.; Doumanov, J. Best1 shot through the eye—structure, functions and clinical implications of bestrophin-1 protein. Biotechnol. Biotechnol. Equip 2013, 27, 3457–3464. [Google Scholar]
- Marmorstein, A.D.; Marmorstein, L.Y.; Rayborn, M.; Wang, X.; Hollyfield, J.G.; Petrukhin, K. Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 2000, 97, 12758–12763. [Google Scholar]
- Milenkovic, V.M.; Rivera, A.; Horling, F.; Weber, B.H. Insertion and topology of normal and mutant bestrophin-1 in the endoplasmic reticulum membrane. J. Biol. Chem 2007, 282, 1313–1321. [Google Scholar]
- Tsunenari, T.; Sun, H.; Williams, J.; Cahill, H.; Smallwood, P.; Yau, K.W.; Nathans, J. Structure-function analysis of the bestrophin family of anion channels. J. Biol. Chem 2003, 278, 41114–41125. [Google Scholar]
- Sun, H.; Tsunenari, T.; Yau, K.W.; Nathans, J. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc. Natl. Acad. Sci. USA 2002, 99, 4008–4013. [Google Scholar]
- O’Driscoll, K.E.; Leblanc, N.; Hatton, W.J.; Britton, F.C. Functional properties of murine bestrophin 1 channel. Biochem. Biophys. Res. Commun 2009, 384, 476–481. [Google Scholar]
- Marmorstein, L.Y.; McLaughlin, P.J.; Stanton, J.B.; Yan, L.; Crabb, J.W.; Marmorstein, A.D. Bestrophin interacts physically and functionally with protein phosphatase 2A. J. Biol. Chem 2002, 277, 30591–30597. [Google Scholar]
- Linsenmeier, R.A.; Steinberg, R.H. Origin and sensitivity of the light peak in the intact cat eye. J. Physiol 1982, 331, 653–673. [Google Scholar]
- Hartzell, C.; Qu, Z.; Putzier, I.; Artinian, L.; Chien, L.T.; Cui, Y. Looking chloride channels straight in the eye: Bestrophins, lipofuscinosis, and retinal degeneration. Physiology (Bethesda) 2005, 20, 292–302. [Google Scholar]
- Marmorstein, A.D.; Stanton, J.B.; Yocom, J.; Bakall, B.; Schiavone, M.T.; Wadelius, C.; Marmorstein, L.Y.; Peachey, N.S. A model of best vitelliform macular dystrophy in rats. Invest. Ophthalmol. Vis. Sci 2004, 45, 3733–3739. [Google Scholar]
- Rosenthal, R.; Bakall, B.; Kinnick, T.; Peachey, N.; Wimmers, S.; Wadelius, C.; Marmorstein, A.; Strauss, O. Expression of bestrophin-1, the product of the VMD2 gene, modulates voltage-dependent Ca2+ channels in retinal pigment epithelial cells. FASEB J 2006, 20, 178–180. [Google Scholar]
- Marmorstein, L.Y.; Wu, J.; McLaughlin, P.; Yocom, J.; Karl, M.O.; Neussert, R.; Wimmers, S.; Stanton, J.B.; Gregg, R.G.; Strauss, O.; et al. The light peak of the electroretinogram is dependent on voltage-gated calcium channels and antagonized by bestrophin (best-1). J. Gen. Physiol 2006, 127, 577–589. [Google Scholar]
- Qu, Z.; Hartzell, H.C. Bestrophin Cl− channels are highly permeable to HCO3. Am. J. Physiol. Cell. Physiol 2008, 294, C1371–C1377. [Google Scholar]
- Woo, D.H.; Han, K.S.; Shim, J.W.; Yoon, B.E.; Kim, E.; Bae, J.Y.; Oh, S.J.; Hwang, E.M.; Marmorstein, A.D.; Bae, Y.C.; et al. TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell 2012, 151, 25–40. [Google Scholar]
- Han, K.S.; Woo, J.; Park, H.; Yoon, B.J.; Choi, S.; Lee, C.J. Channel-mediated astrocytic glutamate release via Bestrophin-1 targets synaptic NMDARs. Mol. Brain 2013, 6, 4. [Google Scholar]
- Rodriguez-Boulan, E.; Powell, S.K. Polarity of epithelial and neuronal cells. Annu. Rev. Cell. Biol 1992, 8, 395–427. [Google Scholar]
- Zegers, M.M.; Hoekstra, D. Mechanisms and functional features of polarized membrane traffic in epithelial and hepatic cells. Biochem. J 1998, 336, 257–269. [Google Scholar]
- Weisz, O.A.; Rodriguez-Boulan, E. Apical trafficking in epithelial cells: signals, clusters and motors. J. Cell. Sci 2009, 122, 4253–4266. [Google Scholar]
- Mullins, R.F.; Oh, K.T.; Heffron, E.; Hageman, G.S.; Stone, E.M. Late development of vitelliform lesions and flecks in a patient with best disease: Clinicopathologic correlation. Arch. Ophthalmol 2005, 123, 1588–1594. [Google Scholar]
- Rodriguez-Boulan, E.; Kreitzer, G.; Musch, A. Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell. Biol 2005, 6, 233–247. [Google Scholar]
- Mellman, I.; Nelson, W.J. Coordinated protein sorting, targeting and distribution in polarized cells. Nat. Rev. Mol. Cell. Biol 2008, 9, 833–845. [Google Scholar]
- Matter, K. Epithelial polarity: Sorting out the sorters. Curr. Biol 2000, 10, R39–R42. [Google Scholar]
- Schuck, S.; Simons, K. Polarized sorting in epithelial cells: Raft clustering and the biogenesis of the apical membrane. J. Cell. Sci 2004, 117, 5955–5964. [Google Scholar]
- Kramer, F.; White, K.; Pauleikhoff, D.; Gehrig, A.; Passmore, L.; Rivera, A.; Rudolph, G.; Kellner, U.; Andrassi, M.; Lorenz, B.; et al. Mutations in the VMD2 gene are associated with juvenile-onset vitelliform macular dystrophy (best disease) and adult vitelliform macular dystrophy but not age-related macular degeneration. Eur. J. Hum. Genet 2000, 8, 286–292. [Google Scholar]
- Lotery, A.J.; Munier, F.L.; Fishman, G.A.; Weleber, R.G.; Jacobson, S.G.; Affatigato, L.M.; Nichols, B.E.; Schorderet, D.F.; Sheffield, V.C.; Stone, E.M. Allelic variation in the VMD2 gene in best disease and age-related macular degeneration. Invest. Ophthalmol. Vis. Sci 2000, 41, 1291–1296. [Google Scholar]
- Davidson, A.E.; Millar, I.D.; Burgess-Mullan, R.; Maher, G.J.; Urquhart, J.E.; Brown, P.D.; Black, G.C.; Manson, F.D. Functional characterization of bestrophin-1 missense mutations associated with autosomal recessive bestrophinopathy. Invest. Ophthalmol. Vis. Sci 2011, 52, 3730–3736. [Google Scholar]
- Milenkovic, V.M.; Rohrl, E.; Weber, B.H.; Strauss, O. Disease-associated missense mutations in bestrophin-1 affect cellular trafficking and anion conductance. J. Cell. Sci 2011, 124, 2988–2996. [Google Scholar]
- Deborde, S.; Perret, E.; Gravotta, D.; Deora, A.; Salvarezza, S.; Schreiner, R.; Rodriguez-Boulan, E. Clathrin is a key regulator of basolateral polarity. Nature 2008, 452, 719–723. [Google Scholar]
- Deora, A.A.; Gravotta, D.; Kreitzer, G.; Hu, J.; Bok, D.; Rodriguez-Boulan, E. The basolateral targeting signal of CD147 (EMMPRIN) consists of a single leucine and is not recognized by retinal pigment epithelium. Mol. Biol. Cell 2004, 15, 4148–4165. [Google Scholar]
- Xiao, Q.; Yu, K.; Cui, Y.Y.; Hartzell, H.C. Dysregulation of human bestrophin-1 by ceramide-induced dephosphorylation. J. Physiol 2009, 587, 4379–4391. [Google Scholar]
- Duran, C.; Chien, L.T.; Hartzell, H.C. Drosophila Bestrophin-1 Currents Are Regulated by Phosphorylation via a CaMKII Dependent Mechanism. PLoS One 2013, 8, e58875. [Google Scholar]
- Hartzell, H.C.; Qu, Z.; Yu, K.; Xiao, Q.; Chien, L.T. Molecular physiology of bestrophins: Multifunctional membrane proteins linked to best disease and other retinopathies. Physiol. Rev 2008, 88, 639–672. [Google Scholar]
- Marmorstein, A.D.; Kinnick, T.R. Focus on molecules: bestrophin (best-1). Exp. Eye Res 2007, 85, 423–424. [Google Scholar]
- Kranjc, A.; Grillo, F.W.; Rievaj, J.; Boccaccio, A.; Pietrucci, F.; Menini, A.; Carloni, P.; Anselmi, C. Regulation of bestrophins by Ca2+: A theoretical and experimental study. PLoS One 2009, 4, e4672. [Google Scholar]
- Milenkovic, V.M.; Krejcova, S.; Reichhart, N.; Wagner, A.; Strauss, O. Interaction of bestrophin-1 and Ca2+ channel beta-subunits: identification of new binding domains on the bestrophin-1 C-terminus. PLoS One 2011, 6, e19364. [Google Scholar]
- Barro Soria, R.; Spitzner, M.; Schreiber, R.; Kunzelmann, K. Bestrophin 1 enables Ca2+ activated Cl- conductance in epithelia. J. Biol. Chem 2006, 284, 29405–29412. [Google Scholar]
- Guziewicz, K.E.; Zangerl, B.; Lindauer, S.J.; Mullins, R.F.; Sandmeyer, L.S.; Grahn, B.H.; Stone, E.M.; Acland, G.M.; Aguirre, G.D. Bestrophin gene mutations cause canine multifocal retinopathy: A novel animal model for best disease. Invest. Ophthalmol. Vis. Sci 2007, 48, 1959–1967. [Google Scholar]
- Kramer, F.; Stohr, H.; Weber, B.H. Cloning and characterization of the murine Vmd2 RFP-TM gene family. Cytogenet. Genome Res 2004, 105, 107–114. [Google Scholar]
- Bakall, B.; Marmorstein, L.Y.; Hoppe, G.; Peachey, N.S.; Wadelius, C.; Marmorstein, A.D. Expression and localization of bestrophin during normal mouse development. Invest. Ophthalmol. Vis. Sci 2003, 44, 3622–3628. [Google Scholar]
- Buk, D.M.; Renner, O.; Graeve, L. Increased association with detergent-resistant membranes/lipid rafts of apically targeted mutants of the interleukin-6 receptor gp80. Eur. J. Cell. Biol 2005, 84, 819–831. [Google Scholar]
- Mullins, R.F.; Kuehn, M.H.; Faidley, E.A.; Syed, N.A.; Stone, E.M. Differential macular and peripheral expression of bestrophin in human eyes and its implication for best disease. Invest. Ophthalmol. Vis. Sci 2007, 48, 3372–3380. [Google Scholar]
- Nandrot, E.F.; Anand, M.; Almeida, D.; Atabai, K.; Sheppard, D.; Finnemann, S.C. Essential role for MFG-E8 as ligand for alphavbeta5 integrin in diurnal retinal phagocytosis. Proc. Natl. Acad. Sci. USA 2007, 104, 12005–12010. [Google Scholar]
- Buk, D.M.; Waibel, M.; Braig, C.; Martens, A.S.; Heinrich, P.C.; Graeve, L. Polarity and lipid raft association of the components of the ciliary neurotrophic factor receptor complex in Madin-Darby canine kidney cells. J. Cell. Sci 2004, 117, 2063–2075. [Google Scholar]
- Doumanov, J.A.; Daubrawa, M.; Unden, H.; Graeve, L. Identification of a basolateral sorting signal within the cytoplasmic domain of the interleukin-6 signal transducer gp130. Cell. Signal 2006, 18, 1140–1146. [Google Scholar]
- Lisanti, M.P.; Sargiacomo, M.; Graeve, L.; Saltiel, A.R.; Rodriguez-Boulan, E. Polarized apical distribution of glycosyl-phosphatidylinositol-anchored proteins in a renal epithelial cell line. Proc. Natl. Acad. Sci. USA 1988, 85, 9557–9561. [Google Scholar]
| Mutants | Primers |
|---|---|
| Best1 Y85H | F 5′-CGTGCTGGGCTTCCACGTGACGCTGGT-3′ R 5′-ACCAGCGTCACGTGGAAGCCCAGCACG-3′ |
| Best1 Q96R | F 5′-CCGCTGGTGGAACCGGTACGAGAACCTGC-3′ R 5′-GCAGGTTCTCGTACCGGTTCCACCAGCGG-3′ |
| Best1 L100R | F 5′-CAGTACGAGAACCGGCCGTGGCCCGAC-3′ R 5′-GTCGGGCCACGGCCGGTTCTCGTACTG-3′ |
| Best1 L207I | F 5′-GGGACCCTATCCTGATCCAGAGCCTGCTG-3′ R 5′-CAGCAGGCTCTGGATCAGGATAGGGTCCC-3′ |
| Best1 Y227N | F 5′-GTGGACACCTGTATGCCAACGACTGGATTAGTATC-3′ R 5′-GATACTAATCCAGTCGTTGGCATACAGGTGTCCAC-3′ |
| Best1 Y227E | F 5′-GTGGACACCTGTATGCCGAGGACTGGATTAGTATCCC-3′ R 5′-GGGATACTAATCCAGTCCTCGGCATACAGGTGTCCAC-3′ |
| Best1 Y227F | F 5′-GTGTGGACACCTGTATGCCTTCGACTGGATTAGT-3′ R 5′-ACTAATCCAGTCGAAGGCATACAGGTGTCCACAC-3′ |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Doumanov, J.A.; Zeitz, C.; Gimenez, P.D.; Audo, I.; Krishna, A.; Alfano, G.; Diaz, M.L.B.; Moskova-Doumanova, V.; Lancelot, M.-E.; Sahel, J.-A.; et al. Disease-Causing Mutations in BEST1 Gene Are Associated with Altered Sorting of Bestrophin-1 Protein. Int. J. Mol. Sci. 2013, 14, 15121-15140. https://doi.org/10.3390/ijms140715121
Doumanov JA, Zeitz C, Gimenez PD, Audo I, Krishna A, Alfano G, Diaz MLB, Moskova-Doumanova V, Lancelot M-E, Sahel J-A, et al. Disease-Causing Mutations in BEST1 Gene Are Associated with Altered Sorting of Bestrophin-1 Protein. International Journal of Molecular Sciences. 2013; 14(7):15121-15140. https://doi.org/10.3390/ijms140715121
Chicago/Turabian StyleDoumanov, Jordan A., Christina Zeitz, Paloma Dominguez Gimenez, Isabelle Audo, Abhay Krishna, Giovanna Alfano, Maria Luz Bellido Diaz, Veselina Moskova-Doumanova, Marie-Elise Lancelot, José-Alain Sahel, and et al. 2013. "Disease-Causing Mutations in BEST1 Gene Are Associated with Altered Sorting of Bestrophin-1 Protein" International Journal of Molecular Sciences 14, no. 7: 15121-15140. https://doi.org/10.3390/ijms140715121
APA StyleDoumanov, J. A., Zeitz, C., Gimenez, P. D., Audo, I., Krishna, A., Alfano, G., Diaz, M. L. B., Moskova-Doumanova, V., Lancelot, M.-E., Sahel, J.-A., Nandrot, E. F., & Bhattacharya, S. S. (2013). Disease-Causing Mutations in BEST1 Gene Are Associated with Altered Sorting of Bestrophin-1 Protein. International Journal of Molecular Sciences, 14(7), 15121-15140. https://doi.org/10.3390/ijms140715121






