Human Prostatic Acid Phosphatase: Structure, Function and Regulation
Abstract
:1. Introduction
2. Biology of Human Prostatic Acid Phosphatase
2.1. Acid Phosphatases (AcPs)
2.2. PAcP Isoforms
2.3. Tissue Distribution of PAcP
2.4. Physiological Levels of PAcP
2.5. PAcP for the Detection of PCa
3. Structure of Human Prostatic Acid Phosphatase
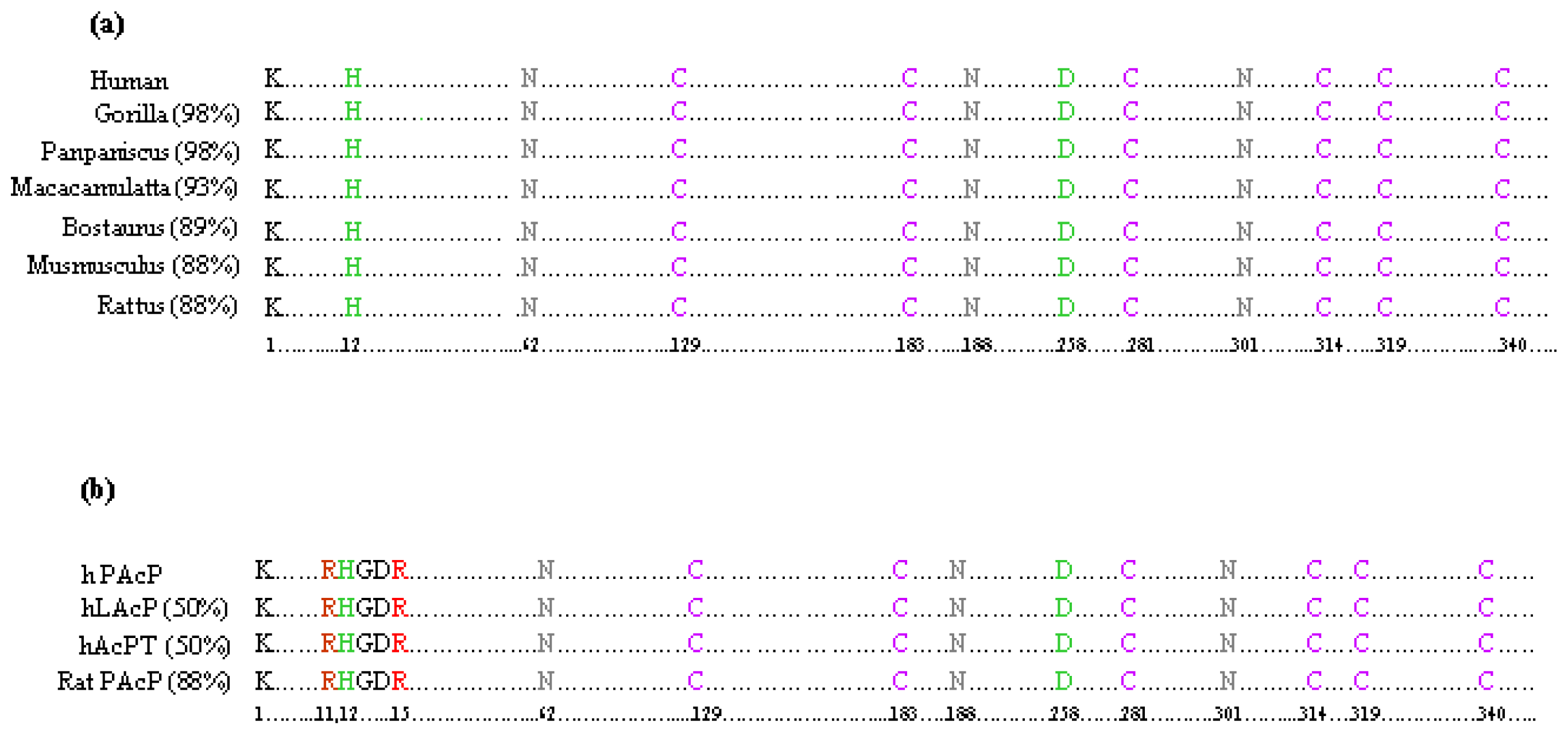
3.1. Homology Alignments of Human PAcP Protein with Other Mammalian Species
3.2. Biochemical Properties of the Human PAcP Gene and mRNA
3.3. Structural Comparison of hPAcP with hLAcP and AcPT at mRNA and Protein Levels
3.4. Structural Analysis of the hPAcP Protein
3.5. Biochemical Characterization of hPAcP Isoforms and Allosteric Regulation
4. Biological Function of Prostatic Acid Phosphatase
4.1. Cellular PAcP as a Tumor Suppressor
4.2. sPAcP: Functions beyond Tumor Suppressor
5. Regulation of PAcP Expression
5.1. Effects of Multi Factors on PAcP Expression
5.2. Transcriptional Regulation of hPAcP Gene Expression
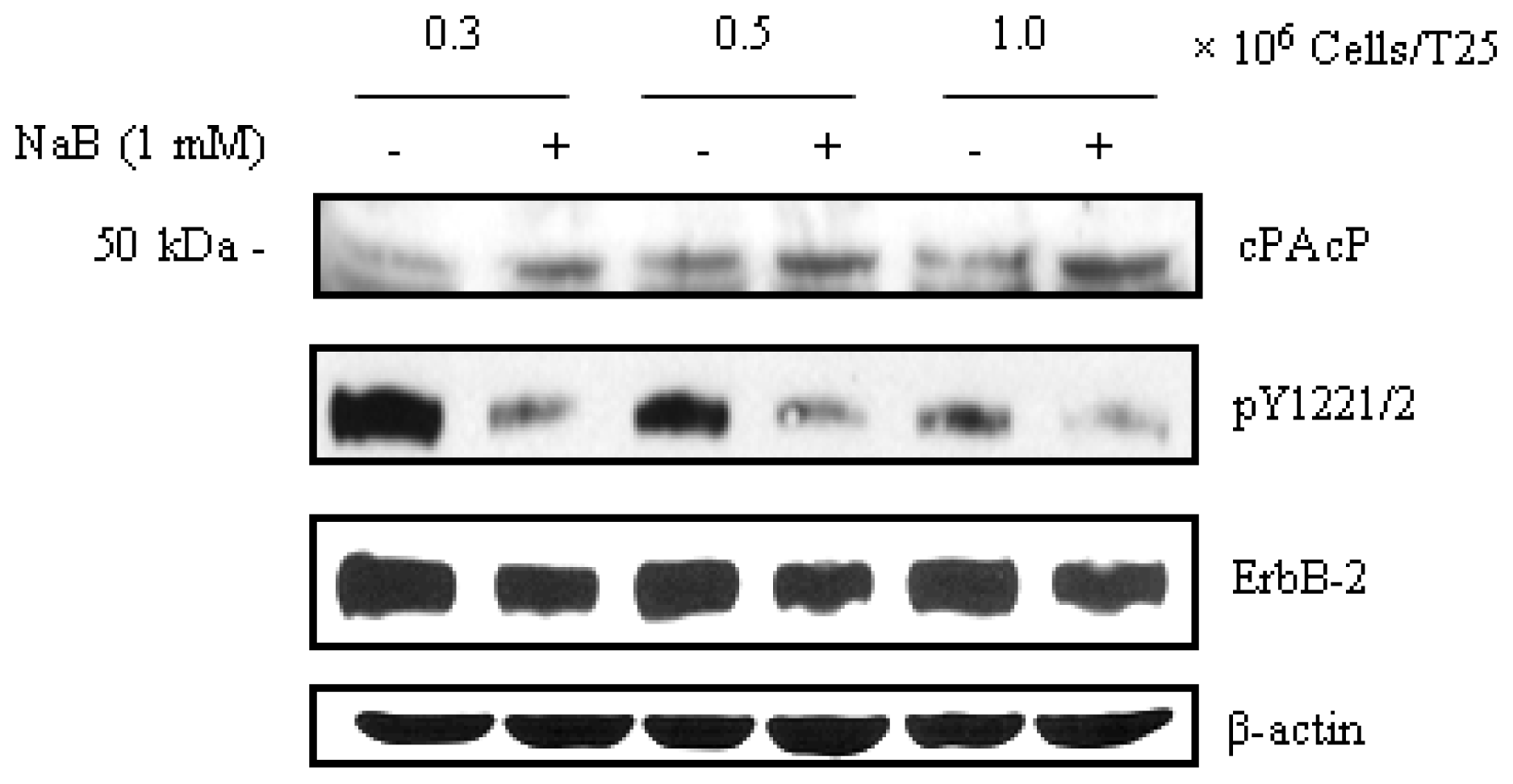
5.3. Epigenetic Regulation of PAcP
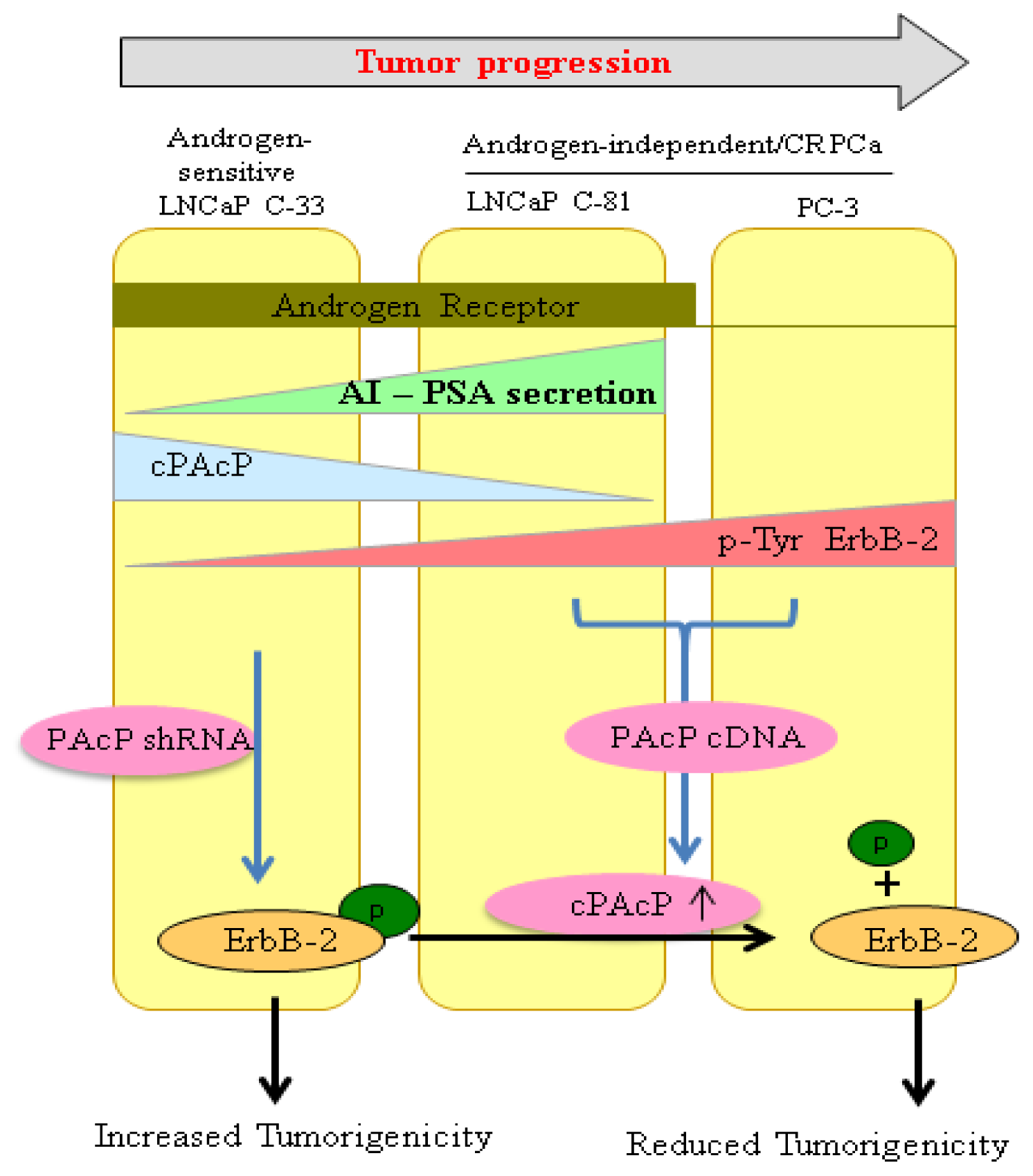
6. ErbB-2/HER-2/neu (ErbB-2) Signaling and Androgen Sensitivity Regulated by cPAcP
7. PAcP as a Therapeutic Agent
8. Conclusions
Acknowledgments
Conflict of Interest
Abbreviations
| AcP | acid phosphatase |
| AcPT | testicular acid phosphatase |
| AI | Androgen-independent |
| AS | Androgen-sensitive |
| cPAcP | cellular prostatic acid phosphatase |
| CR PCa | castration-resistant prostate cancer |
| DHT | 5α-dihydrotestosterone |
| EGF | epidermal growth factor |
| EGFR | EGF receptor |
| HER-2/ErbB-2/neu | human epidermal growth factor receptor-2 |
| FBS | fetal bovine serum |
| HDAC | Histone deacetylase |
| IHC | Immunohistochemistry |
| hLAcP | human lysosomal acid phosphatase |
| hPAcP | human prostatic acid phosphatase |
| PCa | prostate cancer |
| pI | isoelectric point |
| PI3K | phosphoinositide 3-kinase |
| PKC | protein kinase C |
| PSA | prostate-specific antigen |
| PTP | protein tyrosine phosphatase |
| p-Tyr | phosphotyrosine |
| qRT-PCR | quantitative reverse transcription-polymerase chain reaction |
| sPAcP | secretory PAcP |
| TM-PAcP | transmembrane PAcP |
| Tyr-P | tyrosine phosphorylation. |
References
- Gutman, E.B.; Sproul, E.E.; Gutman, A.B. Significance of increased phosphatase activity at the site of osteoplastic metastases secondary to carcinoma of the prostate gland. Am. J. Cancer 1936, 28, 485–495. [Google Scholar]
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer: The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res 1941, 1, 293–297. [Google Scholar]
- Papsidero, L.D.; Wojcieszyn, J.W.; Horoszewicz, J.S.; Leong, S.S.; Murphy, G.P.; Chu, T.M. Isolation of prostatic acid phosphatase-binding immunoglobulin G from human sera and its potential for use as a tumor-localizing reagent. Cancer Res 1980, 40, 3032–3035. [Google Scholar]
- Wang, M.C.; Papsidero, L.D.; Kuriyama, M.; Valenzuela, L.A.; Murphy, G.P.; Chu, T.M. Prostate antigen: A new potential marker for prostatic cancer. Prostate 1981, 2, 89–96. [Google Scholar]
- Chu, T.M.; Lin, M.F. PSA and acid phosphatase in the diagnosis of prostate cancer. J. Clin. Lig. Assay 1998, 21, 24–34. [Google Scholar]
- Zagars, G.K.; von Eschenbach, A.C.; Ayala, A.G. Prognostic factors in prostate cancer. Analysis of 874 patients treated with radiation therapy. Cancer 1993, 72, 1709–1725. [Google Scholar]
- Fowler, J.E., Jr; Pandey, P.; Seaver, L.E.; Feliz, T.P.; Braswell, N.T. Prostate specific antigen regression and progression after androgen deprivation for localized and metastatic prostate cancer. J. Urol 1995, 153, 1860–1865. [Google Scholar]
- Zagars, G.K.; Pollack, A.; von Eschenbach, A.C. Serum testosterone—A significant determinant of metastatic relapse for irradiated localized prostate cancer. Urology 1997, 49, 327–334. [Google Scholar]
- Taira, A.; Merrick, G.; Wallner, K.; Dattoli, M. Reviving the acid phosphatase test for prostate cancer. Oncology (Williston Park) 2007, 21, 1003–1010. [Google Scholar]
- Han, M.; Partin, A.W.; Pound, C.R.; Epstein, J.I.; Walsh, P.C. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol. Clin. North Am 2001, 28, 555–565. [Google Scholar]
- Gyorkey, F. Some aspects of cancer of the prostate gland. Meth. Cancer Res 1973, 10, 279–368. [Google Scholar]
- Yam, L.T. Clinical significance of the human acid phosphatases: A review. Am. J. Med 1974, 56, 604–616. [Google Scholar]
- Meng, T.C.; Lin, M.F. Tyrosine phosphorylation of c-ErbB-2 is regulated by the cellular form of prostatic acid phosphatase in human prostate cancer cells. J. Biol. Chem 1998, 273, 22096–22104. [Google Scholar]
- Veeramani, S.; Yuan, T.C.; Chen, S.J.; Lin, F.F.; Petersen, J.E.; Shaheduzzaman, S.; Srivastava, S.; MacDonald, R.G.; Lin, M.F. Cellular prostatic acid phosphatase: A protein tyrosine phosphatase involved in androgen-independent proliferation of prostate cancer. Endocr. Relat. Cancer 2005, 12, 805–822. [Google Scholar]
- Chuang, T.D.; Chen, S.J.; Lin, F.F.; Veeramani, S.; Kumar, S.; Batra, S.K.; Tu, Y.; Lin, M.F. Human prostatic acid phosphatase, an authentic tyrosine phosphatase, dephosphorylates ErbB-2 and regulates prostate cancer cell growth. J. Biol. Chem 2010, 285, 23598–23606. [Google Scholar]
- Veeramani, S.; Lee, M.S.; Lin, M.F. Revisiting histidine-dependent acid phosphatases: A distinct group of tyrosine phosphatases. Trends Biochem. Sci 2009, 34, 273–278. [Google Scholar]
- Martland, M.; Hansman, F.S.; Robison, R. The phosphoric-esterase of blood. Biochem. J 1924, 18, 1152–1160. [Google Scholar]
- Moss, D.W.; Raymond, F.D.; Wile, D.B. Clinical and biological aspects of acid phosphatase. Crit. Rev. Clin. Lab. Sci 1995, 32, 431–467. [Google Scholar]
- Yousef, G.M.; Diamandis, M.; Jung, K.; Diamandis, E.P. Molecular cloning of a novel human acid phosphatase gene (ACPT) that is highly expressed in the testis. Genomics 2001, 74, 385–395. [Google Scholar]
- Derechin, M.; Ostrowski, W.; Galka, M.; Barnard, E.A. Acid phosphomonoesterase of human prostate: Molecular weight, dissociation and chemical composition. Biochim. Biophys. Acta 1971, 250, 143–154. [Google Scholar]
- Luchter-Wasyl, E.; Ostrowski, W. Subunit structure of human prostatic acid phosphatase. Biochim. Biophys. Acta 1974, 365, 349–359. [Google Scholar]
- Lee, C.L.; Li, S.S.; Chu, T.M. Immunologically reactive tryptic fragments of human prostatic acid phosphatase. Biochem. J 1984, 223, 871–877. [Google Scholar]
- Hakalahti, L.; Vihko, P. Purification of monoclonal antibodies raised against prostate-specific acid phosphatase for use in vivo in radioimaging of prostatic cancer. J. Immunol. Methods 1989, 117, 131–136. [Google Scholar]
- Vihko, P. Human prostatic acid phosphatases: Purification of a minor enzyme and comparisons of the enzymes. Invest. Urol 1979, 16, 349–352. [Google Scholar]
- Lad, P.M.; Learn, D.B.; Cooper, J.F.; Reisinger, D.M. Distribution of prostatic acid phosphatase isoenzymes in normal and cancerous states. Clin. Chim. Acta 1984, 141, 51–65. [Google Scholar]
- Boissonneault, M.; Chapdelaine, A.; Chevalier, S. The enhancement by pervanadate of tyrosine phosphorylation on prostatic proteins occurs through the inhibition of membrane-associated tyrosine phosphatases. Dev Mol. Cell. Biochem 1996, 153, 139–144. [Google Scholar]
- White, K.Y.; Rodemich, L.; Nyalwidhe, J.O.; Comunale, M.A.; Clements, M.A.; Lance, R.S.; Schellhammer, P.F.; Mehta, A.S.; Semmes, O.J.; Drake, R.R. Glycomic characterization of prostatespecific antigen and prostatic acid phosphatase in prostate cancer and benign disease seminal plasma fluids. J. Proteome Res 2009, 8, 620–630. [Google Scholar]
- Xia, X.Z.; Lin, M.F. University of Southern California: Los Angeles, CA, USA, Unpublished work; 1993.
- Vihko, P. Characterization of the principal human prostatic acid phosphatase isoenzyme, purified by affinity chromatography and isoelectric focusing. Part II. Clin Chem 1978, 24, 1783–1787. [Google Scholar]
- Quintero, I.B.; Araujo, C.L.; Pulkka, A.E.; Wirkkala, R.S.; Herrala, A.M.; Eskelinen, E.L.; Jokitalo, E.; Hellstrom, P.A.; Tuominen, H.J.; Hirvikoski, P.P.; et al. Prostatic acid phosphatase is not a prostate specific target. Cancer Res 2007, 67, 6549–6554. [Google Scholar]
- Mori, K.; Wakasugi, C. Immunocytochemical demonstration of prostatic acid phosphatase: Different secretion kinetics between normal, hyperplastic and neoplastic prostates. J. Urol 1985, 133, 877–883. [Google Scholar]
- Hoyhtya, M.; Vihko, P.; Vuolas, L.; Tryggvason, K.; Vihko, R. High-affinity monoclonal antibodies specific for human prostatic acid phosphatase. Clin. Chem 1987, 33, 103–107. [Google Scholar]
- Lilja, H.; Abrahamsson, P.A. Three predominant proteins secreted by the human prostate gland. Prostate 1988, 12, 29–38. [Google Scholar]
- Li, C.Y.; Lam, W.K.; Yam, L.T. Immunohistochemical diagnosis of prostatic cancer with metastasis. Cancer 1980, 46, 706–712. [Google Scholar]
- Shaw, L.M.; Yang, N.; Brooks, J.J.; Neat, M.; Marsh, E.; Seamonds, B. Immunochemical evaluation of the organ specificity of prostatic acid phosphatase. Clin. Chem 1981, 27, 1505–1512. [Google Scholar]
- Yam, L.T.; Janckila, A.J.; Li, C.Y.; Lam, W.K. Presence of “prostatic” acid phosphatase in human neutrophils. Invest. Urol 1981, 19, 34–38. [Google Scholar]
- Waheed, A.; van Etten, R.L.; Gieselmann, V.; von Figura, K. Immunological characterization of human acid phosphatase gene products. Biochem. Genet 1985, 23, 309–319. [Google Scholar]
- Kamoshida, S.; Tsutsumi, Y. Extraprostatic localization of prostatic acid phosphatase and prostate-specific antigen: Distribution in cloacogenic glandular epithelium and sex-dependent expression in human anal gland. Hum. Pathol 1990, 21, 1108–1111. [Google Scholar]
- Drenckhahn, D.; Waheed, A.; van Etten, R. Demonstration of prostatic-type acid phosphatase in non-lysosomal granules in the crypt epithelium of the human duodenum. Histochemistry 1987, 88, 47–52. [Google Scholar]
- Choe, B.K.; Pontes, E.J.; Rose, N.R.; Henderson, M.D. Expression of human prostatic acid phosphatase in a pancreatic islet cell carcinoma. Invest. Urol 1978, 15, 312–318. [Google Scholar]
- Lam, K.W.; Li, C.Y.; Yam, L.T.; Sun, T.; Lee, G.; Ziesmer, S. Improved immunohistochemical detection of prostatic acid phosphatase by a monoclonal antibody. Prostate 1989, 15, 13–21. [Google Scholar]
- Cunha, A.C.; Weigle, B.; Kiessling, A.; Bachmann, M.; Rieber, E.P. Tissue-specificity of prostate specific antigens: Comparative analysis of transcript levels in prostate and non-prostatic tissues. Cancer Lett 2006, 236, 229–238. [Google Scholar]
- Graddis, T.J.; McMahan, C.J.; Tamman, J.; Page, K.J.; Trager, J.B. Prostatic acid phosphatase expression in human tissues. Int J. Clin. Exp. Pathol 2011, 4, 295–306. [Google Scholar]
- Wang, Y.; Harada, M.; Yano, H.; Ogasawara, S.; Takedatsu, H.; Arima, Y.; Matsueda, S.; Yamada, A.; Itoh, K. Prostatic acid phosphatase as a target molecule in specific immunotherapy for patients with nonprostate adenocarcinoma. J. Immunother 2005, 28, 535–541. [Google Scholar]
- Goldfarb, D.A.; Stein, B.S.; Shamszadeh, M.; Petersen, R.O. Age-related changes in tissue levels of prostatic acid phosphatase and prostate specific antigen. J. Urol 1986, 136, 1266–1269. [Google Scholar]
- Ronnberg, L.; Vihko, P.; Sajanti, E.; Vihko, R. Clomiphene citrate administration to normogonadotropic subfertile men: Blood hormone changes and activation of acid phosphatase in seminal fluid. Int. J. Androl 1981, 4, 372–378. [Google Scholar]
- Ahmann, F.R.; Schifman, R.B. Prospective comparison between serum monoclonal prostate specific antigen and acid phosphatase measurements in metastatic prostatic cancer. J. Urol 1987, 137, 431–434. [Google Scholar]
- Simsek, U.; Kutlu, S.; Yavascaouglu, I.; Oktay, B.; Ozyurt, M. Seasonal variation of prostatic acid phosphate and prostate-specific antigen in patients without prostatic malignancy. Eur. Urol 1992, 21, 111–114. [Google Scholar]
- Robles, J.M.; Morell, A.R.; de Torres, J.A.; Rosello, A.S. Clinical behavior of prostatic specific antigen and prostatic acid phosphatase: A comparative study. Int. J. Biol. Markers 1989, 4, 87–94. [Google Scholar]
- Abrahamsson, P.A.; Lilja, H.; Falkmer, S.; Wadström, L.B. Immunohistochemical distribution of the three predominant secretory proteins in the parenchyma of hyperplastic and neoplastic prostate glands. Prostate 1988, 12, 39–46. [Google Scholar]
- Sinha, A.A.; Gleason, D.F.; Wilson, M.J.; Wick, M.R.; Reddy, P.K.; Blackard, C.E. Relationship of prostatic acid phosphatase localization in human prostate by a monoclonal antibody with the Gleason grading system. Prostate 1988, 13, 1–15. [Google Scholar]
- Sakai, H.; Shiraishi, K.; Minami, Y.; Yushita, Y.; Kanetake, H.; Saito, Y. Immunohistochemical prostatic acid phosphatase level as a prognostic factor of prostatic carcinoma. Prostate 1991, 19, 265–272. [Google Scholar]
- Ozu, C.; Nakashima, J.; Horiguchi, Y.; Oya, M.; Ohigashi, T.; Murai, M. Prediction of bone metastases by combination of tartrate-resistant acid phosphatase, alkaline phosphatase and prostate specific antigen in patients with prostate cancer. Int. J. Urol 2008, 15, 419–422. [Google Scholar]
- Clark, N.L.; Swanson, W.J. Pervasive adaptive evolution in primate seminal proteins. PLoS Genet 2005, 1, e35. [Google Scholar]
- ACPP protein [Bos Taurus]. Available online: http://www.ncbi.nlm.nih.gov/protein/AAI46094 (accessed on 28 February 2013).
- Prostatic acid phosphatase [Mus musculus]. Available online: http://www.ncbi.nlm.nih.gov/protein/AAF23171.1 (accessed on 28 February 2013).
- Winqvist, R.; Virkkunen, P.; Grzeschik, K.H.; Vihko, P. Chromosomal localization to 3q21→Qter and two TaqI RFLPs of the human prostate-specific acid phosphatase gene (ACPP). Cytogenet. Cell Genet 1989, 52, 68–71. [Google Scholar]
- Vihko, P.; Virkkunen, P.; Henttu, P.; Roiko, K.; Solin, T.; Huhtala, M.L. Molecular cloning and sequence analysis of cDNA encoding human prostatic acid phosphatase. FEBS Lett 1988, 236, 275–281. [Google Scholar]
- Sharief, F.S.; Li, S.S. Structure of human prostatic acid phosphatase gene. Biochem. Biophys. Res. Commun 1992, 184, 1468–1476. [Google Scholar]
- Li, S.S.; Sharief, F.S. The prostatic acid phosphatase (ACPP) gene is localized to human chromosome 3q21-q23. Genomics 1993, 17, 765–766. [Google Scholar]
- Banas, B.; Blaschke, D.; Fittler, F.; Horz, W. Analysis of the promoter of the human prostatic acid phosphatase gene. Biochim. Biophys. Acta 1994, 1217, 188–194. [Google Scholar]
- Solin, T.; Kontturi, M.; Pohlmann, R.; Vihko, P. Gene expression and prostate specificity of human prostatic acid phosphatase (PAP): Evaluation by RNA blot analyses. Biochim. Biophys. Acta 1990, 1048, 72–77. [Google Scholar]
- Zelivianski, S.; Comeau, D.; Lin, M.F. Cloning and analysis of the promoter activity of the human prostatic acid phosphatase gene. Biochem. Biophys. Res. Comm 1998, 245, 108–112. [Google Scholar]
- Roiko, K.; Janne, O.A.; Vihko, P. Primary structure of rat secretory acid phosphatase and comparison to other acid phosphatases. Gene 1990, 89, 223–229. [Google Scholar]
- Porvari, K.; Kurkela, R.; Kivinen, A.; Vihko, P. Differential androgen regulation of rat prostatic acid phosphatase transcripts. Biochem. Biophys. Res. Commun 1995, 213, 861–868. [Google Scholar]
- Geier, C.; von Figura, K.; Pohlmann, R. Structure of the human lysosomal acid phosphatase gene. Eur. J. Biochem 1989, 183, 611–616. [Google Scholar]
- Virkkunen, P.; Hedberg, P.; Palvimo, J.J.; Birr, E.; Porvari, K.; Ruokonen, M.; Taavitsainen, P.; Jänne, O.A.; Vihko, P. Structural comparison of human and rat prostate-specific acid phosphatase genes and their promoters: Identification of putative androgen response elements. Biochem. Biophys. Res. Commun 1994, 202, 49–57. [Google Scholar]
- Sharief, F.S.; Lee, H.; Leuderman, M.M.; Lundwall, A.; Deaven, L.L.; Lee, C.L.; Li, S.S. Human prostatic acid phosphatase: CDNA cloning, gene mapping and protein sequence homology with lysosomal acid phosphatase. Biochem. Biophys. Res. Commun 1989, 160, 79–86. [Google Scholar]
- Schneider, G.; Lindqvist, Y.; Vihko, P. Three-dimensional structure of rat acid phosphatase. EMBO J 1993, 12, 2609–2615. [Google Scholar]
- Peters, C.; Geier, C.; Pohlmann, R.; Waheed, A.; von Figura, K.; Roiko, K.; Virkkunen, P.; Henttu, P.; Vihko, P. High degree of homology between primary structure of human lysosomal acid phosphatase and human prostatic acid phosphatase. Biol. Chem. Hoppe-Seyler 1989, 370, 177–181. [Google Scholar]
- Lee, H.; Chu, T.M.; Li, S.S.; Lee, C.L. Homodimer and heterodimer subunits of human prostate acid phosphatase. Biochem. J 1991, 277, 759–765. [Google Scholar]
- Ostrowski, W.S.; Kuciel, R. Human prostatic acid phosphatase: Selected properties and practical applications. Clin. Chim. Acta 1994, 226, 121–129. [Google Scholar]
- Kuciel, R.; Bakalova, A.; Mazurkiewicz, A.; Bilska, A.; Ostrowski, W. Is the subunit of prostatic phosphatase active? Reversible denaturation of prostatic acid phosphatase. Biochem. Int 1990, 22, 329–334. [Google Scholar]
- Van Etten, R.L.; Davidson, R.; Stevis, P.E.; MacArthur, H.; Moore, D.L. Covalent structure, disulfide bonding, and identification of reactive surface and active site residues of human prostatic acid phosphatase. J. Biol. Chem 1991, 266, 2313–2319. [Google Scholar]
- Ostanin, K.; Saeed, A.; van Etten, R.L. Heterologous expression of human prostatic acid phosphatase and site-directed mutagenesis of the enzyme active site. J. Biol. Chem 1994, 269, 8971–8978. [Google Scholar]
- Jakob, C.G.; Lewinski, K.; Kuciel, R.; Ostrowski, W.; Lebioda, L. Crystal structure of human prostatic acid phosphatase. Prostate 2000, 42, 211–218. [Google Scholar]
- Murzin, A.G.; Brenner, S.E.; Hubbard, T.; Chothia, C. SCOP: A structural classification of proteins database for the investigation ofsequences and structures. J. Mol. Biol 1995, 247, 536–540. [Google Scholar]
- Ortlund, E.; LaCount, M.W.; Lebioda, L. Crystal structures of human prostatic acid phosphatase in complex with a phosphate ion and a-benzylaminobenzylphosphonic acid update the mechanistic picture and offer new insights into inhibitor design. Biochemistry 2003, 42, 383–389. [Google Scholar]
- Van Etten, R.L. Human prostatic acid phosphatase: A histidine phosphatase. Ann. N.Y. Acad. Sci 1982, 390, 27–51. [Google Scholar]
- Sharma, S.; Rauk, A.; Juffer, A.H. A DFT study on the formation of a phosphohistidine intermediate in prostatic acid phosphatase. J. Am. Chem. Soc 2008, 130, 9708–9716. [Google Scholar]
- Chu, T.M.; Wang, M.C.; Merrin, C.; Valenzuela, L.; Murphy, G.P. Isoenzymes of human prostate acid phosphatase. Oncology 1978, 35, 198–200. [Google Scholar]
- Lin, M.F.; Lee, C.L.; Li, S.S.; Chu, T.M. Purification and characterization of a new human prostatic acid phosphatase isoenzyme. Biochemistry 1983, 22, 1055–1062. [Google Scholar]
- Lam, K.W.; Lee, P.; Eastlund, T.; Yam, L.T. Antigenic and molecular relationship of human prostatic acid phosphatase isoenzymes. Invest. Urol 1980, 18, 209–211. [Google Scholar]
- Moss, D.W. Multiple forms of acid and alkaline phosphatases: Genetics, expression and tissue-specific modification. Clin. Chim. Acta 1986, 161, 123–135. [Google Scholar]
- Luchter-Wasylewska, E.; Wasylewski, M.; Röhm, K.H. Concentration-dependent dissociation/ association of human prostatic acid phosphatase. J. Protein Chem 2003, 22, 243–247. [Google Scholar]
- Porvari, K.S.; Herrala, A.M.; Kurkela, R.M.; Taavitsainen, P.A.; Lindqvist, Y.; Schneider, G.; Vihko, P.T. Site-directed mutagenesis of prostatic acid phosphatase. Catalytically important aspartic acid 258, substrate specificity, and oligomerization. J. Biol. Chem 1994, 269, 22642–22646. [Google Scholar]
- Reif, A.E.; Schlesinger, R.M.; Fish, C.A.; Robinson, C.M. Acid phosphatase isozymes in cancer of the prostate. Cancer 1973, 31, 689–699. [Google Scholar]
- Hakalahti, L.; Vihko, P.; Henttu, P.; Autio-Harmainen, H.; Soini, Y.; Vihko, R. Evaluation of PAP and PSA gene expression in prostatic hyperplasia and prostatic carcinoma using northern-blot analyses, in situ hybridization and immunohistochemical stainings with monoclonal and bispecific antibodies. Int. J. Cancer 1993, 55, 590–597. [Google Scholar]
- Lin, M.F.; Lee, M.S.; Zhou, X.W.; Andressen, J.C.; Meng, T.C.; Johansson, S.L.; West, W.W.; Taylor, R.J.; Anderson, J.R.; Lin, F.F. Decreased expression of cellular prostatic acid phosphatase increases tumorigenicity of human prostate cancer cells. J. Urol 2001, 166, 1943–1950. [Google Scholar]
- Hassan, M.I.; Aijaz, A.; Ahmad, F. Structural and functional analysis of human prostatic acid phosphatase. Expert Rev. Anticancer Ther 2010, 10, 1055–1068. [Google Scholar]
- Lin, M.F.; DaVolio, J.; Garcia-Arenas, R. Expression of human prostatic acid phosphatase activity and the growth of prostate carcinoma cells. Cancer Res 1992, 52, 4600–4607. [Google Scholar]
- Lin, M.F.; Meng, T.C.; Rao, P.S.; Chang, C.; Schonthal, A.H.; Lin, F.F. Expression of human prostatic acid phosphatase correlates with androgen-stimulated cell proliferation in prostate cancer cell lines. J. Biol. Chem 1998, 273, 5939–5947. [Google Scholar]
- Igawa, T.; Lin, F.F.; Lee, M.S.; Karan, D.; Batra, S.K.; Lin, M.F. Establishment and characterization of androgen-independent human prostate cancer LNCaP cell model. Prostate 2002, 50, 222–35. [Google Scholar]
- Yuan, T.C.; Lin, F.F.; Veeramani, S.; Chen, S.J.; Earp, H.S., III; Lin, M.F. ErbB-2 via PYK2 upregulates the adhesive ability of androgen receptor-positive human prostate cancer cells. Oncogene 2007, 26, 7552–7559. [Google Scholar]
- Lin, M.F.; Garcia-Arenas, R.; Xia, X.Z.; Biela, B.; Lin, F.F. The cellular level of prostatic acid phosphatase and the growth of human prostate carcinoma cells. Differentiation 1994, 57, 143–149. [Google Scholar]
- Igawa, T.; Lin, F.F.; Rao, P.; Lin, M.F. Suppression of LNCaP prostate cancer xenograft tumors by a prostate-specific protein tyrosine phosphatase, prostatic acid phosphatase. Prostate 2003, 55, 247–258. [Google Scholar]
- Stewart, L.V.; Lyles, B.; Lin, M.F.; Weigel, N.L. Vitamin D receptor agonists induce prostatic acid phosphatase to reduce cell growth and HER-2 signaling in LNCaP-derived human prostate cancer cells. J. Steroid Biochem. Mol. Biol 2005, 97, 37–46. [Google Scholar]
- Coffey, D.S.; Pienta, K.J. New concepts in studying the control of normal and cancer growth of the prostate. Prog. Clin. Biol. Res 1987, 239, 1–73. [Google Scholar]
- Saha, A.; Basu, J.; Bhattacharyya, A.K. Seminal acid phosphatase from normal, oligospermic, vasectomized, and azoospermic men. Int. J. Fertil 1981, 26, 124–127. [Google Scholar]
- Singh, G.; Adaikan, P.G.; Ng, Y.K. Is seminal prostatic acid phosphatase a reliable marker for male infertility? Singapore Med. J 1996, 37, 598–599. [Google Scholar]
- Münch, J.; Rücker, E.; Ständker, L.; Adermann, K.; Goffinet, C.; Schindler, M.; Wildum, S.; Chinnadurai, R.; Rajan, D.; Specht, A.; et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell 2007, 131, 1059–1071. [Google Scholar]
- Olsen, J.S.; DiMaio, J.T.; Doran, T.M.; Brown, C.; Nilsson, B.L.; Dewhurst, S. Seminal plasma accelerates semen-derived enhancer of viral infection (SEVI) fibril formation by the prostatic acid phosphatase (PAP248–286) peptide. J. Biol. Chem 2012, 287, 11842–11849. [Google Scholar]
- Hunter, T.; Cooper, J.A. Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell 1981, 24, 741–752. [Google Scholar]
- Monget, P.; Pisselet, C.; Monniaux, D. Expression of insulin-like growth factor binding protein-5 by ovine granulosa cells is regulated by cell density and programmed cell death in vitro. J. Cell Physiol 1998, 177, 13–25. [Google Scholar]
- Pfeiffer, D.; Kimmig, R.; Herrmann, J.; Ruge, M.; Fisseler-Eckhoff, A.; Scheidel, P.; Jensen, A.; Schatz, H.; Pfeiffer, A. Epidermal-growth-factor receptor correlates negatively with cell density in cervical squamous epithelium and is down-regulated in cancers of the human uterus. Int. J. Cancer 1998, 79, 49–55. [Google Scholar]
- Chevalier, S.; Bleau, G.; Roberts, K.D.; Chapdelaine, A. Proliferation and differentiation of canine prostatic epithelial cells in culture. Mol. Cell. Endocrinol 1981, 24, 195–208. [Google Scholar]
- Dionne, F.T.; Chevalier, S.; Bleau, G.; Roberts, K.D.; Chapdelaine, A. Induction of acid phosphatase synthesis in canine prostatic epithelial cells in vitro. Mol. Cell. Endocrinol 1983, 33, 113–126. [Google Scholar]
- Lin, M.F.; Garcia-Arenas, R.; Kawachi, M.; Lin, F.F. Regulation of the expression of prostatic acid phosphatase in LNCaP human prostate carcinoma cells. Cell. Mol. Biol. Res 1993, 39, 739–750. [Google Scholar]
- Lin, M.F.; Garcia-Arenas, R. Effect of cell density on androgen regulation of the mRNA level of human prostatic acid phosphatase. Mol. Cell. Endocrinol 1994, 99, R21–24. [Google Scholar]
- Lin, M.F.; Garcia-Arenas, R.; Chao, Y.C.; Lai, M.M.; Patel, P.C.; Xia, X.Z. Regulation of prostatic acid phosphatase expression and secretion by androgen in LNCaP human prostate carcinoma cells. Arch. Biochem. Biophy 1993, 300, 384–390. [Google Scholar]
- Shan, J.D.; Porvari, K.; Ruokonen, M.; Karhu, A.; Launonen, V.; Hedberg, P.; Oikarinen, J.; Vihko, P. Steroid-involved transcriptional regulation of human genes encoding prostatic acid phosphatase, prostate-specific antigen, and prostate-specific glandular kallikrein. Endocrinology 1997, 138, 3764–3770. [Google Scholar]
- Zelivianski, S.; Larson, C.; Seberger, J.; Taylor, R.; Lin, M.F. Expression of human prostatic acid phosphatase gene is regulated by upstream negative and positive elements. Biochim. Biophys. Acta 2000, 1491, 123–132. [Google Scholar]
- Sporn, M.B.; Roberts, A.B.; Wakefield, L.M.; Assoian, R.K. Transforming growth factor-beta: Biological function and chemical structure. Science 1986, 233, 532–534. [Google Scholar]
- Martikainen, P.; Kyprianou, N.; Isaacs, J.T. Effect of transforming growth factor-beta 1 on proliferation and death of rat prostatic cells. Endocrinology 1990, 127, 2963–2968. [Google Scholar]
- Henttu, P.; Vihko, P. Growth factor regulation of gene expression in the human prostatic carcinoma cell line LNCaP. Cancer Res 1993, 53, 1051–1058. [Google Scholar]
- Horoszewicz, J.S.; Leong, S.S.; Kawinski, E.; Karr, J.P.; Rosenthal, H.; Chu, T.M.; Mirand, E.A.; Murphy, G.P. LNCaP model of human prostatic carcinoma. Cancer Res 1983, 43, 1809–1818. [Google Scholar]
- Lin, M.F.; Lee, M.S.; Garcia-Arenas, R.; Lin, F.F. Differential responsiveness of prostatic acid phosphatase and prostate-specific antigen mRNA to androgen in prostate cancer cells. Cell Biol. Int 2000, 24, 681–689. [Google Scholar]
- Henttu, P.; Vihko, P. Steroids inversely affect the biosynthesis and secretion of human prostatic acid phosphatase and prostate-specific antigen in the LNCaP cell line. J. Steroid Biochem. Mol. Biol 1992, 41, 349–360. [Google Scholar]
- Lin, M.F.; Zhang, X.Q.; Dean, J.; Lin, F.F. Protein kinase C pathway is involved in regulating the secretion of prostatic acid phosphatase in human prostate cancer cells. Cell Biol. Int 2001, 25, 1139–1148. [Google Scholar]
- Andrews, P.E.; Young, C.Y.; Montgomery, B.T.; Tindall, D.J. Tumor-promoting phorbol ester down-regulates the androgen induction of prostate-specific antigen in a human prostatic adenocarcinoma cell line. Cancer Res 1992, 52, 1525–1529. [Google Scholar]
- Johnson, J.L.; Ellis, B.A.; Noack, D.; Seabra, M.C.; Catz, S.D. The Rab27a-binding protein, JFC1, regulates androgen-dependent secretion of prostate-specific antigen and prostatic-specific acid phosphatase. Biochem. J 2005, 391, 699–710. [Google Scholar]
- Zelivianski, S.; Igawa, T.; Lim, S.; Taylor, R.; Lin, M.F. Identification and characterization of regulatory elements of the human prostatic acid phosphatase promoter. Oncogene 2002, 21, 3696–3705. [Google Scholar]
- Zelivianski, S.; Glowacki, R.; Lin, M.F. Transcriptional activation of the human prostatic acid phosphatase gene by NF-kappaB via a novel hexanucleotide-binding site. Nucleic Acids Res 2004, 32, 3566–3580. [Google Scholar]
- Chou, Y.W.; Chaturvedi, N.K.; Ouyang, S.; Lin, F.F.; Kaushik, D.; Wang, J.; Kim, I.; Lin, M.F. Histone deacetylase inhibitor valproic acid suppresses the growth and increases the androgen responsiveness of prostate cancer cells. Cancer Lett 2011, 311, 177–186. [Google Scholar]
- Riegman, P.H.; Vlietstra, R.J.; van der Korput, J.A.; Brinkmann, A.O.; Trapman, J. The promoter of the prostate-specific antigen gene contains a functional androgen responsive element. Mol. Endocrinol 1991, 5, 1921–1930. [Google Scholar]
- Cleutjens, K.B.; van Eekelen, C.C.; van der Korput, H.A.; Brinkmann, A.O.; Trapman, J. Two androgen response regions cooperate in steroid hormone regulated activity of the prostate-specific antigen promoter. J. Biol. Chem 1996, 271, 6379–6388. [Google Scholar]
- Gotoh, A.; Ko, S.C.; Shirakawa, T.; Cheon, J.; Kao, C.; Miyamoto, T.; Gardner, T.A.; Ho, L.J.; Cleutjens, C.B.; Trapman, J.; et al. Development of prostate-specific antigen promoter-based gene therapy for androgen-independent human prostate cancer. J. Urol 1998, 160, 220–229. [Google Scholar]
- Miyamoto, S.; Verma, I.M. Rel/NF-kappa B/I kappa B story. Adv. Cancer Res 1995, 66, 255–292. [Google Scholar]
- Perry, A.S. Prostate cancer epigenomics. J. Urol 2013, 189, 10–11. [Google Scholar]
- Albany, C.; Alva, A.S.; Aparicio, A.M.; Singal, R.; Yellapragada, S.; Sonpavde, G.; Hahn, N.M. Epigenetics in prostate cancer. Prostate Cancer 2011, 2011, 580318. [Google Scholar]
- Lin, M.F.; Meng, T.C. Tyrosine phosphorylation of a 185 kDa Phosphoprotein (pp185) inversely correlates with the cellular activity of human prostatic acid phosphatase. Biochem. Biophys. Res. Commun 1996, 226, 206–213. [Google Scholar]
- Li, H.C.; Chernoff, J.; Chen, L.B.; Kirschonbaum, A. A phosphotyrosyl-protein phosphatase activity associated with acid phosphatase from human prostate gland. Eur. J. Biochem 1984, 138, 45–51. [Google Scholar]
- Craft, N.; Shostak, Y.; Carey, M.; Sawyers, C.L. Mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat. Med 1999, 5, 280–285. [Google Scholar]
- Meng, T.C.; Lee, M.S.; Lin, M.F. Interaction between protein tyrosine phosphatase and protein tyrosine kinase is involved in androgen-promoted growth of human prostate cancer cells. Oncogene 2000, 19, 2664–2677. [Google Scholar]
- Lee, M.S.; Igawa, T.; Yuan, T.C.; Zhang, X.Q.; Lin, F.F.; Lin, M.F. ErbB-2 signaling is involved in regulating PSA secretion in androgen-independent human prostate cancer LNCaP C-81 cells. Oncogene 2003, 22, 781–796. [Google Scholar]
- El Sheikh, S.S.; Domin, J.; Abel, P.; Stamp, G.; Lalani, el-N. Phosphorylation of both EGFR and ErbB2 is a reliable predictor of prostate cancer cell proliferation in response to EGF. Neoplasia 2004, 6, 846–853. [Google Scholar]
- Vihko, P.T.; Quintero, I.; Ronka, A.E.; Herrala, A.; Jantti, P.; Porvari, K.; Lindqvist, Y.; Kaija, H.; Pulkka, A.; Vuoristo, J.; et al. Prostatic acid phosphatase (PAcP) is PI(3)P-phosphatase and its inactivation leads to change of cell polarity and invasive prostate cancer. Proc. Amer. Assoc. Cancer Res 2005, 46, 5239. [Google Scholar]
- Zylka, M.J.; Sowa, N.A.; Taylor-Blake, B.; Twomey, M.A.; Herrala, A.; Voikar, V.; Vihko, P. Prostatic acid phosphatase is an ectonucleotidase and suppresses pain by generating adenosine. Neuron 2008, 60, 111–122. [Google Scholar]
- Loor, R.; Wang, M.C.; Valenzuela, L.; Chu, T.M. Expression of prostatic acid phosphatase in human prostate cancer. Cancer Lett 1981, 14, 63–69. [Google Scholar]
- Alaiya, A.A.; Oppermann, M.; Langridge, J.; Roblick, U.; Egevad, L.; Brindstedt, S.; Hellström, M.; Linder, S.; Bergman, T.; Jörnvall, H.; Auer, G. Identification of proteins in human prostate tumor material by two-dimensional gel electrophoresis and mass spectrometry. Cell. Mol. Life Sci 2001, 58, 307–311. [Google Scholar]
- Yeh, S.; Lin, H.K.; Kang, H.Y.; Thin, T.H.; Lin, M.F.; Chang, C. From HER2/Neu signal cascade to androgen receptor and its coactivators: A novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. PNAS 1999, 96, 5458–5463. [Google Scholar]
- Culig, Z.; Hobisch, A.; Cronauer, M.V.; Hittmair, A.; Radmayr, C.; Bartsch, G.; Klocker, H. Activation of the androgen receptor by polypeptide growth factors and cellular regulators. World J. Urol 1995, 13, 285–289. [Google Scholar]
- Grossmann, M.E.; Huang, H.; Tindall, D.J. Androgen receptor signaling in androgen-refractory prostate cancer. J. Natl. Cancer Inst 2001, 93, 1687–1697. [Google Scholar]
- Gioeli, D.; Mandell, J.W.; Petroni, G.R.; Frierson, H.F., Jr; Weber, M.J. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res 1999, 59, 279–284. [Google Scholar]
- Zhang, X.Q.; Lee, M.S.; Zelivianski, S.; Lin, M.F. Characterization of a prostate-specific tyrosine phosphatase by mutagenesis and expression in human prostate cancer cells. J. Biol. Chem 2001, 276, 2544–2550. [Google Scholar]
- Wen, Y.; Hu, M.C.; Makino, K.; Spohn, B.; Bartholomeusz, G.; Yan, D.H.; Hung, M.C. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res 2000, 60, 6841–6845. [Google Scholar]
- Lee, M.S.; Igawa, T.; Lin, M.F. Tyrosine-317 of p52 mediates androgen-stimulated proliferation signals in human prostate cancer cells. Oncogene 2004, 23, 3048–3058. [Google Scholar]
- Tan, S.H.; Dagvadorj, A.; Shen, F.; Gu, L.; Liao, Z.; Abdulghani, J.; Zhang, Y.; Gelmann, E.P.; Zellweger, T.; Culig, Z.; et al. Transcription factor Stat5 synergizes with androgen receptor in prostatecancer cells. Cancer Res 2008, 68, 236–248. [Google Scholar]
- Coffey, D.S.; Isaacs, J.T. Control of prostate growth. Urology 1981, 17, 17–24. [Google Scholar]
- Wang, X.; Yu, J.; Sreekumar, A.; Varambally, S.; Shen, R.; Giacherio, D.; Mehra, R.; Montie, J.E.; Pienta, K.J.; Sanda, M.G.; et al. Autoantibody signatures in prostate cancer. N. Engl. J. Med 2005, 353, 1224–1235. [Google Scholar]
- Taylor, B.S.; Varambally, S.; Chinnaiyan, A.M. Differential proteomic alterations between localised and metastatic prostate cancer. Br. J. Cancer 2006, 95, 425–430. [Google Scholar]
- Rhodes, D.R.; Barrette, T.R.; Rubin, M.A.; Ghosh, D.; Chinnaiyan, A.M. Meta-analysis of microarrays: Interstudy validation of gene expression profiles reveals pathway dysregulation in prostate cancer. Cancer Res 2002, 62, 4427–4433. [Google Scholar]
- Arlen, P.M.; Mohebtash, M.; Madan, R.A.; Gulley, J.L. Promising novel immunotherapies and combinations for prostate cancer. Future Oncol 2009, 5, 187–196. [Google Scholar]
- Drake, C.G.; Jaffee, E.; Pardoll, D.M. Mechanisms of immune evasion by tumors. Adv. Immunol 2006, 90, 51–81. [Google Scholar]
- Fong, L.; Ruegg, C.L.; Brockstedt, D.; Engleman, E.G.; Laus, R. Induction of tissue-speciWc autoimmune prostatitis with prostatic acid phosphatase immunization; implications for immunotherapy of prostate cancer. J. Immunol 1997, 159, 3113–3117. [Google Scholar]
- Johnson, L.E.; Frye, T.P.; Chinnasamy, N.; Chinnasamy, D.; McNeel, D.G. Plasmid DNA vaccine encoding prostatic acid phosphatase is effective in eliciting autologous antigen-specific CD8+ T cells. Cancer Immunol. Immunother 2007, 56, 885–895. [Google Scholar]
- Doehn, C. Immunotherapy of prostate cancer. Eur. Urol 2008, 53, 681–685. [Google Scholar]
- Vieweg, J. Immunotherapy for advanced prostate cancer. Rev. Urol 2007, 9, S29–S38. [Google Scholar]
- Drake, C.G. Prostate cancer as a model for tumour immunotherapy. Nat. Rev. Immunol 2010, 10, 580–593. [Google Scholar]
- McNeel, D.G.; Nguyen, L.D.; Ellis, W.J.; Higano, C.S.; Lange, P.H.; Disis, M.L. Naturally occurring prostate cancer antigen-specific T cell responses of a Th1 phenotype can be detected in patients with prostate cancer. Prostate 2001, 47, 222–229. [Google Scholar]
- Small, E.J.; Fratesi, P.; Reese, D.M.; Strang, G.; Laus, R.; Peshwa, M.V.; Valone, F.H. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J. Clin. Oncol 2000, 18, 3894–3903. [Google Scholar]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Impact study investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med 2010, 363, 411–422. [Google Scholar]
- Garcia, J.A. Sipuleucel-T in patients with metastatic castration-resistant prostate cancer: An insight for oncologists. Ther. Adv. Med. Oncol 2011, 3, 101–108. [Google Scholar]
- Burch, P.A.; Croghan, G.A.; Gastineau, D.A.; Jones, L.A.; Kaur, J.S.; Kylstra, J.W.; Richardson, R.L.; Valone, F.H.; Vuk-Pavlović, S. Immunotherapy (APC8015, Provenge) targeting prostatic acid phosphatase can induce durable remission of metastatic androgen-independent prostate cancer: A Phase 2 trial. Prostate 2004, 60, 197–204. [Google Scholar]
- Beinart, G.; Rini, B.I.; Weinberg, V.; Small, E.J. Antigen-presenting cells 8015 (Provenge) in patients with androgen-dependent, biochemically relapsed prostate cancer. Clin. Prostate Cancer 2005, 4, 55–60. [Google Scholar]
- Rini, B.I.; Weinberg, V.; Fong, L.; Conry, S.; Hershberg, R.M.; Small, E.J. Combination immunotherapy with prostatic acid phosphatase pulsed antigen-presenting cells (provenge) plus bevacizumab in patients with serologic progression of prostate cancer after definitive local therapy. Cancer 2006, 107, 67–74. [Google Scholar]
- Small, E.J.; Higano, C.S.; Kantoff, P.W.; Whitmore, J.B.; Frohlich, M.W.; Petrylak, D.P. Time to disease-related pain after sipuleucel-T in asymptomatic patients with metastatic castrate-resistant prostate cancer (mCRPC): Results from three randomized phase III trials. J. Clin. Oncol 2011, 29, S484. [Google Scholar]
- Sheikh, N.A.; Wesley, J.D.; Chadwick, E.; Perdue, N.; de Rosa, C.P. Characterization of antigen-specific T-cell activation and cytokine expression induced by sipuleucel-T. J. Clin. Oncol 2011, 29, 16–30. [Google Scholar]
- McLeod, D.G.; Quinn, D.I.; Whitmore, M.; Tabesh, M. Sipuleucel-T in African Americans: A subgroup analysis of three phase III sipuleucel-T in advanced prostate cancer. J. Clin. Oncol 2011, 29, e15148. [Google Scholar]
- Paller, C.J.; Antonarakis, E.S. Sipuleucel-T for the treatment of metastatic prostate cancer: Promise and challenges. Hum. Vaccin. Immunother 2012, 8, 509–519. [Google Scholar]
- Schellhammer, P.F.; Chodak, G.; Whitmore, J.B.; Sims, R.; Frohlich, M.W.; Kantoff, P.W. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the immunotherapy for prostate adenocarcinoma treatment (IMPACT) trial. Urology 2013, in press. [Google Scholar]


© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Muniyan, S.; Chaturvedi, N.K.; Dwyer, J.G.; LaGrange, C.A.; Chaney, W.G.; Lin, M.-F. Human Prostatic Acid Phosphatase: Structure, Function and Regulation. Int. J. Mol. Sci. 2013, 14, 10438-10464. https://doi.org/10.3390/ijms140510438
Muniyan S, Chaturvedi NK, Dwyer JG, LaGrange CA, Chaney WG, Lin M-F. Human Prostatic Acid Phosphatase: Structure, Function and Regulation. International Journal of Molecular Sciences. 2013; 14(5):10438-10464. https://doi.org/10.3390/ijms140510438
Chicago/Turabian StyleMuniyan, Sakthivel, Nagendra K. Chaturvedi, Jennifer G. Dwyer, Chad A. LaGrange, William G. Chaney, and Ming-Fong Lin. 2013. "Human Prostatic Acid Phosphatase: Structure, Function and Regulation" International Journal of Molecular Sciences 14, no. 5: 10438-10464. https://doi.org/10.3390/ijms140510438
APA StyleMuniyan, S., Chaturvedi, N. K., Dwyer, J. G., LaGrange, C. A., Chaney, W. G., & Lin, M.-F. (2013). Human Prostatic Acid Phosphatase: Structure, Function and Regulation. International Journal of Molecular Sciences, 14(5), 10438-10464. https://doi.org/10.3390/ijms140510438





