Two Distinct Channels Mediated by m2mAChR and α9nAChR Co-Exist in Type II Vestibular Hair Cells of Guinea Pig
Abstract
:1. Introduction
2. Results and Discussion
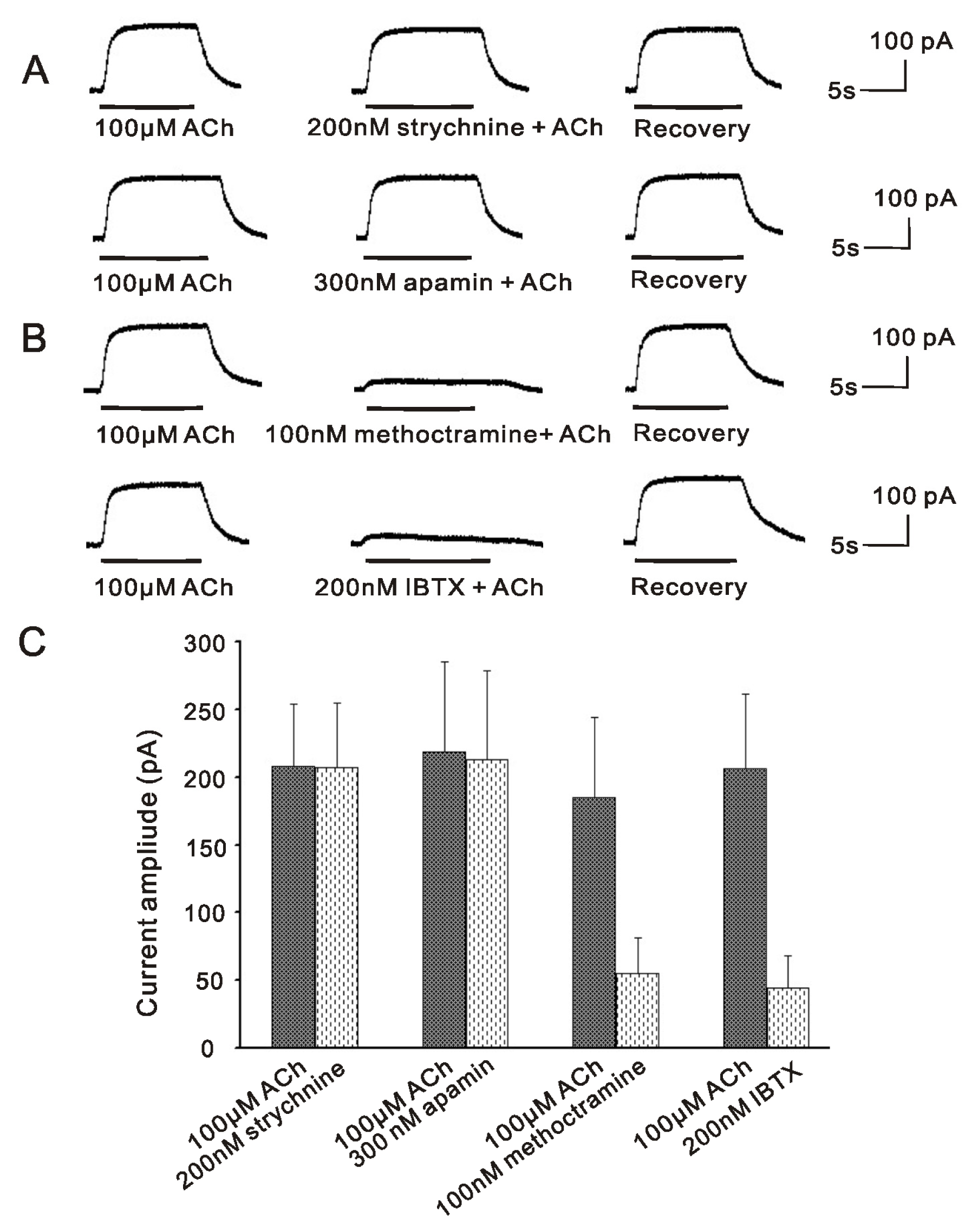
2.1. m2mAChR Sensitive BK Currents Exist in VHCs II Isolated by Collagenase IA
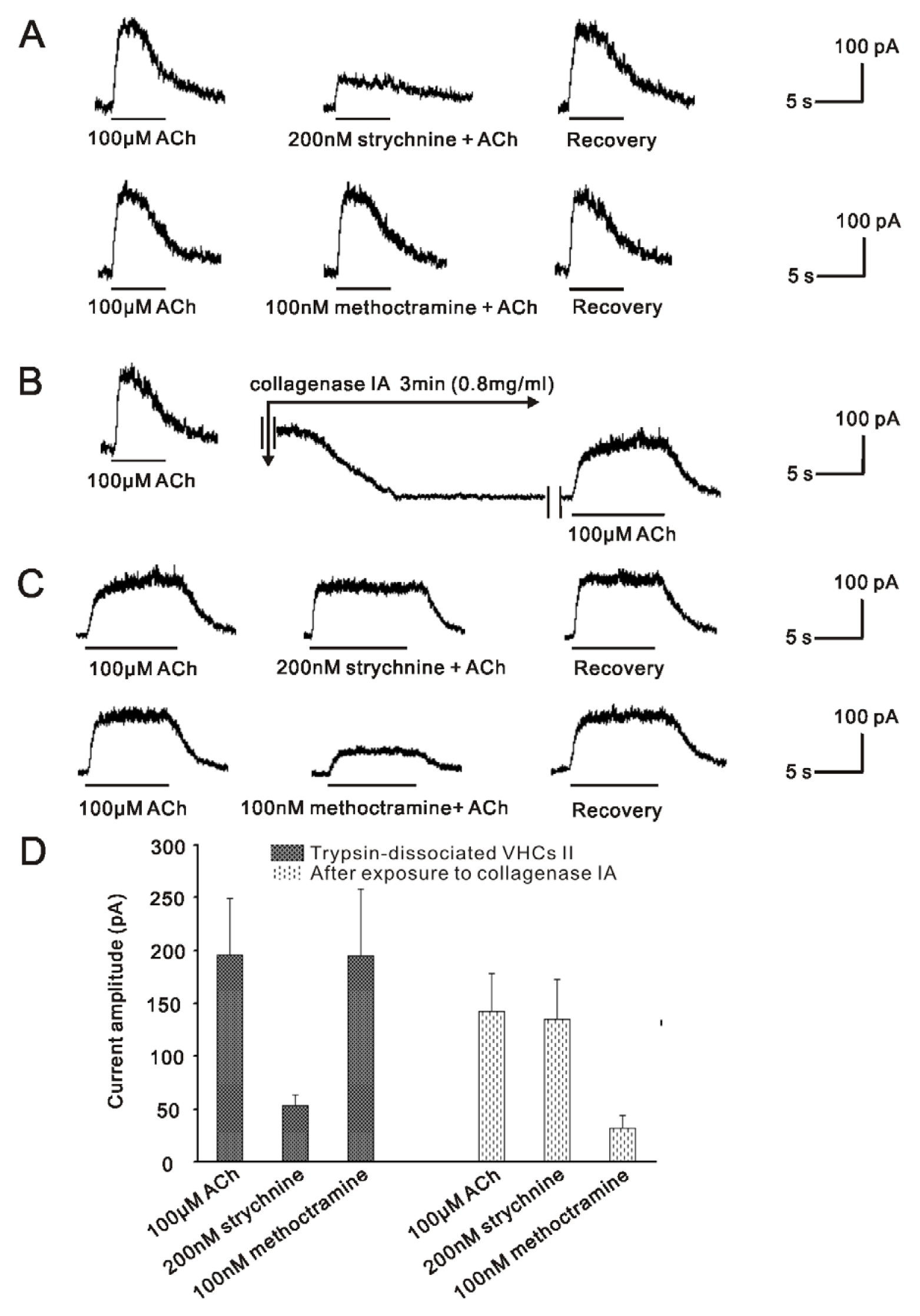
2.2. α9nAChR Sensitive SK2 Currents Exist in VHCs II Isolated by Trypsin
2.3. m2mAChR Sensitive BK Currents and α9nAChR Sensitive SK2 Currents Co-Exist in VHCs II
2.4. Discussion
2.4.1. BK Channel and SK2 Channel Co-Exist in the Same VHCs II, but do Not Play Their Roles at the Same Time in Vestibular Efferent Inhibition as in OHCs
2.4.2. The Mechanisms Underlying the Changes of the ACh Response in Trypsined Cells after Exposure to Collagenase IA
3. Experimental Section
3.1. Animal Procedures and VHCs II Preparation
3.2. VHCs II Isolated by Collagenase IA
3.3. VHCs II Isolated by Trypsin
3.4. Electrophysiology
3.5. Drugs
3.6. Data Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Scarfone, E.; Ulfendahl, M.; Lofstrand, P.; Flock, A. Light- and electron microscopy of isolated vestibular hair cells from the guinea pig. Cell Tissue Res 1991, 266, 51–58. [Google Scholar]
- Godfrey, D.A.; Park, J.L.; Ross, C.D. Choline acetyltransferase and acetylcholinesterase in centrifugal labyrinthine bundles of rats. Hear. Res 1984, 14, 93–106. [Google Scholar]
- Kong, W.J.; Egg, G.; Hussl, B.; Spoendlin, H.; Schrott-Fischer, A. Localization of chat-like immunoreactivity in the vestibular endorgans of the rat. Hear. Res 1994, 75, 191–200. [Google Scholar]
- Kong, W.J.; Hussl, B.; Thumfart, W.F.; Schrott-Fischer, A. Ultrastructural localization of ChAT-like i mMunoreactivity in the human vestibular periphery. Hear. Res 1998, 119, 96–103. [Google Scholar]
- Kong, W.J.; Scholtz, A.W.; Hussl, B.; Kammen-Jolly, K.; Schrott-Fischer, A. Localization of efferent neurotransmitters in the inner ear of the homozygous Bronx waltzer mutant mouse. Hear. Res 2002, 167, 136–155. [Google Scholar]
- Guth, P.S.; Perin, P.; Norris, C.H.; Valli, P. The vestibular hair cells: Post-transductional signal processing. Prog. Neurobiol 1998, 54, 193–247. [Google Scholar]
- Caulfield, M.P. Muscarinic receptors—Characterization, coupling and function. Pharmacol. Ther 1993, 58, 319–379. [Google Scholar]
- Wess, J. Molecular biology of muscarinic acetylcholine receptors. Crit. Rev. Neurobiol 1996, 10, 69–99. [Google Scholar]
- Anderson, A.D.; Troyanovskaya, M.; Wackym, P.A. Differential expression of alpha2–7, alpha9 and beta2–4 nicotinic acetylcholine receptor subunit mRNA in the vestibular end-organs and Scarpa’s ganglia of the rat. Brain. Res 1997, 778, 409–413. [Google Scholar]
- Wackym, P.A.; Chen, C.T.; Ishiyama, A.; Pettis, R.M.; Lopez, I.A.; Hoffman, L. Muscarinic acetylcholine receptor subtype mRNAs in the human and rat vestibular periphery. Cell Biol. Int 1996, 2, 187–192. [Google Scholar]
- Ishiyama, A.; Lopez, I.; Wackym, P.A. Molecular characterization of muscarinic receptors in the human vestibular periphery. Implications for pharmacotherapy. Am. J. Otol 1997, 18, 648–654. [Google Scholar]
- Li, G.Q.; Kevetter, G.A.; Leonard, R.B.; Prusak, D.J.; Wood, T.G.; Correia, M.J. Muscarinic acetylcholine receptor subtype expression in avian vestibular hair cells, nerve terminals and ganglion cells. Neuroscience 2007, 146, 384–402. [Google Scholar]
- Yao, Q.; Cheng, H.; Guo, C.; Zhou, T.; Huang, X.; Kong, W. Muscarinic acetylcholine receptor subtype expression in type vestibular hair cells of guinea pigs. J. Huazhong. Uni. Sci. Technol 2011, 31, 682–686. [Google Scholar]
- Yoshida, N.; Shigemoto, T.; Sugai, T.; Ohmori, H. The role of inositol trisphosphate on ACh-induced outward currents in bullfrog saccular hair cells. Brain. Res 1994, 644, 90–100. [Google Scholar]
- Elgoyhen, A.B.; Vetter, D.E.; Katz, E.; Rothlin, C.V.; Heinemann, S.F.; Boulter, J. alpha10: A determinant of nicotinic cholinergic receptor function in ma mMalian vestibular and cochlear mechanosensory hair cells. Proc. Natl. Acad. Sci. USA 2001, 98, 3501–3506. [Google Scholar]
- Holt, J.C.; Lioudyno, M.; Athas, G.; Garcia, M.M.; Perin, P.; Guth, P.S. The effect of proteolytic enzymes on the alpha9-nicotinic receptor-mediated response in isolated frog vestibular hair cells. Hear. Res 2001, 152, 25–42. [Google Scholar]
- Kong, W.J.; Guo, C.K.; Zhang, S.; Hao, J.; Wang, Y.J.; Li, Z.W. The properties of ACh-induced BK currents in guinea pig type II vestibular hair cells. Hear. Res 2005, 209, 1–9. [Google Scholar]
- Kong, W.J.; Guo, C.K.; Zhang, X.W.; Chen, X.; Zhang, S.; Li, G.Q.; Li, Z.W.; van Cauwenberge, P. The coupling of acetylcholine-induced BK channel and calcium channel in guinea pig saccular type II vestibular hair cells. Brain. Res 2007, 1129, 110–115. [Google Scholar]
- Guo, C.K.; Wang, Y.; Zhou, T.; Yu, H.; Zhang, W.J.; Kong, W.J. M2 muscarinic ACh receptors sensitive BK channels mediate cholinergic inhibition of type II vestibular hair cells. Hear. Res 2012, 285, 13–19. [Google Scholar]
- Kong, W.J.; Guo, C.K.; Zhang, S.; Zhang, X.W.; Wang, Y.J.; Li, Z.W. Fast cholinergic efferent inhibition in guinea pig outer hair cells. Brain. Res 2006, 1102, 103–108. [Google Scholar]
- Darbon, P.; Wright, D.J.; Evans, M.G. Conductance properties of the acetylcholine receptor current of Guinea pig outer hair cells. J. Assoc. Res. Otolaryngol 2011, 12, 59–70. [Google Scholar]
- Armstrong, C.E.; Roberts, W.M. Electrical properties of frog saccular hair cells: Distortion by enzymatic dissociation. J. Neurosci 1998, 18, 2962–2973. [Google Scholar]
- Armstrong, C.E.; Roberts, W.M. Rapidly inactivating and non-inactivating calcium-activated potassium currents in frog saccular hair cells. J. Physiol 2001, 536, 49–65. [Google Scholar]
- Eybalin, M. Neurotransmitters and neuromodulators of the ma mMalian cochlea. Physiol. Rev 1993, 73, 309–373. [Google Scholar]
- Schweizer, F.E.; Savin, D.; Luu, C.; Sultemeier, D.R.; Hoffman, L.F. Distribution of high-conductance calcium-activated potassium channels in rat vestibular epithelia. J. Comp. Neuro 2009, 517, 134–145. [Google Scholar]
- Wersinger, E.; McLean, W.J.; Fuchs, P.A.; Pyott, S.J. BK channels mediate cholinergic inhibition of high frequency cochlear hair cells. PloS One 2010, 5, e13836. [Google Scholar]
- Maison, S.F.; Pyott, S.J.; Meredith, A.L.; Liberman, M.C. Olivocochlear suppression of outer hair cells in vivo: Evidence for combined action of BK and SK2 channels throughout the cochlea. J. Neurophysiol 2013. [Google Scholar] [CrossRef]
- Engel, J.; Braig, C.; Ruttiger, L.; Kuhn, S.; Zi mMermann, U.; Blin, N.; Sausbier, M.; Kalbacher, H.; Munkner, S.; Rohbock, K.; et al. Two classes of outer hair cells along the tonotopic axis of the cochlea. Neuroscience 2006, 143, 837–849. [Google Scholar]
- Hafidi, A.; Beurg, M.; Dulon, D. Localization and developmental expression of BK channels in ma mMalian cochlear hair cells. Neuroscience 2005, 130, 475–484. [Google Scholar]
- Maison, S.F.; Liu, X.P.; Vetter, D.E.; Eatock, R.A.; Nathanson, N.M.; Wess, J.; Liberman, M.C. Muscarinic signaling in the cochlea: presynaptic and postsynaptic effects on efferent feedback and afferent excitability. J. Neurosci 2010, 30, 6751–6762. [Google Scholar]
- Vetter, D.E.; Liberman, M.C.; Mann, J.; Barhanin, J.; Boulter, J.; Brown, M.C.; Saffiote-Kolman, J.; Heinemann, S.F.; Elgoyhen, A.B. Role of alpha9 nicotinic ACh receptor subunits in the development and function of cochlear efferent innervation. Neuron 1999, 23, 93–103. [Google Scholar]
- Vetter, D.E.; Katz, E.; Maison, S.F.; Taranda, J.; Turcan, S.; Ballestero, J.; Liberman, M.C.; Elgoyhen, A.B.; Boulter, J. The alpha10 nicotinic acetylcholine receptor subunit is required for normal synaptic function and integrity of the olivocochlear system. Proc. Natl. Acad. Sci. USA 2007, 104, 20594–20599. [Google Scholar]
- Lewis, R.S.; Hudspeth, A.J. Voltage- and ion-dependent conductances in solitary vertebrate hair cells. Nature 1983, 304, 538–541. [Google Scholar]
- Steinacker, A.; Rojas, L. Acetylcholine modulated potassium channel in the hair cell of the toadfish saccule. Hear. Res. 1988, 35, 265–269. [Google Scholar]
- Housley, G.D.; Norris, C.H.; Guth, P.S. Cholinergically-induced changes in outward currents in hair cells isolated from the semicircular canal of the frog. Hear. Res 1990, 43, 121–133. [Google Scholar]
- Holt, J.R.; Eatock, R.A. Inwardly rectifying currents of saccular hair cells from the leopard frog. Neurophysiology 1995, 73, 1484–1502. [Google Scholar]
- Erostegui, C.; Norris, C.H.; Bobbin, R.P. In vitro pharmacologic characterization of a cholinergic receptor on outer hair cells. Hear. Res 1994, 74, 135–147. [Google Scholar]
- Nenov, A.P.; Norris, C.; Bobbin, R.P. Acetylcholine response in guinea pig outer hair cells. I. Properties of the response. Hear. Res 1996, 101, 132–148. [Google Scholar]
- Sugasawa, M.; Erostegui, C.; Blanchet, C.; Dulon, D. ATP activates a cation conductance and Ca(2+)-dependent Cl− conductance in Hensen cells of guinea pig cochlea. Am. J. Physiol 1996, 271, C1817–1827. [Google Scholar]
- Nenov, A.P.; Chen, C.; Bobbin, R.P. Outward rectifying potassium currents are the dominant voltage activated currents present in Deiters’ cells. Hear. Res 1998, 123, 168–182. [Google Scholar]
- Gille, A.M.; Imhoff, J.M.; Keil, B. á-Clostripain. J. Biol. Chem 1979, 10, 1462–1468. [Google Scholar]
- Hermodson, M.A.; Ericsson, L.H.; Neurath, H.; Walsh, K.A. Determination of the amino acid sequence of porcine trypsin by sequenator analysis. Biochemistry 1973, 12, 3146–3153. [Google Scholar]
- Catterall, W.A. Structure and function of voltage-sensitive ion channels. Science 1988, 242, 50–61. [Google Scholar]
- Desai, R.; Peretz, A.; Idelson, H.; Lazarovici, P.; Attali, B. Ca2+-activated K+ channels in human leukemic Jurkat T cells. Molecular cloning, biochemical and functional characterization. J. Biol. Chem 2000, 275, 39954–39963. [Google Scholar]
- Smyth, D.G. Techniques in enzymic hydrolysis. Methods Enzymol 1967, 11, 214–231. [Google Scholar]
- Chilton, L.; Ohya, S.; Freed, D.; George, E.; Drobic, V.; Shibukawa, Y.; Maccannell, K.A.; Imaizumi, Y.; Clark, R.B.; Dixon, I.M.; et al. K+ currents regulate the resting membrane potential, proliferation, and contractile responses in ventricular fibroblasts and myofibroblasts. Am. J. Physiol. Heart. Circ. Physiol 2005, 288, H2931–H2939. [Google Scholar]
- Yuan, X.J. Voltage-gated K+ currents regulate resting membrane potential and [Ca2+]i in pulmonary arterial myocytes. Cir. Res 1995, 77, 370–378. [Google Scholar]
- Armstrong, C.E.; Roberts, W.M. Papain alters the resonant frequency of frog saccular hair cells. Biophys. J. Abstr 1996, 70, A346. [Google Scholar]
- Armstrong, C.E.; Roberts, W.M. At least three K+ currents are involved in resonance in frog saccular hair cells. Biophys. J. Abstr 1997, 72, A355. [Google Scholar]

© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhou, T.; Wang, Y.; Guo, C.-K.; Zhang, W.-J.; Yu, H.; Zhang, K.; Kong, W.-J. Two Distinct Channels Mediated by m2mAChR and α9nAChR Co-Exist in Type II Vestibular Hair Cells of Guinea Pig. Int. J. Mol. Sci. 2013, 14, 8818-8831. https://doi.org/10.3390/ijms14058818
Zhou T, Wang Y, Guo C-K, Zhang W-J, Yu H, Zhang K, Kong W-J. Two Distinct Channels Mediated by m2mAChR and α9nAChR Co-Exist in Type II Vestibular Hair Cells of Guinea Pig. International Journal of Molecular Sciences. 2013; 14(5):8818-8831. https://doi.org/10.3390/ijms14058818
Chicago/Turabian StyleZhou, Tao, Yi Wang, Chang-Kai Guo, Wen-Juan Zhang, Hong Yu, Kun Zhang, and Wei-Jia Kong. 2013. "Two Distinct Channels Mediated by m2mAChR and α9nAChR Co-Exist in Type II Vestibular Hair Cells of Guinea Pig" International Journal of Molecular Sciences 14, no. 5: 8818-8831. https://doi.org/10.3390/ijms14058818



