Noninvasive Metabolomic Profiling of Human Embryo Culture Media Using a Simple Spectroscopy Adjunct to Morphology for Embryo Assessment in in Vitro Fertilization (IVF)
Abstract
:1. Introduction
2. Results and Discussion
2.1. A Preliminary Analysis of the 45 Samples
2.2. The Verification of Preliminary Results
3. Experimental Section
3.1. Patient Selection, Treatment and Sample Collection
3.1.1. Patient Selection and Treatment
3.1.2. Sample Collection
3.2. Raman Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Mosher, W.D.; Pratt, W.F. Fecundity and infertility in the United Sates: Incidence and trends. Feril. Steril 1991, 56, 192–193. [Google Scholar]
- Luke, B.; Brown, M.B. Contemporary risks of maternal morbidity and adverse outcomes with increasing maternal age and plurality. Fertil. Steril 2007, 88, 283–293. [Google Scholar]
- Luke, B.; Martin, J. The rise in multiple births in the United States: Who, what, when, where, and why. Clin. Obstet. Gynecol 2004, 47, 118–133. [Google Scholar]
- Templeton, A. Avoiding multiple pregnancies in ART: Replace as many embryos as you like-one at a time. Hum. Reprod 2000, 15, 1662. [Google Scholar]
- Adamson, G.D.; de Mouzon, J. World abortive report on in vitro fertilization, 2000. Fertil. Steril 2006, 85, 1586–1622. [Google Scholar]
- Sakkas, D.; Gardner, D.K. Noninvasive methods to assess embryo quality. Curr. Opin. Obstet. Gynecol 2005, 17, 283–288. [Google Scholar]
- Roseboom, T.J.; Vermeiden, J.P. The probability of pregnancy after embryo transfer is affected by the age of the patient, cause of infertility, number of embryos transferred and the average morphology score, as revealed by multiple logistic regression analysis. Hum. Reprod 1995, 10, 3035–3041. [Google Scholar]
- Bromer, J.G.; Seli, E. Assessment of embryo viability in assisted reproductive technologies: Shortcomings of current approaches and the emerging role of metabolomics. Curr. Opin. Obstet. Gynecol 2008, 20, 234–241. [Google Scholar]
- Brison, D.R.; Houghton, F.D. Identification of viable embryos in IVF by on-invasive measurement of amino acid trunover. Hum. Reprod 2004, 19, 2319–2324. [Google Scholar]
- Steptoe, P.C.; Edwards, R.G. Birth after the reimplantation of a human embryo. Lancet 1978, 2, 366. [Google Scholar]
- Toner, J.P. Progress we can be proud of: U.S. trends in assisted reproduction over the first 20 years. Fertil. Steril 2002, 78, 943–950. [Google Scholar]
- Brinster, R.L. Studies on the development of the mouse embryos in vitro. II. The effect of energy source. J. Exp. Zool 1965, 158, 59–68. [Google Scholar]
- Gardner, D.K.; Leese, H.J. Non-invasive measurement of nutrient uptake by single cultured preimplantation mouse embryos. Hum. Reprod 1986, 1, 25–27. [Google Scholar]
- Hardy, K.; Hooper, M.A.K. Non-invasive measurement of glucose and pyruvate uptake by individual human oocytes and preimplantation embryos. Hum. Reprod 1989, 4, 188–191. [Google Scholar]
- Conaqhan, J.; Handyside, A.H. Effects of pyruvate and glucose on the development of the human preimplantation embryo. J. Reprod. Fertil 1993, 99, 87–95. [Google Scholar]
- Casslen, B.G. Free amino acidcs in human uterine fluid. possible role of high tanrine concentration. Rrod. Med 1987, 32, 181–184. [Google Scholar]
- Miller, J.G.; Schultz, G.A. Amino acid content of preimplantation rabbit embryos and fluids of the reprodictive tract. Biol. Reprod 1987, 36, 125–129. [Google Scholar]
- Gardner, D.K. Changes in requirement and utlization of nutrients during mammalian preimplantation embryo development and their significance in embryo culture. Theriogenology 1998, 49, 83–102. [Google Scholar]
- Gardner, D.K.; Lane, M. Embryo Culture Systems. In Handbook of in vitro Fertilization; Trounson, A., Gardner, D.K., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 195–254. [Google Scholar]
- Houghton, F.D.; Hawkhead, J.A. Noninvaive amino acid turnover predicts human embrvo developmental capacity. Hum. Reprod 2002, 17, 999–1005. [Google Scholar]
- Sturmey, R.G.; Hawkhead, J.A. DNA damage and metabolic activity in the preimplantation embryo. Hum. Reprod 2009, 24, 81–91. [Google Scholar]
- Seli, E.; Botros, L. Noninvasive metabolomic profiling of embryo culture media using proton nuclear magnetic correlates with reproductive potential of embryos in women undergoing in vitrofertilization. Fertil. Steril. 2008, 90, 2183–2189. [Google Scholar]
- Steer, C.V.; Mills, C.L. The cumulative embryo score: A predictive embryo scoring technique to select the optimal number of embryos to transfer in an in vitro fertilization and embryo transfer programme. Hum. Reprod 1992, 7, 117–119. [Google Scholar]
- Van Royan, E.; Mangelschots, K. Characterization of a top quality embryo, a step towards single-embryo transfer. Hum. Reprod 1999, 14, 2345–2349. [Google Scholar]
- Veeck, L. An Atlas of Human Gametes and Conceptuses: An Illustrated Reference for Assisted Reproductive Technology; Parthenon; New York, NY, USA, 1999. [Google Scholar]
- Sakkas, D.; Percival, G. Assessment of early cleaving in vitro fertilized human embryos at the 2-cell stage before transfer improves embryo selection. Fertil. Steril 2001, 76, 1150–1156. [Google Scholar]
- Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine. Preimplantation genetic testing: A Practice Committee opinion. Fertil. Steril. 2007, 88, 1497–1504.
- Lane, M.; Gardner, D.K. Embryo culture medium: Which is the best? Best Pract. Res. Clin. Obstet. Gynaecol 2007, 21, 83–100. [Google Scholar]
- Gardner, D.K.; Lane, M. Ex vivo early embryo development and effects on gene expression and imprinting. Reprod. Fertil. Dev 2005, 17, 361–370. [Google Scholar]
- Vergouw, C.G.; Botros, L.L. Metabolomic profiling by near-infrared spectroscopy as a tool to assess embryo viability: A non-invasive method for embryo selection. Hum. Reprod 2008, 23, 1499–1504. [Google Scholar]
- Hardarson, T.; Ahlstrom, A. Non-invasive metabolomic profiling of Day 2 and 5 embryo culture medium: A prospective randomized trial. Hum. Reprod 2012, 27, 89–96. [Google Scholar]
- Nadal-Desbarats, L.; Veau, S. Is NMR metabolic profiling of spent embryo culture media useful to assist in vitrohuman embryo selection? MAGMA 2012. [Google Scholar] [CrossRef]
- Wiener-Megnazi, Z.; Shiloh, H. Oxidative parameters of embryo culture media may predict treatment outcome in vitro fertilization: A novel applicable tool for improving embryo selection. Fertil. Steril 2011, 95, 979–984. [Google Scholar]
- Seli, E.; Sakkas, D. Noninvasive metabolomic profiling of embryo culture media using Raman and near-infrared spectroscopy correlates with reproductive potential of embryos in women undergoing in vitro fertilization. Fertil. Steril 2007, 88, 1350–1357. [Google Scholar]
- Scott, R.; Seli, E. Noninvasive metabolomic profiling of human embryo culture media using Raman spectroscopy predicts embryonic reproductive potential: A prospective blinded pilot study. Fertil. Steril 2008, 90, 77–83. [Google Scholar]
- Ben-Yosef, D.; Yogev, L. Testicular sperm retrieval and cryopreservation prior to initiating ovarian stimulation as the first line approach in patients with non-obstructive azoospermia. Hum. Reprod 1999, 14, 1794–1801. [Google Scholar]
- Cummins, J.M.; Breen, T.M. A formula for scoring human embryo growth rates in vitro fertilization: Its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J. In Vitro Fert. Embryo Transf 1986, 3, 284–295. [Google Scholar]
- Stone, N.; Kendall, C. Raman spectroscopy for identification of epithelial cancers. Faraday Discuss 2004, 126, 141–157. [Google Scholar]
 ) and the fitting spectrum(…).
) and the fitting spectrum(…).

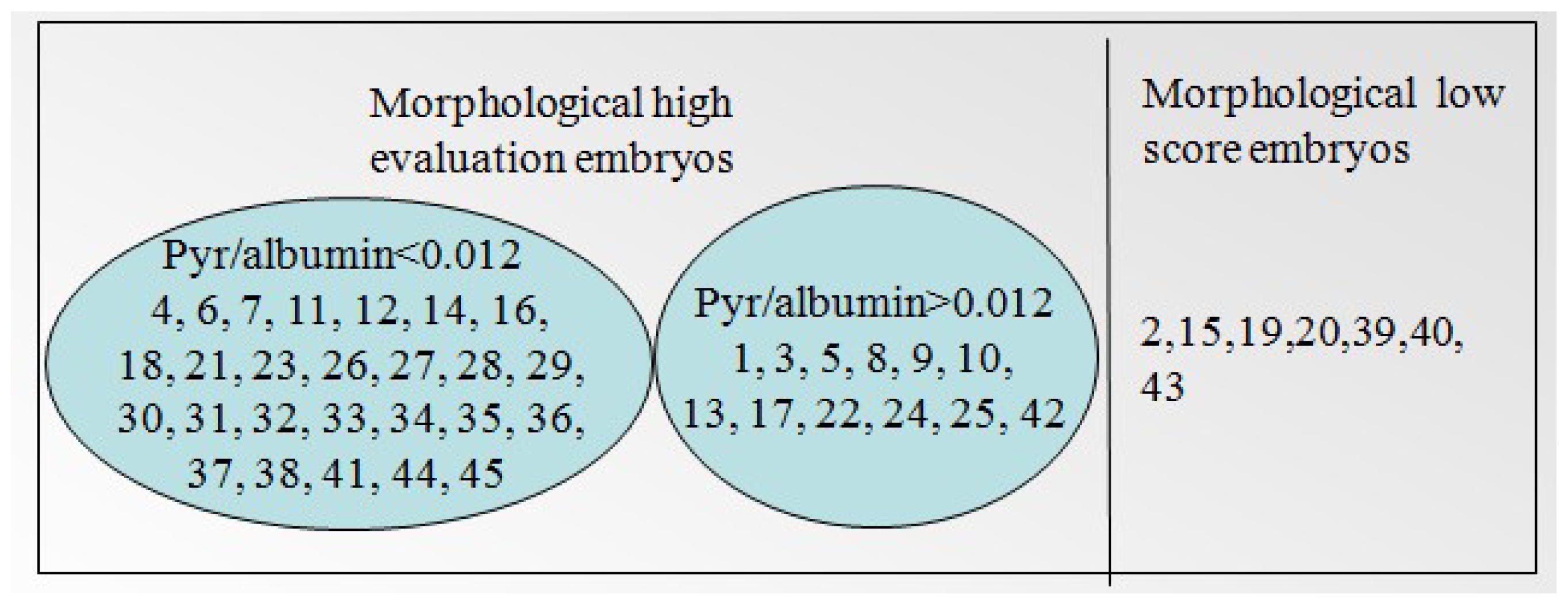
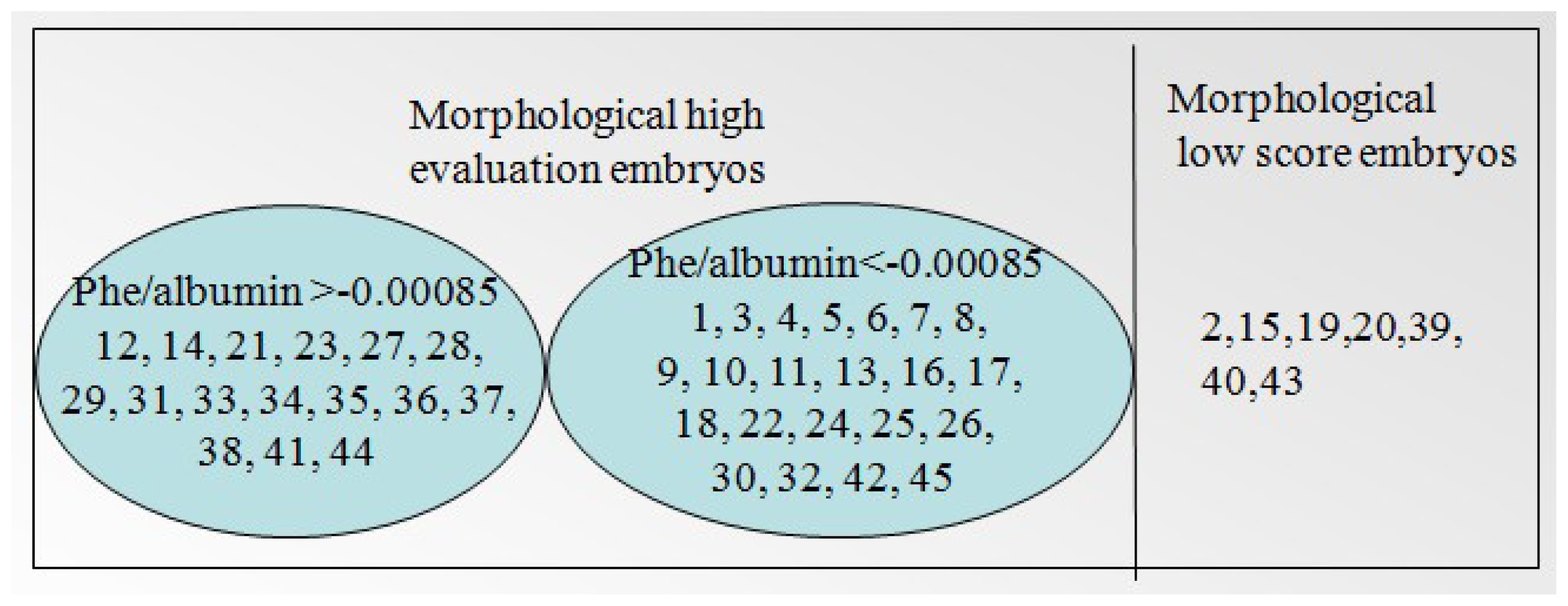



| Relative concentration (C′) | Range of C′ | Embryo developmental capacity | Number of sample |
|---|---|---|---|
| Sodium pyruvate/albumin | <0.012 | + | 4, 6, 7, 11, 12, 14, 16, 18, 21, 23, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 41, 44, 45 |
| >0.012 | — | 1, 3, 5, 8, 9, 10, 13, 17, 22, 24, 25, 42 | |
| Phenylalanine/albumin | ≥0.00085 | + | 12, 14, 21, 23, 27, 28, 29, 31, 33, 34, 35, 36, 37, 38, 41, 44 |
| ≤0.00085 | — | 1, 3, 4, 5, 6, 7, 8, 9, 10, 11, 13, 16, 17, 18, 22, 24, 25, 26, 30, 32, 42, 45 | |
| Age (years) | Endometrial thickness (cm) | No. of oocytes retrieved | No. of high-quality embryos | Mean No. of transferred embryos per cycle |
|---|---|---|---|---|
| 30.02 ± 3.12 | 0.93 ± 0.42 | 13.52 ± 4.10 | 9.73 ± 4.82 | 2.16 ± 0.78 |
| Sample number | The number of embryonic | Morphological classification |
|---|---|---|
| 1 | 8 | A |
| 2 | 5 | C |
| 3 | 8 | B |
| 4 | 8 | A |
| 5 | 8 | A |
| 6 | 8 | A |
| 7 | 8 | B |
| 8 | 8 | A |
| 9 | 8 | B |
| 10 | 6 | B |
| 11 | 8 | B |
| 12 | 8 | B |
| 13 | 6 | B |
| 14 | 8 | B |
| 15 | 6 | C |
| 16 | 8 | B |
| 17 | 8 | B |
| 18 | 5 | B |
| 19 | 8 | C |
| 20 | 5 | C |
| 21 | 7 | B |
| 22 | 4 | B |
| 23 | 4 | B |
| 24 | 7 | B |
| 25 | 8 | A |
| 26 | 8 | B |
| 27 | 8 | B |
| 28 | 7 | B |
| 29 | 6 | B |
| 30 | 5 | B |
| 31 | 6 | B |
| 32 | 3 | B |
| 33 | 5 | B |
| 34 | 5 | B |
| 35 | 7 | B |
| 36 | 7 | B |
| 37 | 5 | B |
| 38 | 8 | B |
| 39 | 4 | C |
| 40 | 3 | C |
| 41 | 7 | B |
| 42 | 8 | B |
| 43 | 4 | C |
| 44 | 7 | B |
| 45 | 7 | B |
| 46 | 8 | B |
| 47 | 6 | B |
| 48 | 8 | C |
| 49 | 8 | A |
| 50 | 8 | A |
| 51 | — | fragment |
| 52 | 8 | B |
| 53 | 8 | B |
| 54 | 4 | B |
| 55 | 8 | B |
| 56 | 8 | B |
| 57 | 8 | C |
| Component | Concentration (mM) |
|---|---|
| Sodium bicarbonate | 25.0 |
| Potassium chloride | 2.5 |
| Potassium phosphate | 0.35 |
| Calcium chloride, anhydrous | 1.7 |
| Sodium chloride | 101.5 |
| Magnesium sulfate, anhydrous | 0.2 |
| Sodium bicarbonate | 25.0 |
| Sodium pyruvate | 0.2 |
| Glucose | 0.5 |
| Sodium citrate | 1.0 |
| Sodium lactate (D/L) | 20 |
| EDTA, disodium, dihydrate | 10 μm |
| Alanine | 0.05 |
| Arginine | 0.3 |
| Alanyl-glutamine | 1.0 |
| Asparagine | 0.05 |
| Aspartic acid | 0.05 |
| Cysteine | 0.05 |
| Glutamic acid | 0.05 |
| Glycine | 0.05 |
| Histidine | 0.1 |
| Isoleucine | 0.2 |
| Leucine | 0.2 |
| Lysine | 0.2 |
| Methionine | 0.05 |
| Phenylalanine | 0.1 |
| Proline | 0.05 |
| Serine | 0.05 |
| Taurine | 0.05 |
| Threonine | 0.2 |
| Tryptophan | 0.02 |
| Tyrosine | 0.1 |
| Valine | 0.2 |
| Phenol red | 4.8 mg/L |
| Gentamicin | 10 ug/mL |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhao, Q.; Yin, T.; Peng, J.; Zou, Y.; Yang, J.; Shen, A.; Hu, J. Noninvasive Metabolomic Profiling of Human Embryo Culture Media Using a Simple Spectroscopy Adjunct to Morphology for Embryo Assessment in in Vitro Fertilization (IVF). Int. J. Mol. Sci. 2013, 14, 6556-6570. https://doi.org/10.3390/ijms14046556
Zhao Q, Yin T, Peng J, Zou Y, Yang J, Shen A, Hu J. Noninvasive Metabolomic Profiling of Human Embryo Culture Media Using a Simple Spectroscopy Adjunct to Morphology for Embryo Assessment in in Vitro Fertilization (IVF). International Journal of Molecular Sciences. 2013; 14(4):6556-6570. https://doi.org/10.3390/ijms14046556
Chicago/Turabian StyleZhao, Qinghong, Tailang Yin, Jin Peng, Yujie Zou, Jing Yang, Aiguo Shen, and Jiming Hu. 2013. "Noninvasive Metabolomic Profiling of Human Embryo Culture Media Using a Simple Spectroscopy Adjunct to Morphology for Embryo Assessment in in Vitro Fertilization (IVF)" International Journal of Molecular Sciences 14, no. 4: 6556-6570. https://doi.org/10.3390/ijms14046556
APA StyleZhao, Q., Yin, T., Peng, J., Zou, Y., Yang, J., Shen, A., & Hu, J. (2013). Noninvasive Metabolomic Profiling of Human Embryo Culture Media Using a Simple Spectroscopy Adjunct to Morphology for Embryo Assessment in in Vitro Fertilization (IVF). International Journal of Molecular Sciences, 14(4), 6556-6570. https://doi.org/10.3390/ijms14046556




