Batch and Continuous Ultrasound Assisted Extraction of Boldo Leaves (Peumus boldus Mol.)
Abstract
:1. Introduction
2. Results and Discussion
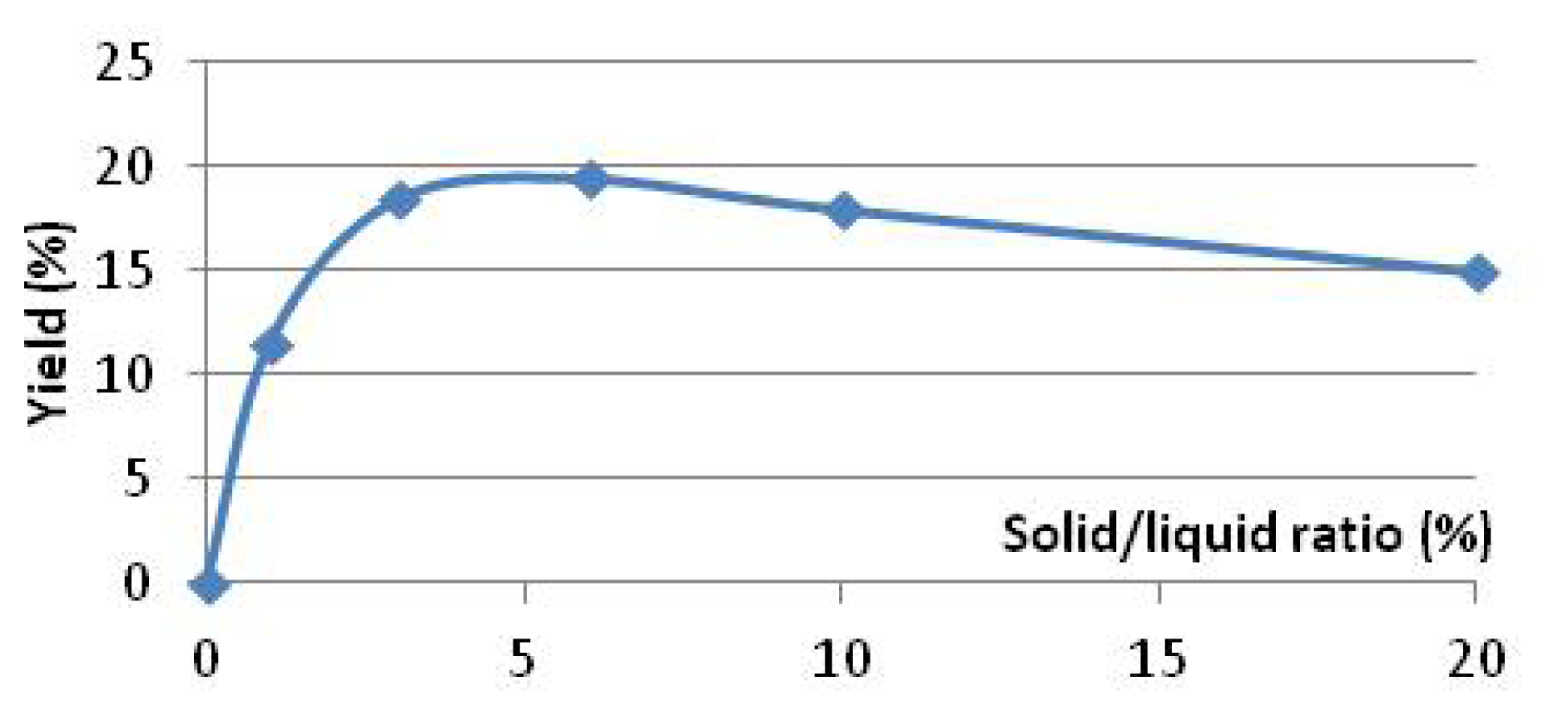
2.1. Preliminary Study
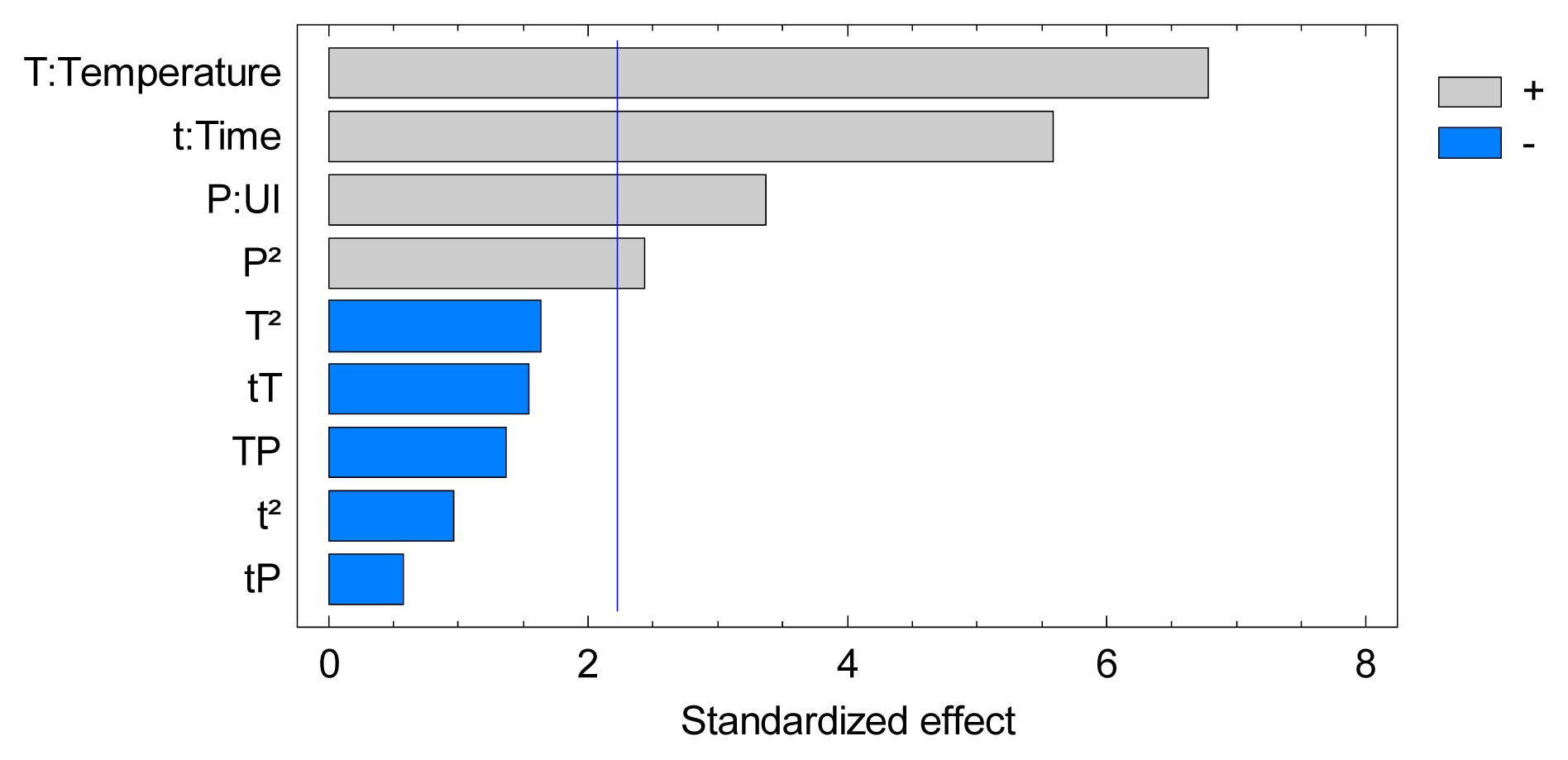
2.2. Experimental Design Studies
2.2.1. Results for Soluble Material Extraction
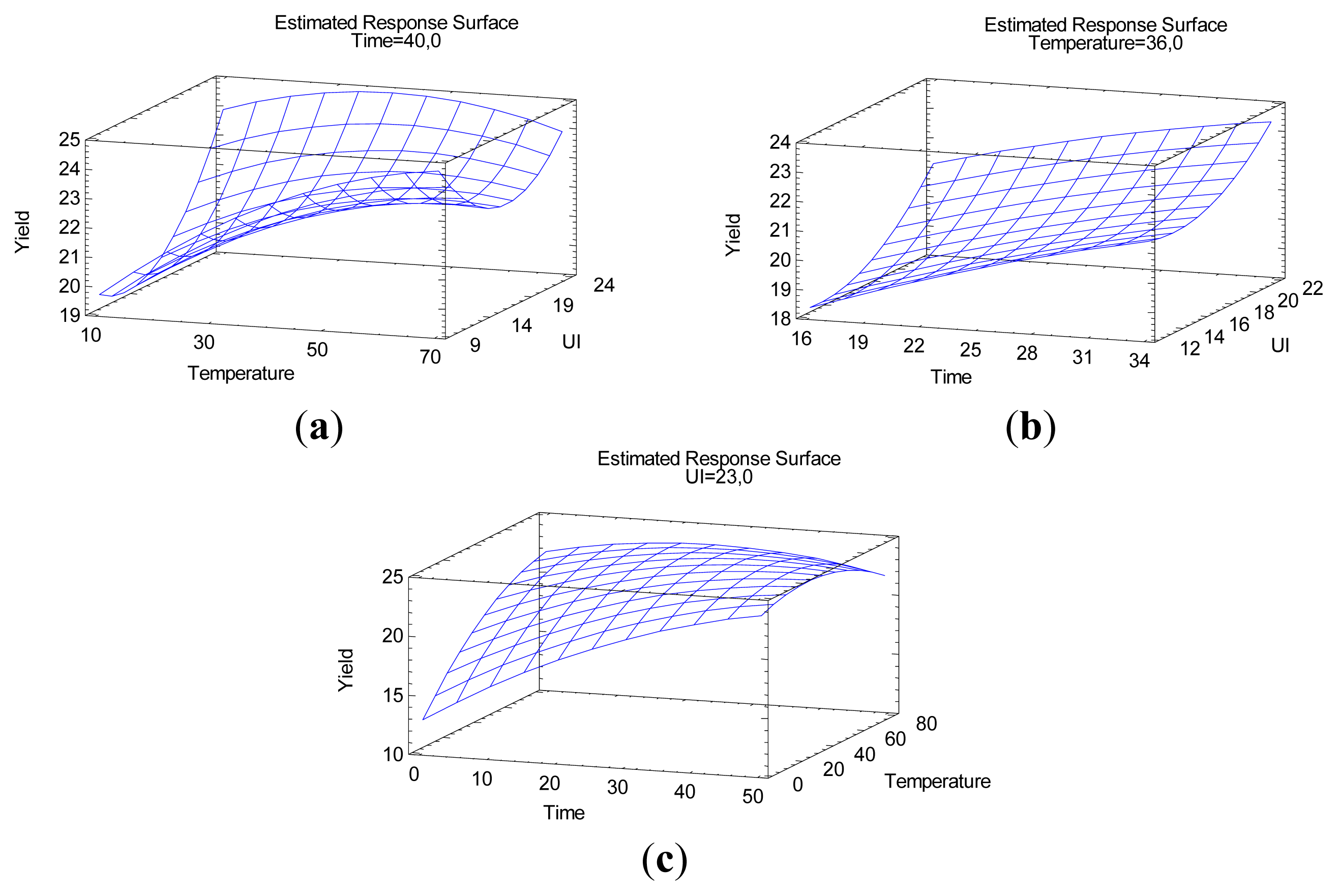
2.2.2. Optimization for Yield of Extraction
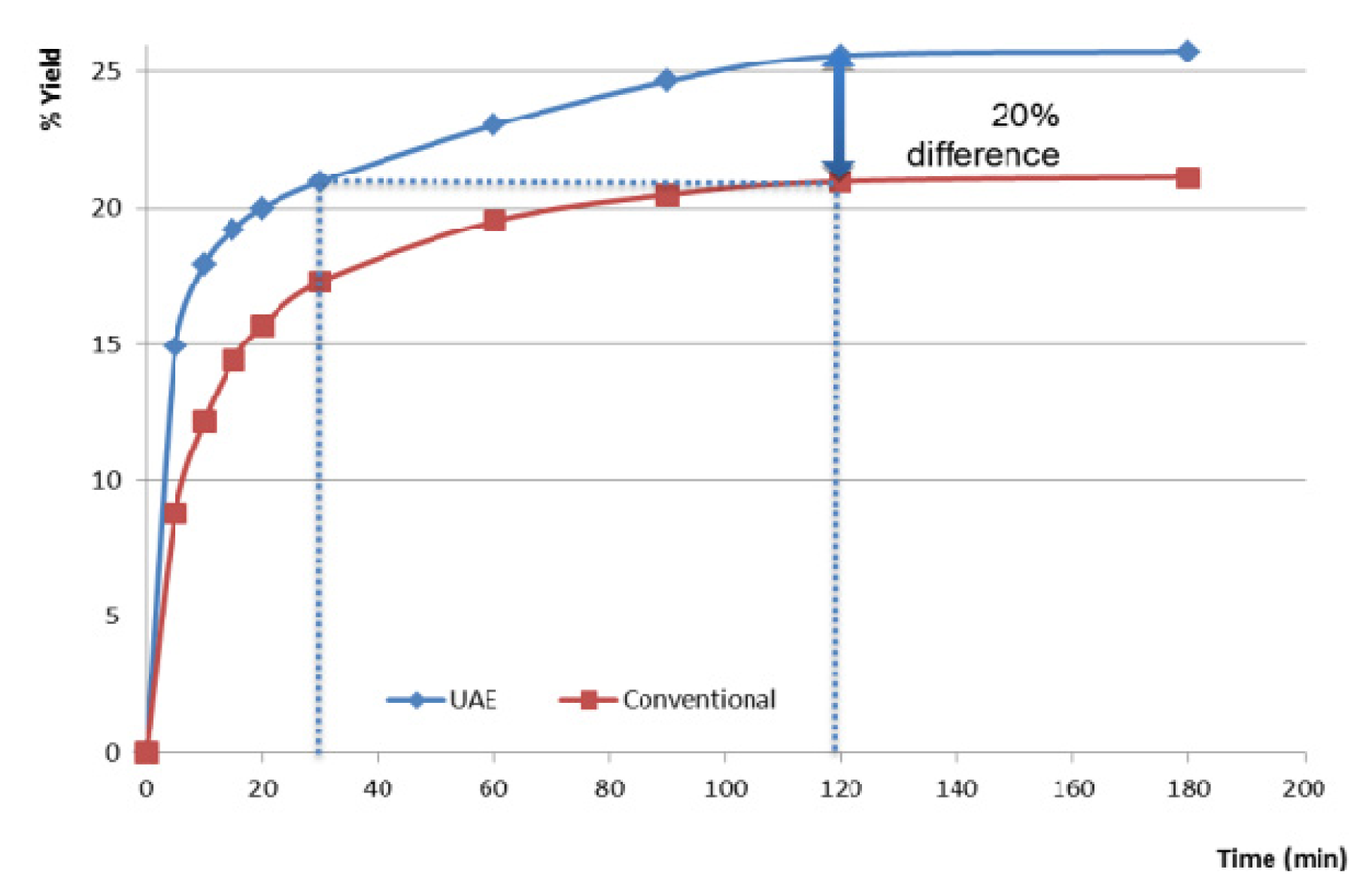
2.3. Comparison Study between Ultrasound Assisted Extraction and Conventional Maceration
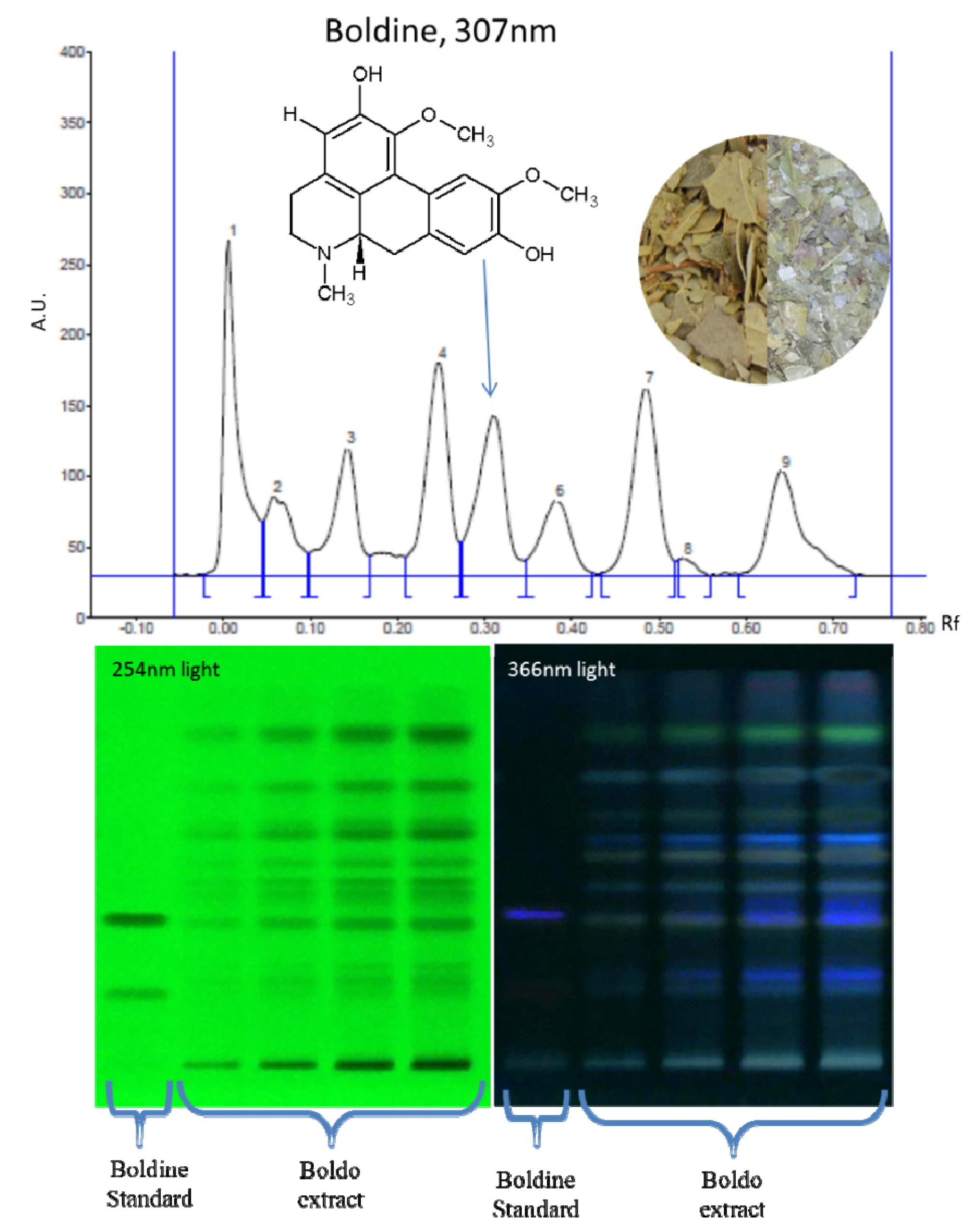
2.4. Ultrasounds Effects on Extracted Molecules
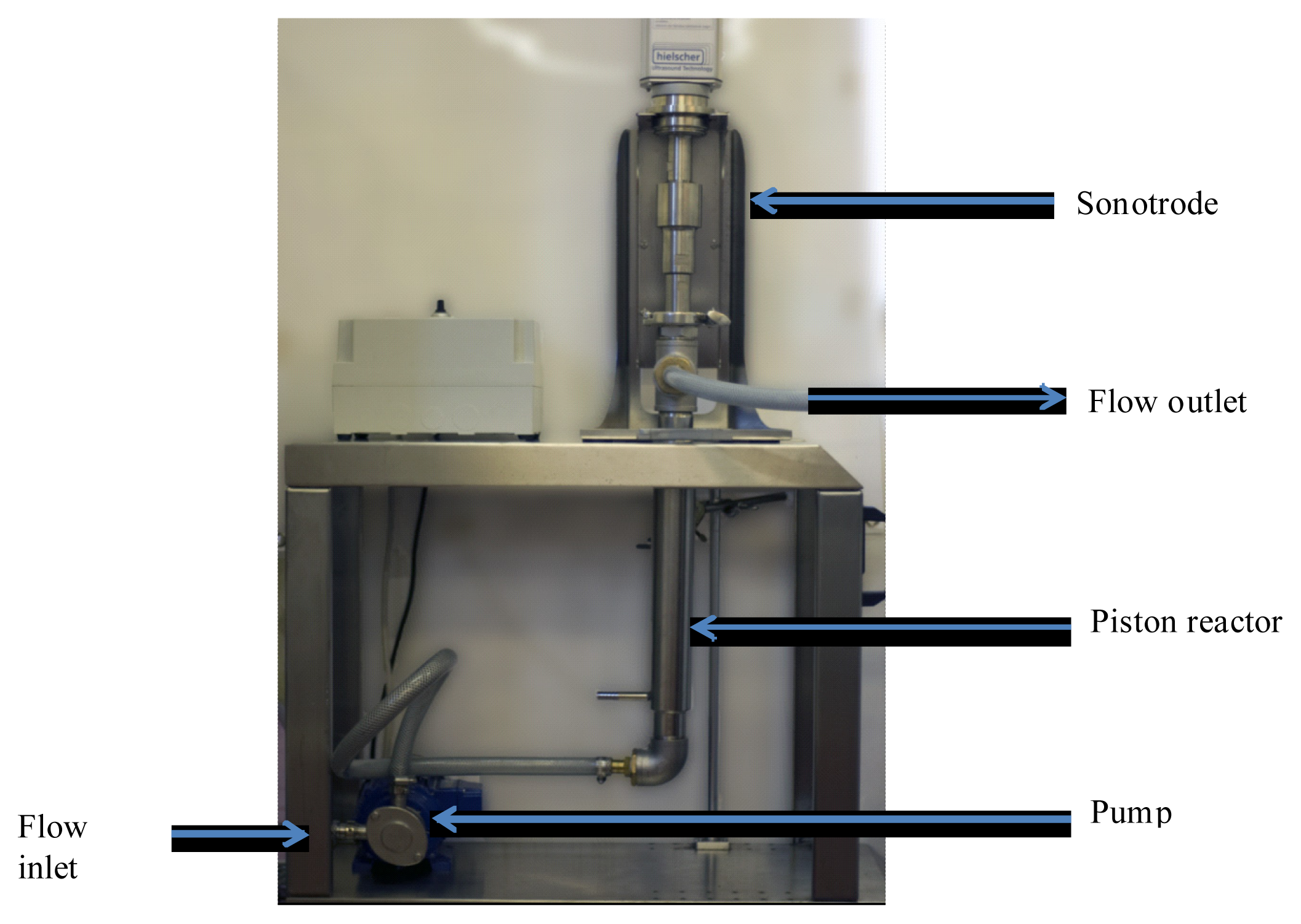
2.5. From Batch to Continuous System of Extraction
3. Experimental Section
3.1. Plant Material and Chemicals
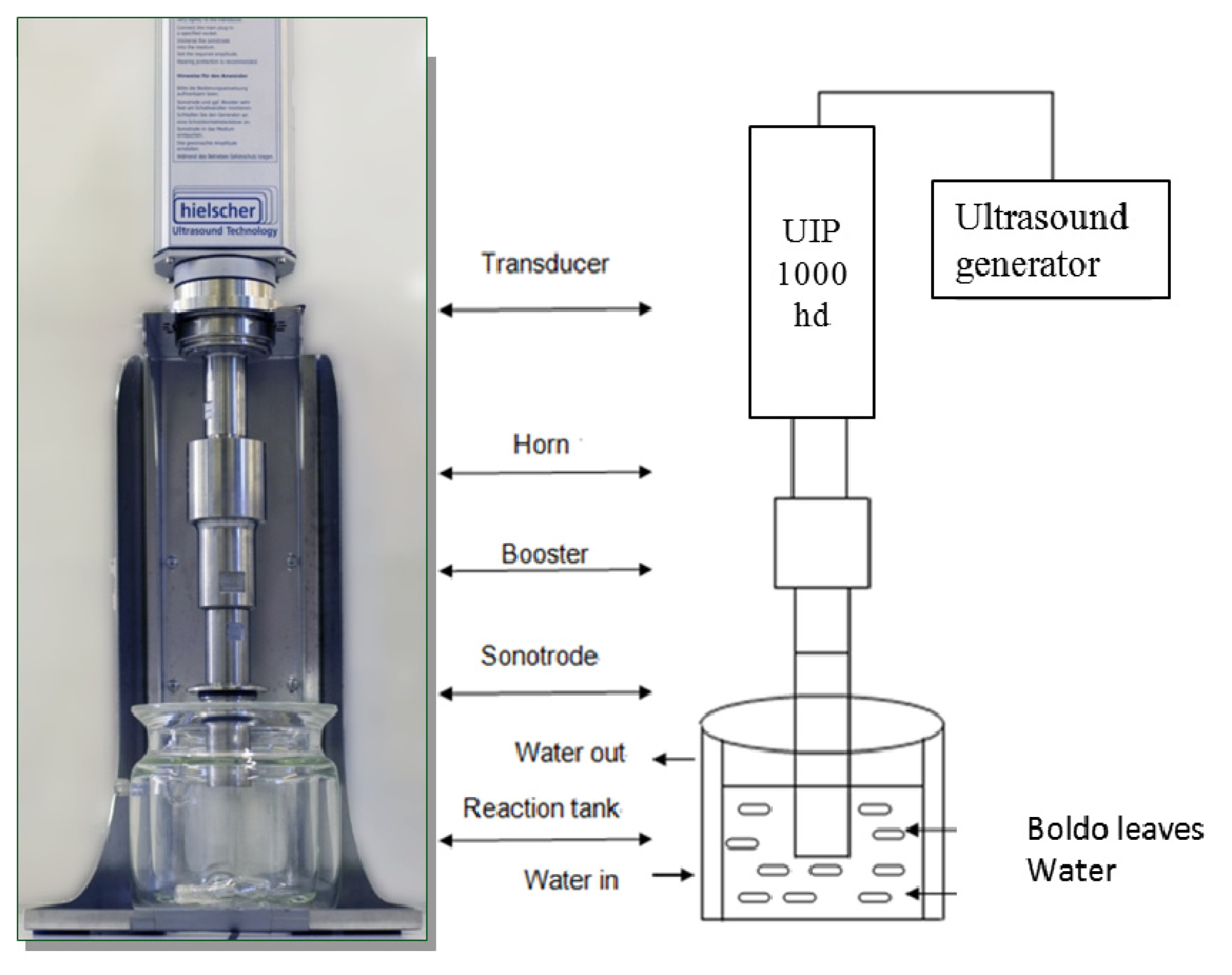
3.2. Extraction Procedures
3.3. Yield of Extraction Determination
3.4. Isolated Compound Study
3.5. Boldine Analysis
3.5.1. Hydrolysis
3.5.2. HPTLC analysis
3.6. Experimental Design
3.7. Scanning Electron Microscopy
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Vogel, H.; González, B.; Razmilic, I. Boldo (Peumus boldus) cultivated under different light conditions, soil humidity and plantation density. Ind. Crops Prod 2011, 34, 1310–1312. [Google Scholar]
- Speisky, H.; Cassels, B.K. Boldo and boldine: An emerging case of natural drug development. Pharmacological research. Pharmacol. Soc 1994, 29, 1–12. [Google Scholar]
- Del Valle, J.M.; Rogalinski, T.; Zetzl, C.; Brunner, G. Extraction of boldo (Peumus boldus M.) leaves with supercritical CO2 and hot pressurized water. Food Res. Int 2005, 38, 203–213. [Google Scholar]
- Schmeda-Hirschmann, G.; Rodriguez, J.A.; Theoduloz, C.; Astudillo, S.L.; Feresin, G.E.; Tapia, A. Free-radical scavengers and antioxidants from Peumus boldus Mol. (“Boldo”). Free Rad. Res 2003, 37, 447–452. [Google Scholar]
- Quezada, N.; Asencio, M.; del Valle, J.M.; Aguilera, J.M.; Gómez, B. Antioxidant activity of crude extract, alkaloid fraction, and flavonoid fraction from Boldo (Peumus boldus Molina) leaves. J. Food Sci 2004, 69, C371–C376. [Google Scholar]
- Cederbaum, A.I.; Ukielka, E.K.; Speiskyf, H. Inhibition of rat liver microsomal lipid peroxidation by boldine. Biochem. Pharmacol 1992, 44, 1765–1772. [Google Scholar]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci 2012, 13, 8615–8627. [Google Scholar]
- Caldeira, I.; Pereira, R.; Clímaco, M.C.; Belchior, A.; Bruno de Sousa, R. Improved method for extraction of aroma compounds in aged brandies and aqueous alcoholic wood extracts using ultrasound. Anal. Chim. Acta 2004, 513, 125–134. [Google Scholar]
- Xia, T.; Shi, S.; Wan, X. Impact of ultrasonic-assisted extraction on the chemical and sensory quality of tea infusion. J. Food Eng 2006, 74, 557–560. [Google Scholar]
- Ma, Y.-Q.; Chen, J.-C.; Liu, D.-H.; Ye, X.-Q. Simultaneous extraction of phenolic compounds of citrus peel extracts: Effect of ultrasound. Ultrason. Sonochem 2009, 16, 57–62. [Google Scholar]
- Virot, M.; Tomao, V.; Le Bourvellec, C.; Renard, C.M.C.G.; Chemat, F. Towards the industrial production of antioxidants from food processing by-products with ultrasound-assisted extraction. Ultrason. Sonochem 2010, 17, 1066–1074. [Google Scholar]
- Chen, F.; Sun, Y.; Zhao, G.; Liao, X.; Hu, X.; Wu, J.; Wang, Z. Optimization of ultrasound-assisted extraction of anthocyanins in red raspberries and identification of anthocyanins in extract using high-performance liquid chromatography-mass spectrometry. Ultrason. Sonochem 2007, 14, 767–778. [Google Scholar]
- Barbero, G.F.; Liazid, A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of capsaicinoids from peppers. Talanta 2008, 75, 1332–1337. [Google Scholar]
- Khan, M.K.; Abert-Vian, M.; Fabiano-Tixier, A.-S.; Dangles, O.; Chemat, F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem 2010, 119, 851–858. [Google Scholar]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem 2001, 8, 303–313. [Google Scholar]
- Legallais. Available online: http://www.legallais-labo.fr/ accessed on 6 March 2013.
- Hielsher. Available online: http://www.hielscher.com/ accessed on 6 March 2013.

| Run | Sonication time (min) | Temperature (°C) | UI * (W/cm2) | Yield (%) |
|---|---|---|---|---|
| 1 | 25.0 | 40.0 | 16.5 | 20.6 |
| 2 | 25.0 | 40.0 | 16.5 | 20.6 |
| 3 | 25.0 | 40.0 | 16.5 | 21.2 |
| 4 | 25.0 | 40.0 | 16.5 | 20.4 |
| 5 | 25.0 | 40.0 | 16.5 | 20.7 |
| 6 | 25.0 | 40.0 | 16.5 | 20.3 |
| 7 | 16.0 | 22.0 | 12.6 | 16.5 |
| 8 | 16.0 | 58.0 | 12.6 | 21.7 |
| 9 | 34.0 | 22.0 | 12.6 | 20.7 |
| 10 | 34.0 | 58.0 | 12.6 | 23.0 |
| 11 | 16.0 | 22.0 | 20.4 | 19.5 |
| 12 | 16.0 | 58.0 | 20.4 | 22.0 |
| 13 | 34.0 | 22.0 | 20.4 | 21.9 |
| 14 | 34.0 | 58.0 | 20.4 | 23.8 |
| 15 | 25.0 | 9.7 | 16.5 | 16.5 |
| 16 | 25.0 | 70.3 | 16.5 | 21.4 |
| 17 | 9.9 | 40.0 | 16.5 | 17.3 |
| 18 | 40.1 | 40.0 | 16.5 | 21.4 |
| 19 | 25.0 | 40.0 | 9.9 | 20.0 |
| 20 | 25.0 | 40.0 | 23.1 | 22.8 |
| Source | Sum of squares | Df | Mean square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| A:Time | 20.166 | 1 | 20.166 | 31.25 | 0.0002 |
| B:Temperature | 29.7028 | 1 | 29.7028 | 46.03 | 0 |
| C:UI * | 7.34062 | 1 | 7.34062 | 11.37 | 0.0071 |
| AA | 0.597778 | 1 | 0.597778 | 0.93 | 0.3585 |
| AB | 1.53125 | 1 | 1.53125 | 2.37 | 0.1545 |
| AC | 0.21125 | 1 | 0.21125 | 0.33 | 0.5799 |
| BB | 1.72989 | 1 | 1.72989 | 2.68 | 0.1326 |
| BC | 1.20125 | 1 | 1.20125 | 1.86 | 0.2024 |
| CC | 3.82983 | 1 | 3.82983 | 5.93 | 0.0351 |
| Total error | 6.45333 | 10 | 0.645333 | ||
| Total (corr.) | 73.4655 | 19 |
| Method of extraction | Time of extraction (min) | Yield of extraction (% of leaves boldo solubilized in the extract) | Boldine (μg of boldine/g of boldo leaves) |
|---|---|---|---|
| UAE | 30 | 21.8 | 100 |
| Conventional | 30 | 18.0 | 51.7 |
| UAE | 120 | 26.7 | 148 |
| Conventional | 120 | 21.5 | 99.5 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Petigny, L.; Périno-Issartier, S.; Wajsman, J.; Chemat, F. Batch and Continuous Ultrasound Assisted Extraction of Boldo Leaves (Peumus boldus Mol.). Int. J. Mol. Sci. 2013, 14, 5750-5764. https://doi.org/10.3390/ijms14035750
Petigny L, Périno-Issartier S, Wajsman J, Chemat F. Batch and Continuous Ultrasound Assisted Extraction of Boldo Leaves (Peumus boldus Mol.). International Journal of Molecular Sciences. 2013; 14(3):5750-5764. https://doi.org/10.3390/ijms14035750
Chicago/Turabian StylePetigny, Loïc, Sandrine Périno-Issartier, Joël Wajsman, and Farid Chemat. 2013. "Batch and Continuous Ultrasound Assisted Extraction of Boldo Leaves (Peumus boldus Mol.)" International Journal of Molecular Sciences 14, no. 3: 5750-5764. https://doi.org/10.3390/ijms14035750






