MiR-590-5P Inhibits Growth of HepG2 Cells via Decrease of S100A10 Expression and Inhibition of the Wnt Pathway
Abstract
:1. Introduction
2. Results and Discussion
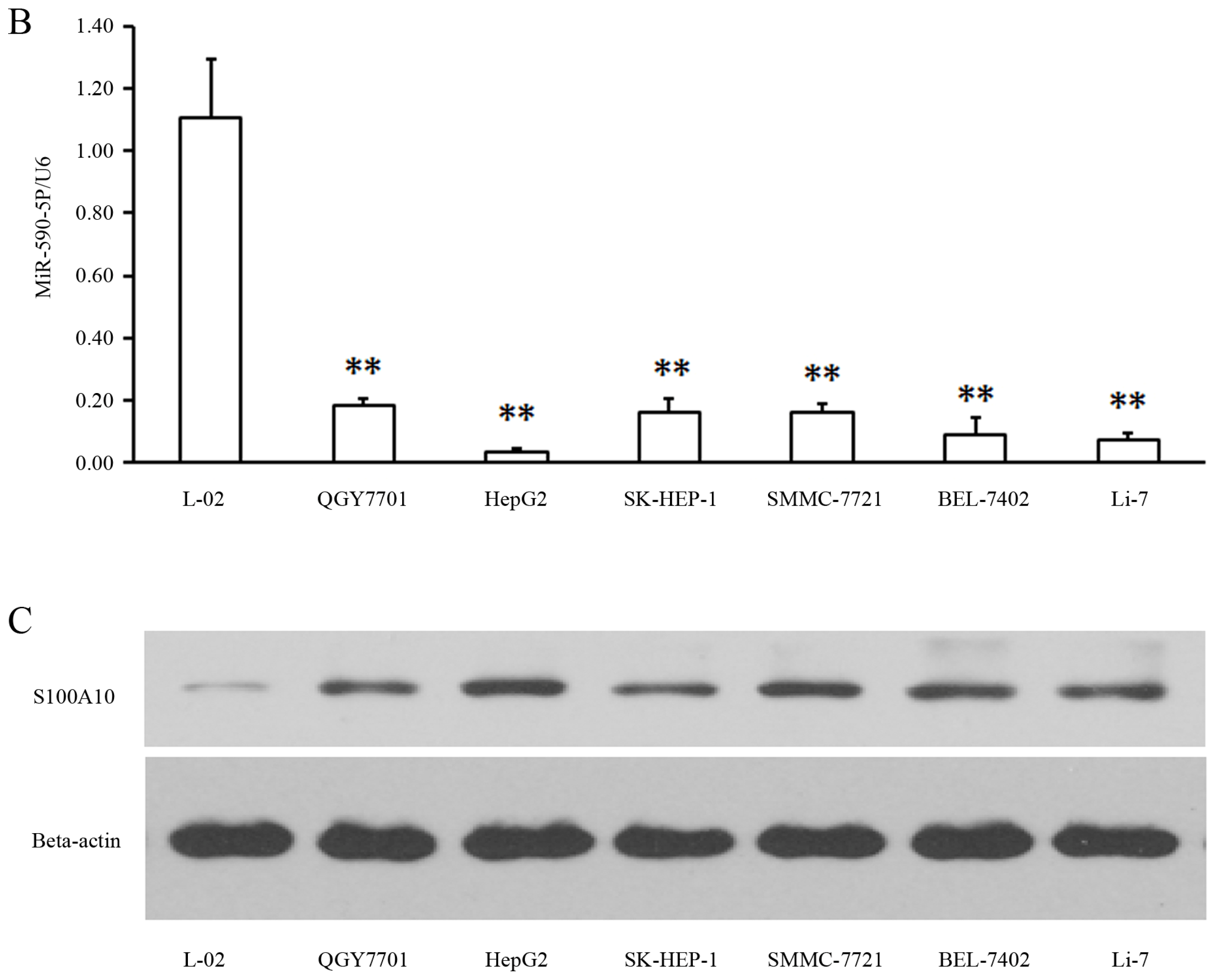
2.1. MiR-590-5P Is Down-Regulated and S100A10 Is Enhanced in Liver Cancer Cell Lines
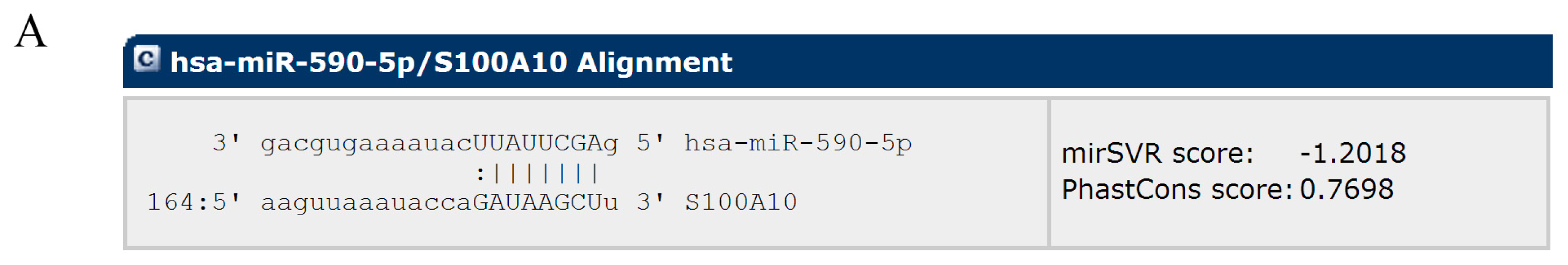
2.2. MiR-590-5P Inhibits S100A10 Expression by Interacting with 3′UTR of S100A10 mRNA
2.3. The Over-Expression of miR-590-5P Inhibits Cellular Proliferation and Causes Cell Cycle Arrest in HepG2
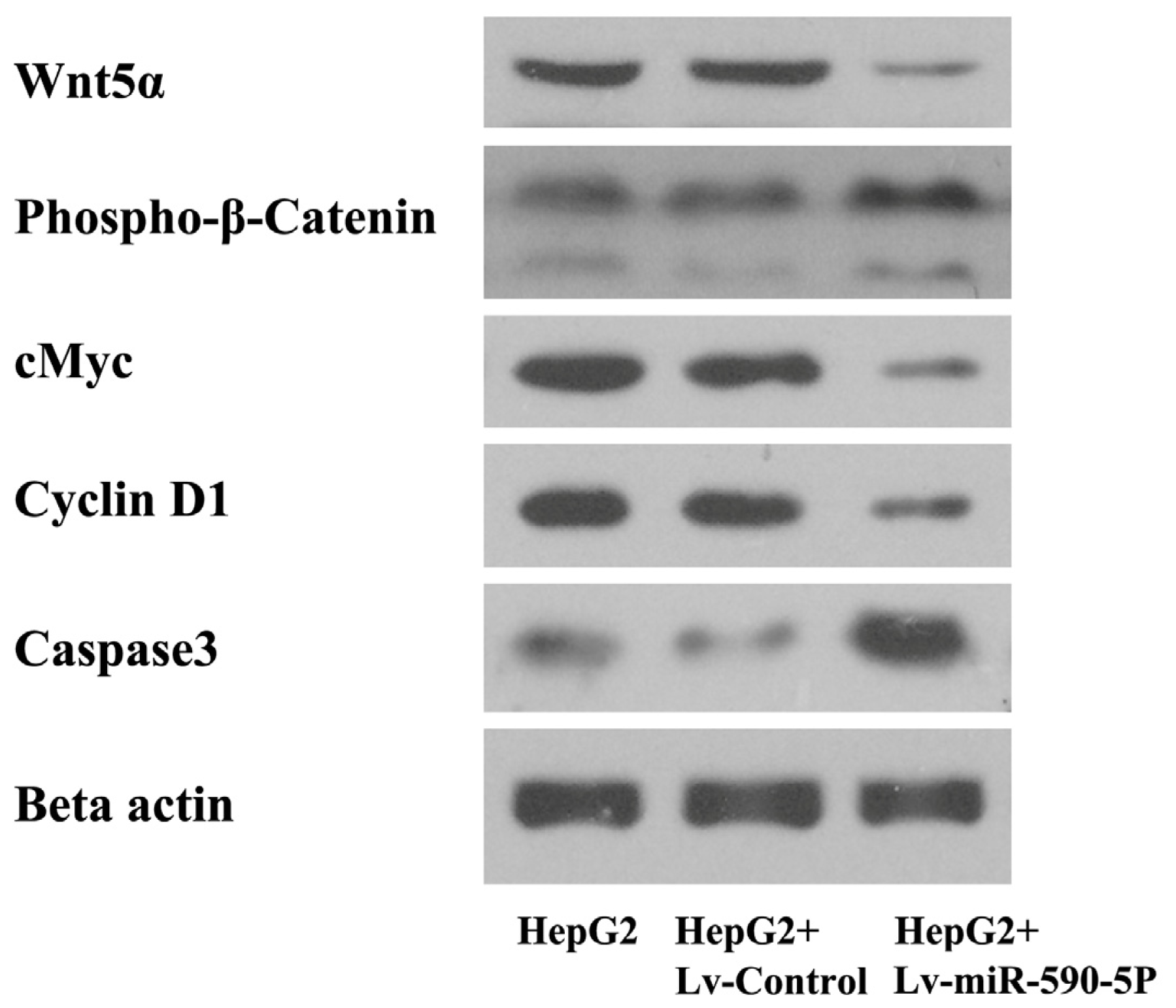
2.4. The Over-Expression of miR-590-5P Affects Wnt Pathway Signaling in HepG2 Cells
3. Experimental Section
3.1. Cell Culture
3.2. Plasmid Construction
3.3. Cell Transfection and Luciferase Assay
3.4. Lentivirus Package and Cell Infection
3.5. Real Time PCR
3.6. Western Blotting
3.7. Cell Proliferation Assays
3.8. Statistical Analysis
4. Conclusions
References
- Chen, J.G.; Zhang, S.W. Liver cancer epidemic in China: Past, present and future. Semin. Cancer Biol 2011, 21, 59–69. [Google Scholar]
- Santamaria-Kisiel, L.; Shaw, G.S. Identification of regions responsible for the open conformation of S100A10 using chimaeric S100A11-S100A10 proteins. Biochem. J 2011, 434, 37–48. [Google Scholar]
- Glenney, J.R., Jr; Tack, BF. Amino-terminal sequence of p36 and associated p10: Identification of the site of tyrosine phosphorylation and homology with S-100. Proc. Natl. Acad. Sci. USA 1985, 82, 7884–7888. [Google Scholar]
- Gerke, V.; Weber, K. Identity of p36K phosphorylated upon Rous sarcoma virus transformation with a protein purified from brush borders; calcium-dependent binding to non-erythroid spectrin and F-actin. EMBO J 1984, 3, 227–233. [Google Scholar]
- Waisman, D.M.; Annexin, II. tetramer: Structure and function. Mol. Cell. Biochem. 1995, 149–150, 301–322. [Google Scholar]
- Gerke, V.; Moss, S.E. Annexins and membrane dynamics. Biochim. Biophys. Acta 1997, 1357, 129–154. [Google Scholar]
- Gerke, V.; Weber, K. The regulatory chain in the p36-kd substrate complex of viral tyrosine-specific protein kinases is related in sequence to the S-100 protein of glial cells. EMBO J 1985, 4, 2917–2920. [Google Scholar]
- Svenningsson, P.; Chergui, K.; Rachleff, I.; Flajolet, M.; Zhang, X.; El Yacoubi, M.; Vaugeois, J.M.; Nomikos, G.G.; Greengard, P. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science 2006, 311, 77–80. [Google Scholar]
- Warner-Schmidt, J.L.; Flajolet, M.; Maller, A.; Chen, E.Y.; Qi, H.; Svenningsson, P.; Greengard, P. Role of p11 in cellular and behavioral effects of 5-HT4 receptor stimulation. J. Neurosci 2009, 29, 1937–1946. [Google Scholar]
- Girard, C.; Tinel, N.; Terrenoire, C.; Romey, G.; Lazdunski, M.; Borsotto, M. p11, an annexin II subunit, an auxiliary protein associated with the background K+ channel, TASK-1. EMBO J 2002, 21, 4439–4448. [Google Scholar]
- Renigunta, V.; Yuan, H.; Zuzarte, M.; Rinne, S.; Koch, A.; Wischmeyer, E.; Gao, Y.; Karschin, A.; Jacob, R.; Schwappach, B.; et al. The retention factor p11 confers an endoplasmic reticulum-localization signal to the potassium channel TASK-1. Traffic 2006, 7, 168–181. [Google Scholar]
- Van de Graaf, S.F.J.; Hoenderop, J.G.J.; Gkika, D.; Lamers, D.; Prenen, J.; Rescher, U.; Gerke, V.; Staub, O.; Nilius, B.; Bindels, R.J. Functional expression of the epithelial Ca2+ channels (TRPV5 and TRPV6) requires association of the S100A10 annexin 2 complex. EMBO J 2003, 22, 1478–1487. [Google Scholar]
- MacLeod, T.J.; Kwon, M.; Filipenko, N.R.; Waisman, D.M. Phospholipid-associated annexin A2- S100A10 heterotetramer and its subunits: Characterization of the interaction with tissue plasminogen activator, plasminogen, and plasmin. J. Biol. Chem 2003, 278, 25577–25584. [Google Scholar]
- Phipps, K.D.; Surette, A.P.; O’Connell, P.A.; Waisman, D.M. Plasminogen receptor S100A10 is essential for the migration of tumor-promoting macrophages into tumor sites. Cancer Res 2011, 71, 6676–6683. [Google Scholar]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet 2004, 5, 522–531. [Google Scholar]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar]
- Farh, K.K.; Grimson, A.; Jan, C.; Lewis, B.P.; Johnston, W.K.; Lim, L.P.; Burge, C.B.; Bartel, D.P. The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science 2005, 310, 1817–1821. [Google Scholar]
- Pauli, A.; Rinn, J.L.; Schier, A.F. Non-coding RNAs as regulators of embryogenesis. Nat. Rev. Genet 2011, 12, 136–149. [Google Scholar]
- Leaman, D.; Chen, P.Y.; Fak, J.; Yalcin, A.; Pearce, M.; Unnerstall, U.; Marks, D.S.; Sander, C.; Tuschl, T.; Gaul, U. Antisense-mediated depletion reveals essential and specific functions of microRNAs in Drosophila development. Cell 2005, 121, 1097–1108. [Google Scholar]
- Shivdasani, R.A. MicroRNAs: Regulators of gene expression and cell differentiation. Blood 2006, 108, 3646–3653. [Google Scholar]
- Hwang, H.W.; Mendell, J.T. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br. J. Cancer 2006, 94, 776–780. [Google Scholar]
- Alvarez-Saavedra, M.; Antoun, G.; Yanagiya, A.; Oliva-Hernandez, R.; Cornejo-Palma, D.; Perez-Iratxeta, C.; Sonenberg, N.; Cheng, H.Y. miRNA-132 orchestrates chromatin remodeling and translational control of the circadian clock. Hum. Mol. Genet 2011, 20, 731–751. [Google Scholar]
- Pogribny, I.P.; Tryndyak, V.P.; Boyko, A.; Rodriguez-Juarez, R.; Beland, F.A.; Kovalchuk, O. Induction of microRNAome deregulation in rat liver by long-term tamoxifen exposure. Mutat. Res 2007, 619, 30–37. [Google Scholar]
- Meng, F.; Henson, R.; Wehbe-Janek, H.; Ghoshal, K.; Jacob, S.T.; Patel, T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 2007, 133, 647–658. [Google Scholar]
- Heneghan, H.M.; Miller, N.; Kerin, M.J. MiRNAs as biomarkers and therapeutic targets in cancer. Curr. Opin. Pharmacol 2010, 10, 543–550. [Google Scholar]
- Negrini, M.; Calin, G.A. Breast cancer metastasis: A microRNA story. Breast Cancer Res 2008, 10, 203. [Google Scholar]
- Wang, X.; Tang, S.; Le, S.Y.; Lu, R.; Rader, J.S.; Meyers, C.; Zheng, Z.M. Aberrant expression of oncogenic and tumor-suppressive microRNAs in cervical cancer is required for cancer cell growth. PLoS One 2008, 3, e2557. [Google Scholar]
- Wang, X.C.; Tian, L.L.; Jiang, X.Y.; Wang, Y.Y.; Li, D.G.; She, Y.; Chang, J.H.; Meng, A.M. The expression and function of miRNA-451 in non-small cell lung cancer. Cancer Lett 2011, 311, 203–209. [Google Scholar]
- Singh, P.; Soon, P.S.; Feige, J.J.; Chabre, O.; Zhao, J.T.; Cherradi, N.; Lalli, E.; Sidhu, S.B. Dysregulation of microRNAs in adrenocortical tumors. Mol. Cell. Endocrinol 2012, 351, 118–128. [Google Scholar]
- Zheng, Z.M.; Wang, X. Regulation of cellular miRNA expression by human papillomaviruses. Biochim. Biophys. Acta 2011, 1809, 668–677. [Google Scholar]
- Pfeifer, A.; Ikawa, M.; Dayn, Y.; Verma, I.M. Transgenesis by lentiviral vectors, Lack of gene silencing in mammalian embryonic stem cells and preimp lantation embryos. Proc. Natl. Acad. Sci. USA 2002, 99, 2140–2145. [Google Scholar]
- Yu, X.; Zhan, X.; D’Costa, J.; Tanavde, V.M.; Ye, Z.; Peng, T.; Malehorn, M.T.; Yang, X.; Civin, C.I.; Cheng, L. Lentiviral vectors with two independent internal promoters transfer high-level expression of multiple transgenes to human hematopoietic stem-progenitor cells. Mol. Ther 2003, 7, 827–838. [Google Scholar]
- Lois, C.; Hong, E.J.; Pease, S.; Brown, E.J.; Baltimore, D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 2002, 295, 868–872. [Google Scholar]
- Lai, Z.; Brady, R.O. Gene transfer into the central nervous system in vivo using a recombinant lentivirus vector. J. Neurosci. Res 2002, 67, 363–371. [Google Scholar]
- Noda, T.; Oki, S.; Kitajima, K.; Harada, T.; Komune, S.; Meno, C. Restriction of Wnt signaling in the dorsal otocyst determines semicircular canal formation in the mouse embryo. Dev. Biol 2012, 362, 83–93. [Google Scholar]
- Veeck, J.; Dahl, E. Targeting the Wnt pathway in cancer: The emerging role of Dickkopf-3. Biochim. Biophys. Acta 2012, 1825, 18–28. [Google Scholar]
- Camilli, T.C.; Weeraratna, A.T. Striking the target in Wnt-y conditions: Intervening in Wnt signaling during cancer progression. Biochem. Pharmacol 2010, 80, 702–711. [Google Scholar]
- Myant, K.; Sansom, O.J. Wnt/Myc interactions in intestinal cancer: Partners in crime. Exp. Cell. Res 2011, 317, 2725–2731. [Google Scholar]
- Daniel, J.M.; Reynolds, A.B. Tyrosine phosphorylation and cadherin/catenin function. Bioessays 1997, 19, 883–891. [Google Scholar]




© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shan, X.; Miao, Y.; Fan, R.; Qian, H.; Chen, P.; Liu, H.; Yan, X.; Li, J.; Zhou, F. MiR-590-5P Inhibits Growth of HepG2 Cells via Decrease of S100A10 Expression and Inhibition of the Wnt Pathway. Int. J. Mol. Sci. 2013, 14, 8556-8569. https://doi.org/10.3390/ijms14048556
Shan X, Miao Y, Fan R, Qian H, Chen P, Liu H, Yan X, Li J, Zhou F. MiR-590-5P Inhibits Growth of HepG2 Cells via Decrease of S100A10 Expression and Inhibition of the Wnt Pathway. International Journal of Molecular Sciences. 2013; 14(4):8556-8569. https://doi.org/10.3390/ijms14048556
Chicago/Turabian StyleShan, Xiangxiang, Yufeng Miao, Rengen Fan, Haixin Qian, Ping Chen, Hongqi Liu, Xiaomei Yan, Jianping Li, and Fen Zhou. 2013. "MiR-590-5P Inhibits Growth of HepG2 Cells via Decrease of S100A10 Expression and Inhibition of the Wnt Pathway" International Journal of Molecular Sciences 14, no. 4: 8556-8569. https://doi.org/10.3390/ijms14048556



