Presence of proNGF-Sortilin Signaling Complex in Nigral Dopamine Neurons and Its Variation in Relation to Aging, Lactacystin and 6-OHDA Insults
Abstract
:1. Introduction
2. Results and Discussion
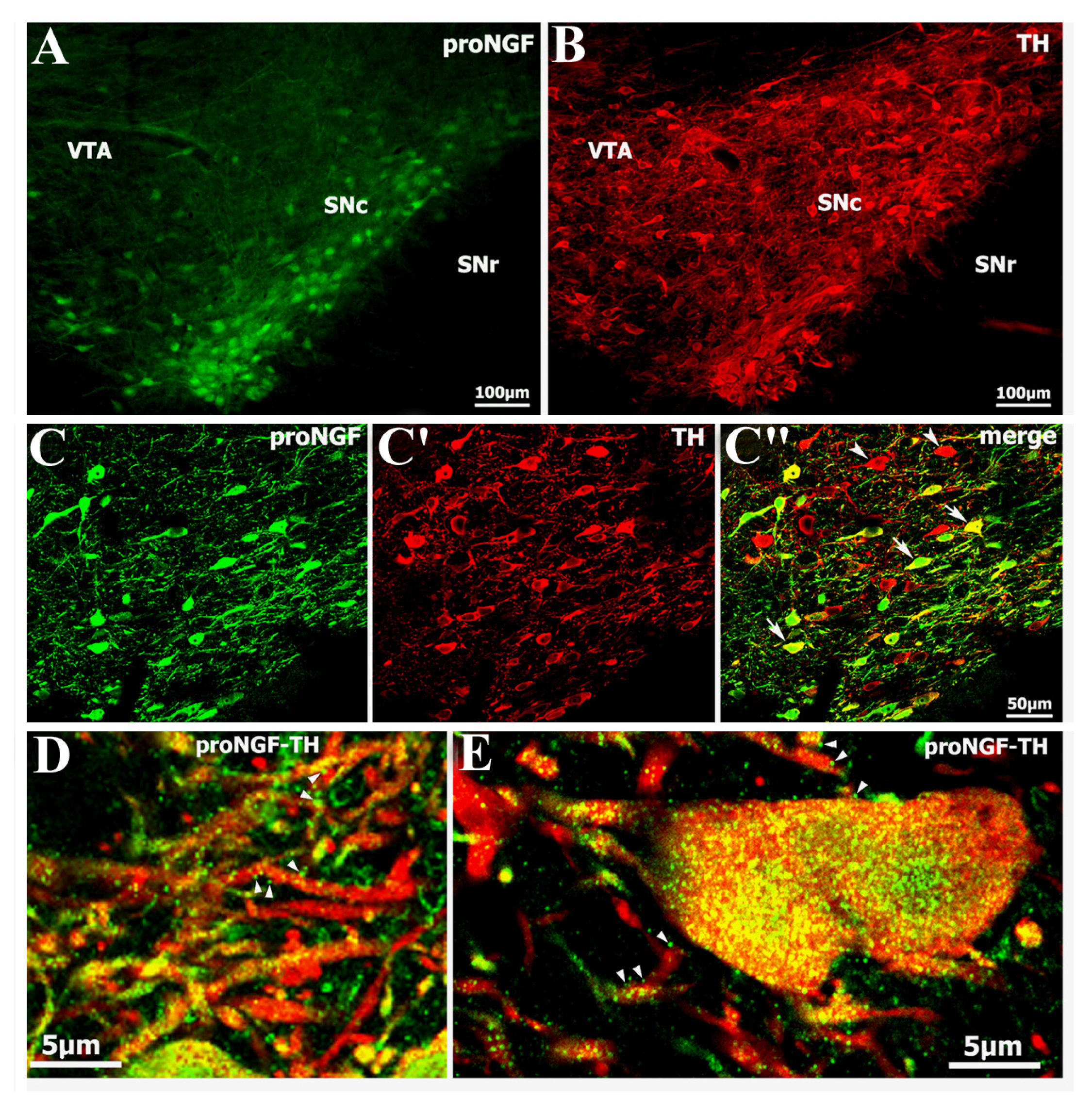
2.1. Identification of Abundant proNGF in A9 Ventral Tier Dopamine Neurons of the Substantia Nigra
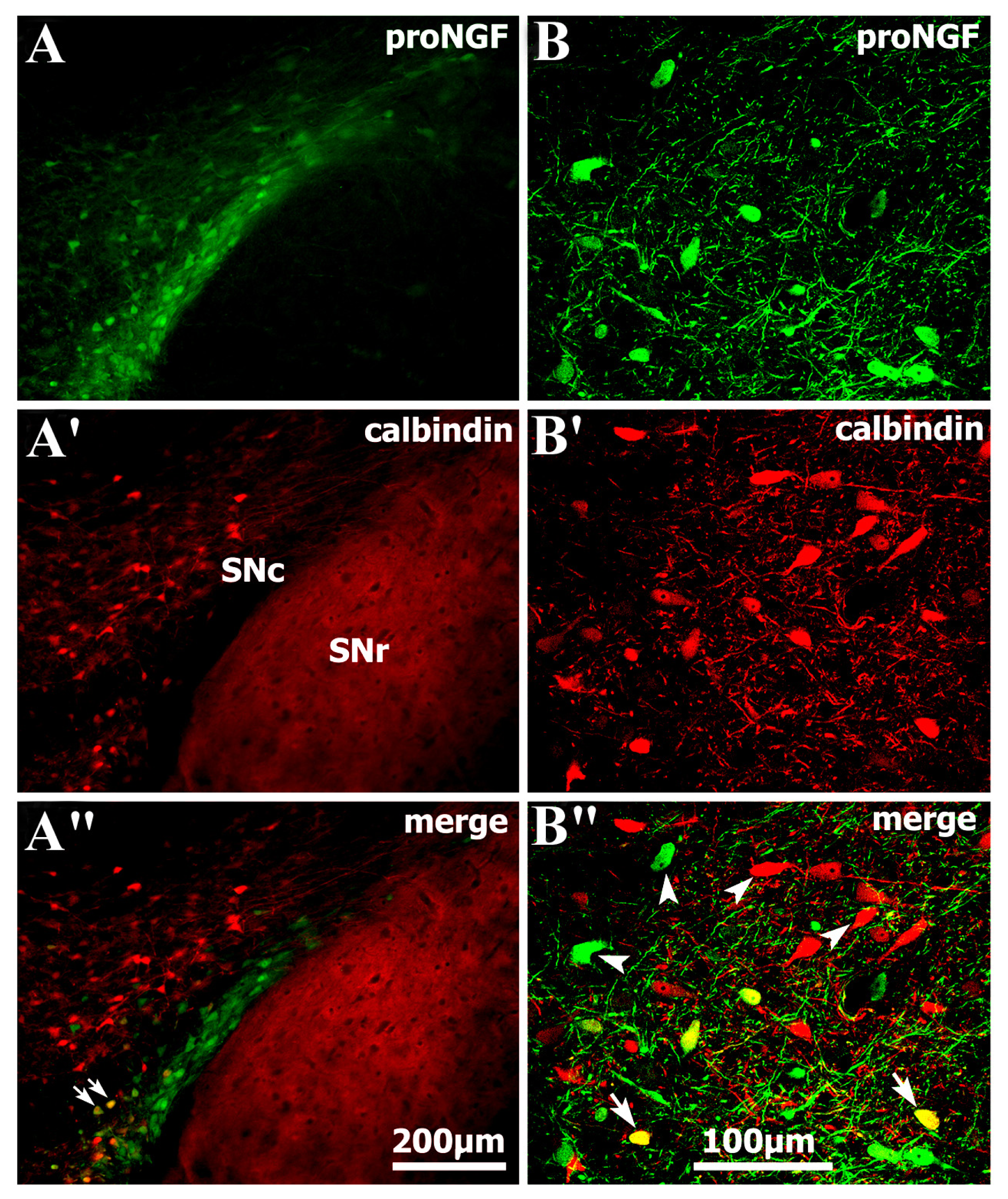
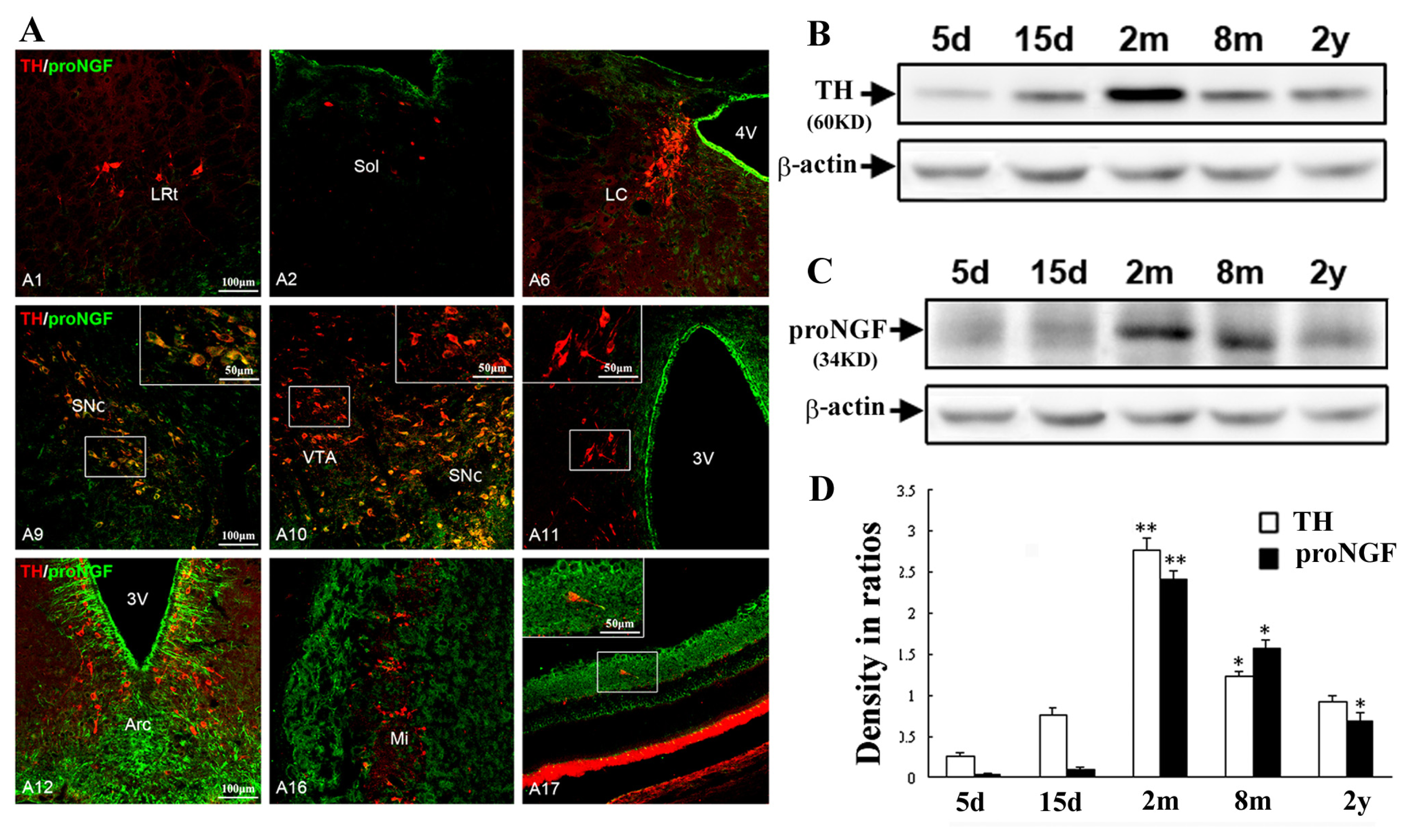
2.2. Selective Distribution of proNGF/TH Neurons in Substantia Nigra and Age-Related Changes
2.3. Abundant Distribution of Sortilin/TH Neurons in Substantia Nigra and Age-Related Changes
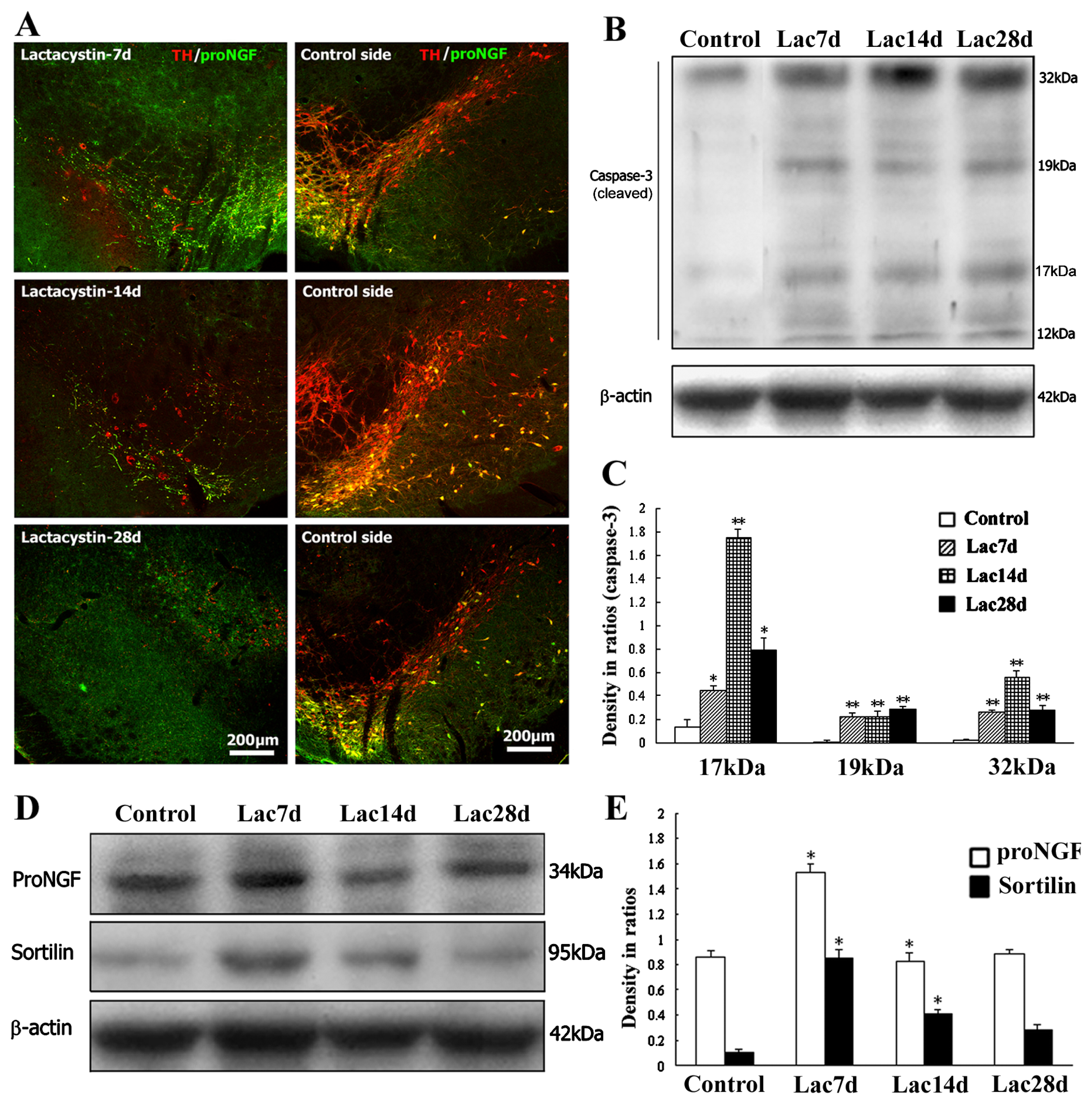
2.4. Neurodegenerative or Dying Sensitivity of proNGF-Containing Neurons to Lactacystin Insult
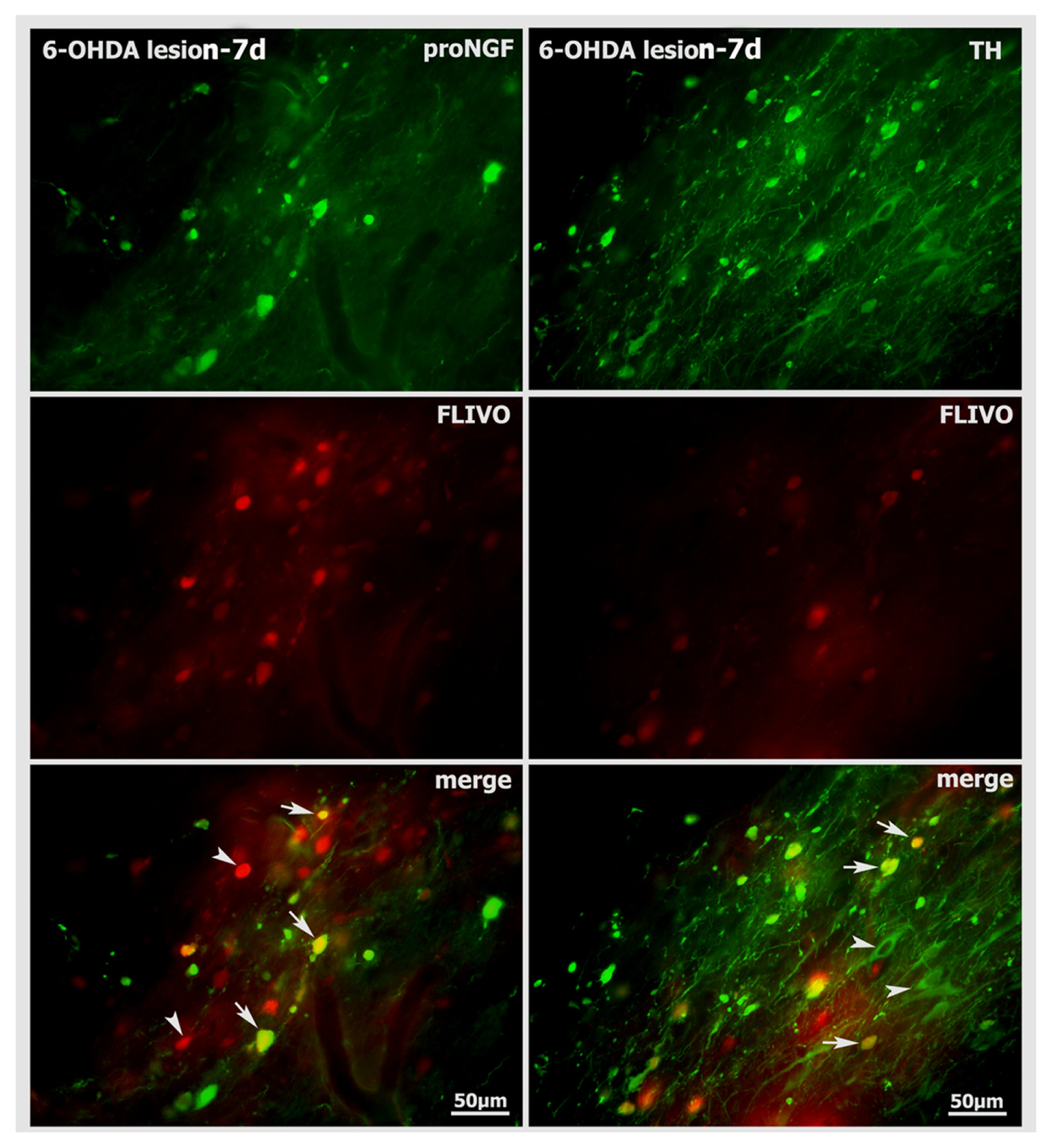
2.5. Neurodegenerative Vulnerability of proNGF-Containing Neurons to 6-OHDA Insult
3. Experimental Section
3.1. Animals and Animal Models
3.2. Brain Tissue Preparation
3.3. Immunofluorescence
3.4. Immunoelectron Microscopy
3.5. Western Blot
3.6. FLIVO Staining for Neuronal Degeneration
3.7. Statistical Analysis
4. Conclusions
Acknowledgements
Conflict of Interest
Abbreviations
| 6-OHDA | 6-hydroxydopamine |
| AD | Alzheimer’s disease |
| ALS | amyotrophic lateral sclerosis |
| BDNF | brain-derived neurotrophic factor |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| NGF | nerve growth factor |
| NT-3 | neurotrophin-3 |
| p75NTR | p75 neurotrophin receptor |
| PBS | phosphate buffered saline |
| PD | Parkinson’s disease |
| proBDNF | proform of brain-derived neurotrophic factor |
| proNGF | proform of nerve growth factor |
| TH | tyrosine hydroxylase |
References
- Fernandez-Espejo, E. Pathogenesis of Parkinson’s disease: Prospects of neuroprotective and restorative therapies. Mol. Neurobiol 2004, 29, 15–30. [Google Scholar]
- Rangasamy, S.B.; Soderstrom, K.; Bakay, R.A.; Kordower, J.H. Neurotrophic factor therapy for Parkinson’s disease. Prog. Brain Res 2010, 184, 237–264. [Google Scholar]
- Yacoubian, T.A.; Standaert, D.G. Targets for neuroprotection in Parkinson’s disease. Biochim. Biophys. Acta 2009, 1792, 676–687. [Google Scholar]
- Chen, L.W.; Zhang, J.P.; Shum, D.K.Y.; Chan, Y.S. Localization of NGF, NT3 and GDNF in nestin-expressing reactive astrocytes in the caudate-putamen of MPTP-treated C57/BL mice. J. Comp. Neurol 2006, 497, 898–909. [Google Scholar]
- Chung, E.K.; Chen, L.W.; Chan, Y.S.; Yung, K.K. Downregulation of glial glutamate transporters after dopamine denervation in the striatum of 6-hydroxydopamine-lesioned rats. J. Comp. Neurol 2008, 511, 421–437. [Google Scholar]
- Wu, J.; Yu, W.; Chen, Y.; Su, Y.; Ding, Z.; Ren, H.; Jiang, Y.; Wang, J. Intrastriatal transplantation of GDNF-engineered BMSCs and its neuroprotection in lactacystin-induced Parkinsonian rat model. Neurochem. Res 2010, 35, 495–502. [Google Scholar]
- Chen, L.W.; Yung, K.K.; Chan, Y.S.; Shum, D.K.; Bolam, J.P. The proNGF-p75NTR-sortilin signalling complex as new target for the therapeutic treatment of Parkinson’s disease. CNS Neurol. Disord. Drug Targets 2008, 7, 512–523. [Google Scholar]
- Wang, Y.Q.; Bian, G.L.; Bai, Y.; Cao, R.; Chen, L.W. Identification and kainic acid-induced up-regulation of low-affinity p75 neurotrophin receptor (p75NTR) in the nigral dopamine neurons of adult rats. Neurochem. Int 2008, 53, 56–62. [Google Scholar]
- Barrett, G.L. The p75 neurotrophin receptor and neuronal apoptosis. Prog. Neurobiol 2000, 61, 205–229. [Google Scholar]
- Dechant, G.; Barde, Y.A. The neurotrophin receptor p75(NTR): Novel functions and implications for diseases of the nervous system. Nat. Neurosci 2002, 5, 1131–1136. [Google Scholar]
- Fahnestock, M.; Yu, G.; Coughlin, M.D. ProNGF: A neurotrophic or an apoptotic molecule? Prog. Brain Res 2004, 146, 101–110. [Google Scholar]
- Nykjaer, A.; Lee, R.; Teng, K.K.; Jansen, P.; Madsen, P.; Nielsen, M.S.; Jacobsen, C.; Kliemannel, M.; Schwarz, E.; Willnow, T.E.; et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature 2004, 427, 843–848. [Google Scholar]
- Nykjaer, A.; Willnow, T.E.; Petersen, C.M. p75NTR-live or let die. Curr. Opin. Neurobiol 2005, 15, 49–57. [Google Scholar]
- Al-Shawi, R.; Hafner, A.; Chun, S.; Raza, S.; Crutcher, K.; Thrasivoulou, C.; Simons, P.; Cowen, T. ProNGF, sortilin, and age-related neurodegeneration. Ann. N.Y. Acad. Sci 2007, 1119, 208–215. [Google Scholar]
- Arnett, M.G.; Ryals, J.M.; Wright, D.E. Pro-NGF, sortilin, and p75NTR: Potential mediators of injury-induced apoptosis in the mouse dorsal root ganglion. Brain Res 2007, 1183, 32–42. [Google Scholar]
- Cuello, A.C.; Bruno, M.A. The failure in NGF maturation and its increased degradation as the probable cause for the vulnerability of cholinergic neurons in Alzheimer’s disease. Neurochem. Res 2007, 32, 1041–1045. [Google Scholar]
- Di Maria, E.; Giorgio, E.; Uliana, V.; Bonvicini, C.; Faravelli, F.; Cammarata, S.; Novello, M.C.; Galimberti, D.; Scarpini, E.; Zanetti, O.; et al. Possible influence of a non-synonymous polymorphism located in the NGF precursor on susceptibility to late-onset Alzheimer’s disease and mild cognitive impairment. J. Alzheimers Dis. 2012, 29, 699–705. [Google Scholar]
- Harrington, A.W.; Leiner, B.; Blechschmitt, C.; Arevalo, J.C.; Lee, R.; Mörl, K.; Meyer, M.; Hempstead, B.L.; Yoon, S.O.; Giehl, K.M. Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc. Natl. Acad. Sci. USA 2004, 101, 6226–6230. [Google Scholar]
- Küst, B.M.; Brouwer, N.; Mantingh, I.J.; Boddeke, H.W.; Copray, J.C. Reduced p75NTR expression delays disease onset only in female mice of a transgenic model of familial amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord 2003, 4, 100–105. [Google Scholar]
- Griffin, R.J.; Williams, B.W.; Bischof, J.C.; Olin, M.; Johnson, G.L.; Lee, B.W. Use of a fluorescently labeled poly-caspase inhibitor for in vivo detection of apoptosis related to vascular-targeting agent arsenic trioxide for cancer therapy. Technol. Cancer Res. Treat 2007, 6, 651–654. [Google Scholar]
- Hennigan, A.; O’Callaghan, R.M.; Kelly, A.M. Neurotrophins and their receptors: Roles in plasticity, neurodegeneration and neuroprotection. Biochem. Soc. Trans 2007, 35, 424–427. [Google Scholar]
- Al-Shawi, R.; Hafner, A.; Olson, J.; Chun, S.; Raza, S.; Thrasivoulou, C.; Lovestone, S.; Killick, R.; Simons, P.; Cowen, T. Neurotoxic and neurotrophic roles of proNGF and the receptor sortilin in the adult and aging nervous system. Eur. J. Neurosci 2008, 27, 2103–2114. [Google Scholar]
- Masoudi, R.; Ioannou, M.S.; Coughlin, M.D.; Pagadala, P.; Neet, K.E.; Clewes, O.; Allen, S.J.; Dawbarn, D.; Fahnestock, M. Biological activity of nerve growth factor precursor is dependent upon relative levels of its receptors. J. Biol. Chem 2009, 284, 18424–18433. [Google Scholar]
- Volosin, M.; Song, W.; Almeida, R.D.; Kaplan, D.R.; Hempstead, B.L.; Friedman, W.J. Interaction of survival and death signaling in basal forebrain neurons: Roles of neurotrophins and proneurotrophins. J. Neurosci 2006, 26, 7756–7766. [Google Scholar]
- Wang, Y.J.; Valadares, D.; Sun, Y.; Wang, X.; Zhong, J.H.; Liu, X.H.; Majd, S.; Chen, L.; Gao, C.Y.; Chen, S.; et al. Effects of proNGF on neuronal viability, neurite growth and amyloid-beta metabolism. Neurotox. Res 2010, 17, 257–267. [Google Scholar]
- Nakamura, K.; Namekata, K.; Harada, C.; Harada, T. Intracellular sortilin expression pattern regulates proNGF-induced naturally occurring cell death during development. Cell Death Differ 2007, 14, 1552–1554. [Google Scholar]
- Rogers, M.L.; Bailey, S.; Matusica, D.; Nicholson, I.; Muyderman, H.; Pagadala, P.C.; Neet, K.E.; Zola, H.; Macardle, P.; Rush, R.A. ProNGF mediates death of Natural Killer cells through activation of the p75NTR-sortilin complex. J. Neuroimmunol 2010, 226, 93–103. [Google Scholar]
- Santos, A.M.; López-Sánchez, N.; Martín-Oliva, D.; de la Villa, P.; Cuadros, M.A.; Frade, J.M. Sortilin participates in light-dependent photoreceptor degeneration in vivo. PLoS One 2012, 7, e36243. [Google Scholar]
- Sobottka, B.; Reinhardt, D.; Brockhaus, M.; Jacobsen, H.; Metzger, F. ProNGF inhibits NGF-mediated TrkA activation in PC12 cells. J. Neurochem 2008, 107, 1294–1303. [Google Scholar]
- Song, W.; Volosin, M.; Cragnolini, A.B.; Hempstead, B.L.; Friedman, W.J. ProNGF induces PTEN via p75NTR to suppress Trk-mediated survival signaling in brain neurons. J. Neurosci 2010, 30, 15608–15615. [Google Scholar]
- Coulson, E.J.; May, L.M.; Osborne, S.L.; Reid, K.; Underwood, C.K.; Meunier, F.A.; Bartlett, P.F.; Sah, P. p75 neurotrophin receptor mediates neuronal cell death by activating GIRK channels through phosphatidylinositol 4,5-bisphosphate. J. Neurosci 2008, 28, 315–324. [Google Scholar]
- Bhakar, A.L.; Howell, J.L.; Paul, C.E.; Salehi, A.H.; Becker, E.B.; Said, F.; Bonni, A.; Barker, P.A. Apoptosis induced by p75NTR overexpression requires Jun kinase-dependent phosphorylation of Bad. J. Neurosci. 2003, 23, 11373–11381. [Google Scholar]
- Linggi, M.S.; Burke, T.L.; Williams, B.B.; Harrington, A.; Kraemer, R.; Hempstead, B.L.; Yoon, S.O.; Carter, B.D. Neurotrophin receptor interacting factor (NRIF) is an essential mediator of apoptotic signaling by the p75 neurotrophin receptor. J. Biol. Chem 2005, 280, 13801–13808. [Google Scholar]
- Florez-McClure, M.L.; Linseman, D.A.; Chu, C.T.; Barker, P.A.; Bouchard, R.J.; Le, S.S.; Laessig, T.A.; Heidenreich, K.A. The p75 neurotrophin receptor can induce autophagy and death of cerebellar Purkinje neurons. J. Neurosci 2004, 24, 4498–4509. [Google Scholar]
- Lee, R.; Kermani, P.; Teng, K.K.; Hempstead, B.L. Regulation of cell survival by secreted proneurotrophins. Science 2001, 294, 1945–1948. [Google Scholar]
- Srinivasan, B.; Roque, C.H.; Hempstead, B.L.; Al-Ubaidi, M.R.; Roque, R.S. Microglia-derived pronerve growth factor promotes photoreceptor cell death via p75 neurotrophin receptor. J. Biol. Chem 2004, 279, 41839–41845. [Google Scholar]
- Yune, T.Y.; Lee, J.Y.; Jung, G.Y.; Kim, S.J.; Jiang, M.H.; Kim, Y.C.; Oh, Y.J.; Markelonis, G.J.; Oh, T.H. Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury. J. Neurosci 2007, 27, 7751–7761. [Google Scholar]
- Pehar, M.; Cassina, P.; Vargas, M.R.; Castellanos, R.; Viera, L.; Beckman, J.S.; Estévez, A.G.; Barbeito, L. Astrocytic production of nerve growth factor in motor neuron apoptosis: Implications for amyotrophic lateral sclerosis. J. Neurochem 2004, 89, 464–473. [Google Scholar]
- Kalous, A.; Nangle, M.R.; Anastasia, A.; Hempstead, B.L.; Keast, J.R. Neurotrophic actions initiated by proNGF in adult sensory neurons may require peri-somatic glia to drive local cleavage to NGF. J. Neurochem 2012, 122, 523–536. [Google Scholar]
- Takamura, A.; Sato, Y.; Watabe, D.; Okamoto, Y.; Nakata, T.; Kawarabayashi, T.; Oddo, S.; Laferla, F.M.; Shoji, M.; Matsubara, E. Sortilin is required for toxic action of Aβ oligomers (AβOs): Extracellular AβOs trigger apoptosis, and intraneuronal AβOs impair degradation pathways. Life Sci 2012, 91, 1177–1186. [Google Scholar]
- Tauris, J.; Gustafsen, C.; Christensen, E.I.; Jansen, P.; Nykjaer, A.; Nyengaard, J.R.; Teng, K.K.; Schwarz, E.; Ovesen, T.; Madsen, P.; et al. Proneurotrophin-3 may induce sortilin-dependent death in inner ear neurons. Eur. J. Neurosci 2011, 33, 622–631. [Google Scholar]
- Teng, H.K.; Teng, K.K.; Lee, R.; Wright, S.; Tevar, S.; Almeida, R.D.; Kermani, P.; Torkin, R.; Chen, Z.Y.; Lee, F.S.; et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J. Neurosci 2005, 25, 5455–5463. [Google Scholar]
- Jansen, P.; Giehl, K.; Nyengaard, J.R.; Teng, K.; Lioubinski, O.; Sjoegaard, S.S.; Breiderhoff, T.; Gotthardt, M.; Lin, F.; Eilers, A.; et al. Roles for the pro-neurotrophin receptor sortilin in neuronal development, aging and brain injury. Nat. Neurosci 2007, 10, 1449–1457. [Google Scholar]
- Bierl, M.A.; Isaacson, L.G. Increased NGF proforms in aged sympathetic neurons and their targets. Neurobiol. Aging 2007, 28, 122–134. [Google Scholar]
- Terry, A.V., Jr.; Kutiyanawalla, A.; Pillai, A. Age-dependent alterations in nerve growth factor (NGF)-related proteins, sortilin, and learning and memory in rats. Physiol. Behav. 2011, 102, 149–157. [Google Scholar]
- Greferath, U.; Mallard, C.; Roufail, E.; Rees, S.M.; Barrett, G.L.; Bartlett, P.F. Expression of the p75 neurotrophin receptor by striatal cholinergic neurons following global ischemia in rats is associated with neuronal degeneration. Neurosci. Lett 2002, 332, 57–60. [Google Scholar]
- Provenzano, M.J.; Xu, N.; ver Meer, M.R.; Clark, J.J.; Hansen, M.R. p75NTR and sortilin increase after facial nerve injury. Laryngoscope 2008, 118, 87–93. [Google Scholar]
- Capsoni, S.; Brandi, R.; Arisi, I.; D’Onofrio, M.; Cattaneo, A. A dual mechanism linking NGF/proNGF imbalance and early inflammation to Alzheimer’s disease neurodegeneration in the AD11 anti-NGF mouse model. CNS Neurol. Disord. Drug Targets 2011, 10, 635–647. [Google Scholar]
- Cuello, A.C.; Bruno, M.A.; Allard, S.; Leon, W.; Iulita, M.F. Cholinergic involvement in Alzheimer’s disease A link with NGF maturation and degradation. J. Mol. Neurosci 2010, 40, 230–235. [Google Scholar]
- Counts, S.E.; Mufson, E.J. The role of nerve growth factor receptors in cholinergic basal forebrain degeneration in prodromal Alzheimer disease. J. Neuropathol. Exp. Neurol 2005, 64, 263–272. [Google Scholar]
- Ali, T.K.; Al-Gayyar, M.M.; Matragoon, S.; Pillai, B.A.; Abdelsaid, M.A.; Nussbaum, J.J.; El-Remessy, A.B. Diabetes-induced peroxynitrite impairs the balance of pro-nerve growth factor and nerve growth factor, and causes neurovascular injury. Diabetologia 2011, 54, 657–668. [Google Scholar]
- Stoica, G.; Lungu, G.; Kim, H.T.; Wong, P.K. Up-regulation of pro-nerve growth factor, neurotrophin receptor p75, and sortilin is associated with retrovirus-induced spongiform encephalomyelopathy. Brain Res 2008, 1208, 204–216. [Google Scholar]
- Turner, B.J.; Murray, S.S.; Piccenna, L.G.; Lopes, E.C.; Kilpatrick, T.J.; Cheema, S.S. Effect of p75 neurotrophin receptor antagonist on disease progression in transgenic amyotrophic lateral sclerosis mice. J. Neurosci. Res 2004, 78, 193–199. [Google Scholar]
- Wang, T.; Liu, Y.Y.; Wang, X.; Yang, N.; Zhu, H.B.; Zuo, P.P. Protective effects of octacosanol on 6-hydroxydopamine-induced Parkinsonism in rats via regulation of ProNGF and NGF signaling. Acta Pharmacol. Sin 2010, 31, 765–774. [Google Scholar]
- Hempstead, B.L. Commentary: Regulating proNGF action: Multiple targets for therapeutic intervention. Neurotox. Res 2009, 16, 255–260. [Google Scholar]



© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xia, Y.; Chen, B.-Y.; Sun, X.-L.; Duan, L.; Gao, G.-D.; Wang, J.-J.; Yung, K.K.-L.; Chen, L.-W. Presence of proNGF-Sortilin Signaling Complex in Nigral Dopamine Neurons and Its Variation in Relation to Aging, Lactacystin and 6-OHDA Insults. Int. J. Mol. Sci. 2013, 14, 14085-14104. https://doi.org/10.3390/ijms140714085
Xia Y, Chen B-Y, Sun X-L, Duan L, Gao G-D, Wang J-J, Yung KK-L, Chen L-W. Presence of proNGF-Sortilin Signaling Complex in Nigral Dopamine Neurons and Its Variation in Relation to Aging, Lactacystin and 6-OHDA Insults. International Journal of Molecular Sciences. 2013; 14(7):14085-14104. https://doi.org/10.3390/ijms140714085
Chicago/Turabian StyleXia, Yi, Bei-Yu Chen, Xiao-Long Sun, Li Duan, Guo-Dong Gao, Jing-Jie Wang, Ken Kam-Lin Yung, and Liang-Wei Chen. 2013. "Presence of proNGF-Sortilin Signaling Complex in Nigral Dopamine Neurons and Its Variation in Relation to Aging, Lactacystin and 6-OHDA Insults" International Journal of Molecular Sciences 14, no. 7: 14085-14104. https://doi.org/10.3390/ijms140714085



