Cantharidin Impedes Activity of Glutathione S-Transferase in the Midgut of Helicoverpa armigera Hübner
Abstract
:1. Introduction
2. Results and Discussion
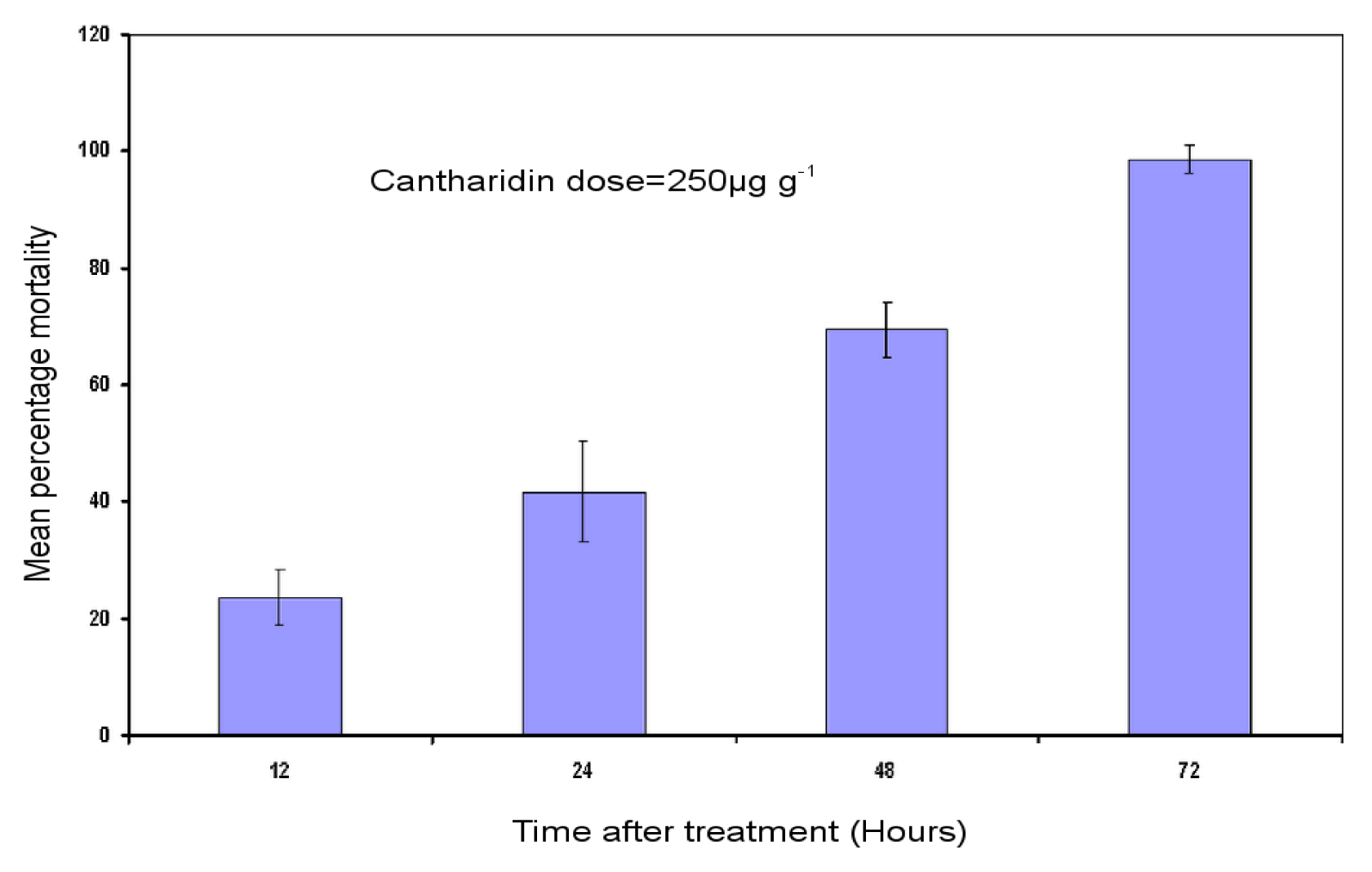
2.1. Bioassay
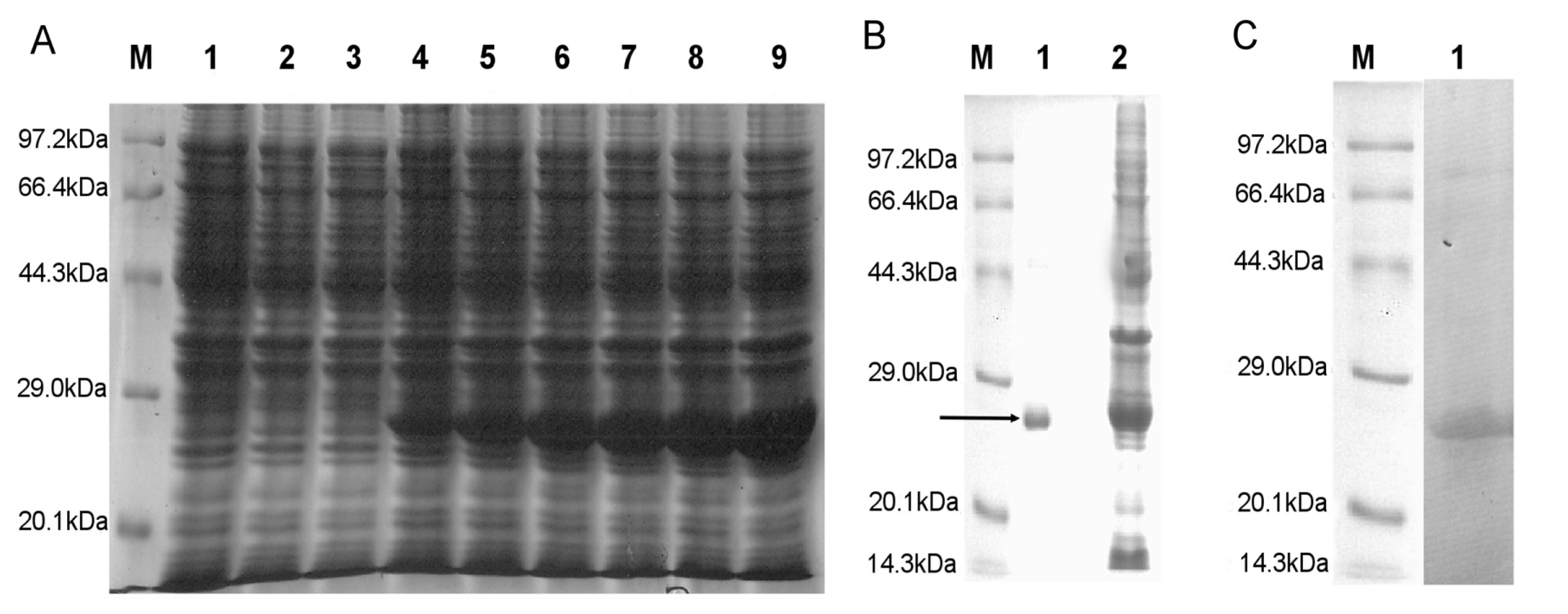
2.2. SDS-PAGE Analysis
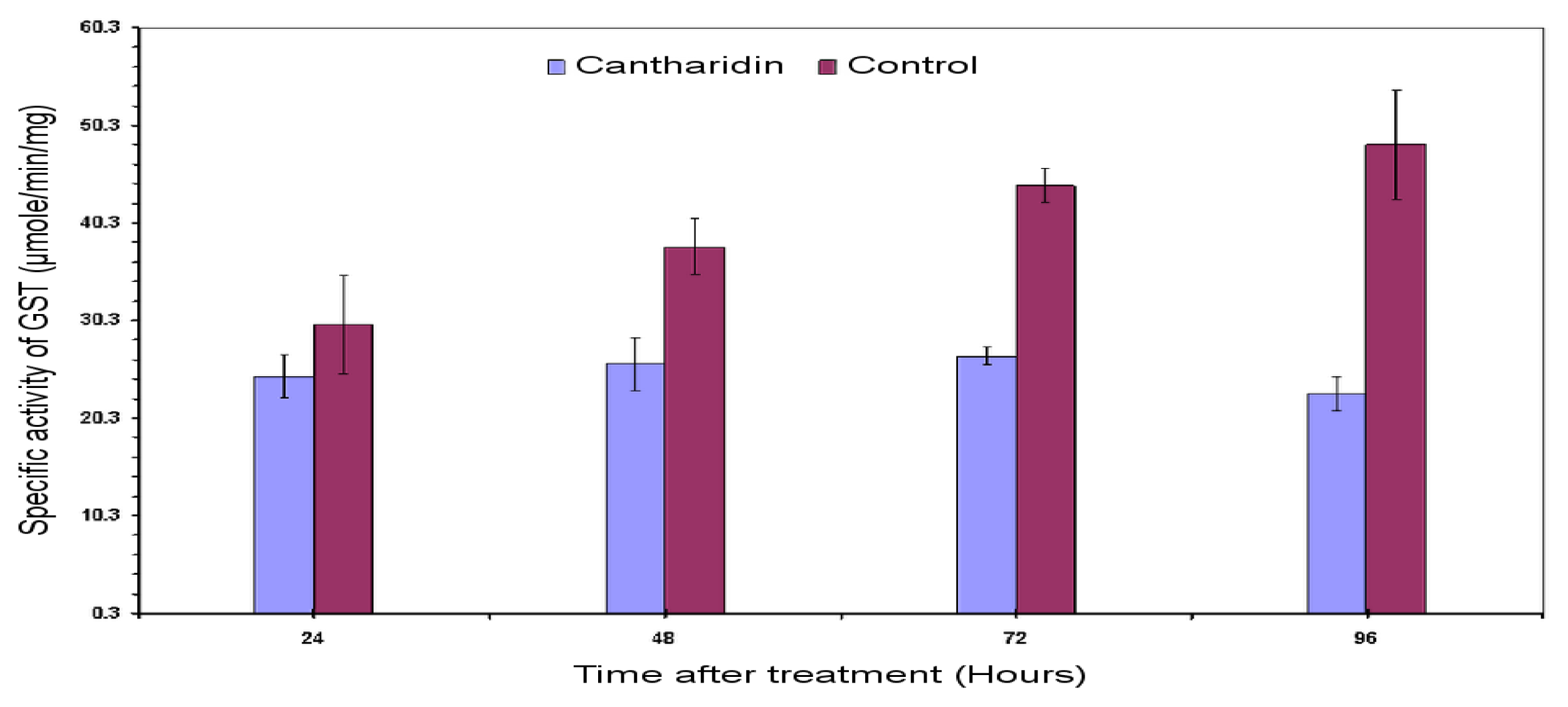
2.3. Specific Activity of HaGST
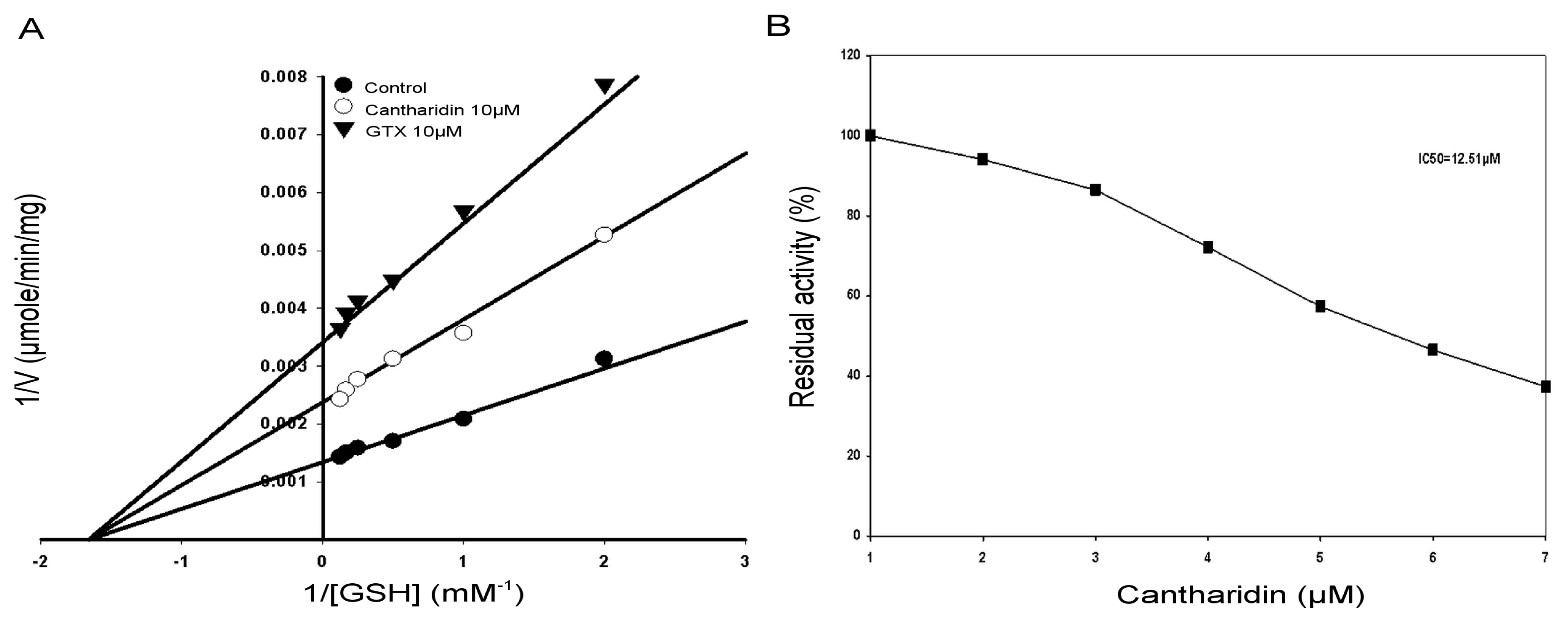
2.4. Kinetic Properties of GSTs
2.5. Kinetic Properties of Purified rHaGST
2.6. IC50 of Cantharidin
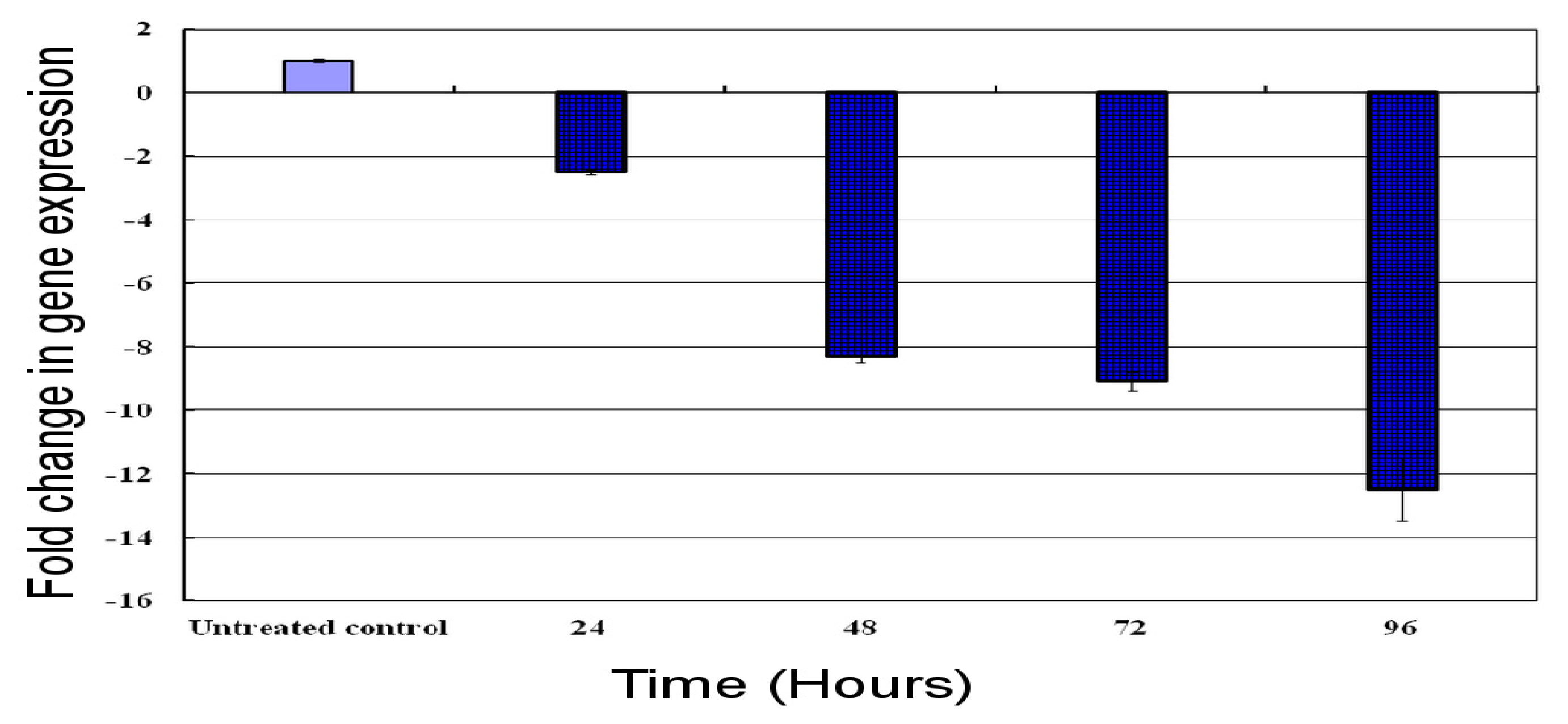
2.7. Time Course Expression Profile of the HaGST Gene by Real-Time qPCR
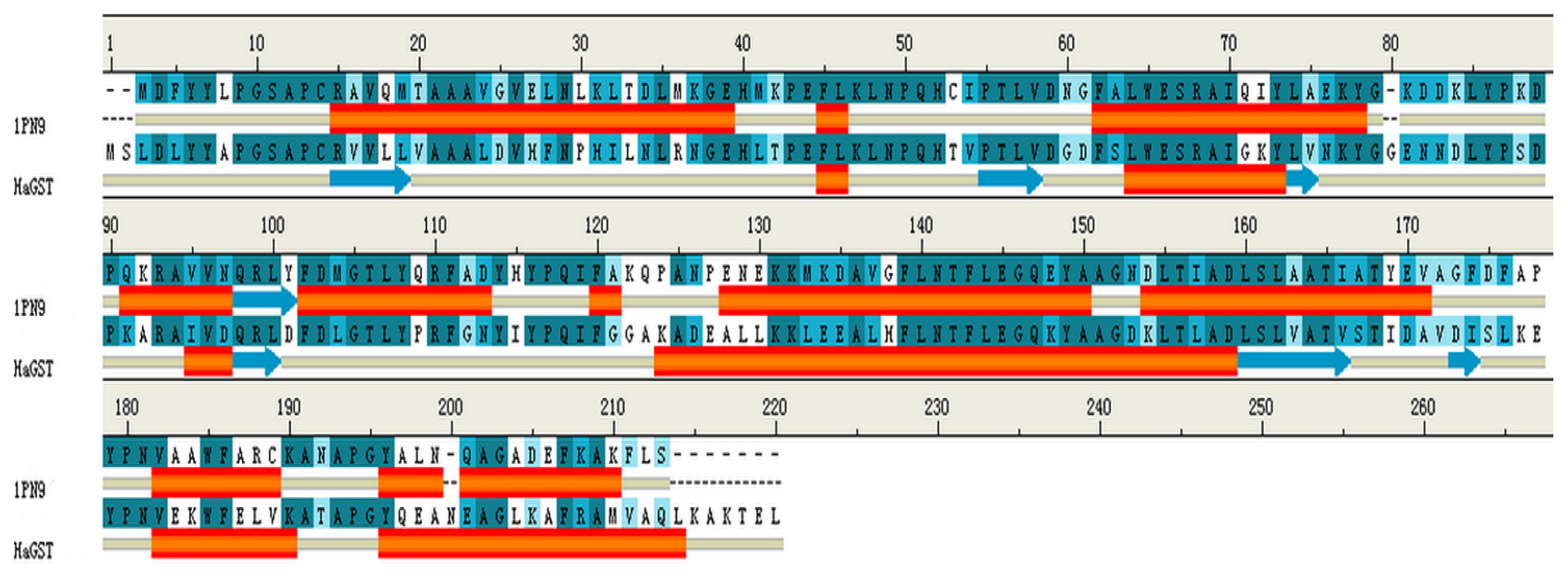
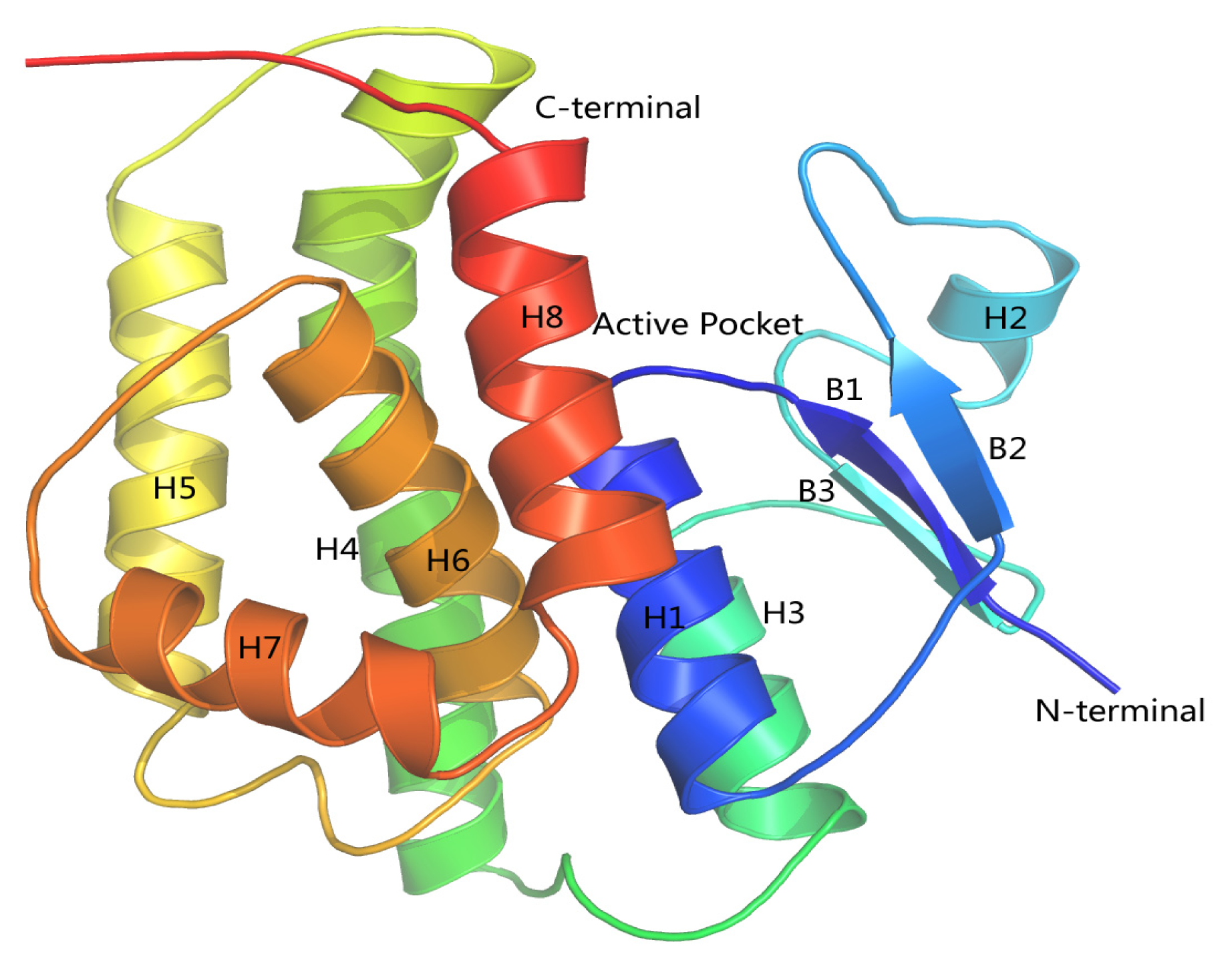
2.8. Homology Modeling of HaGST
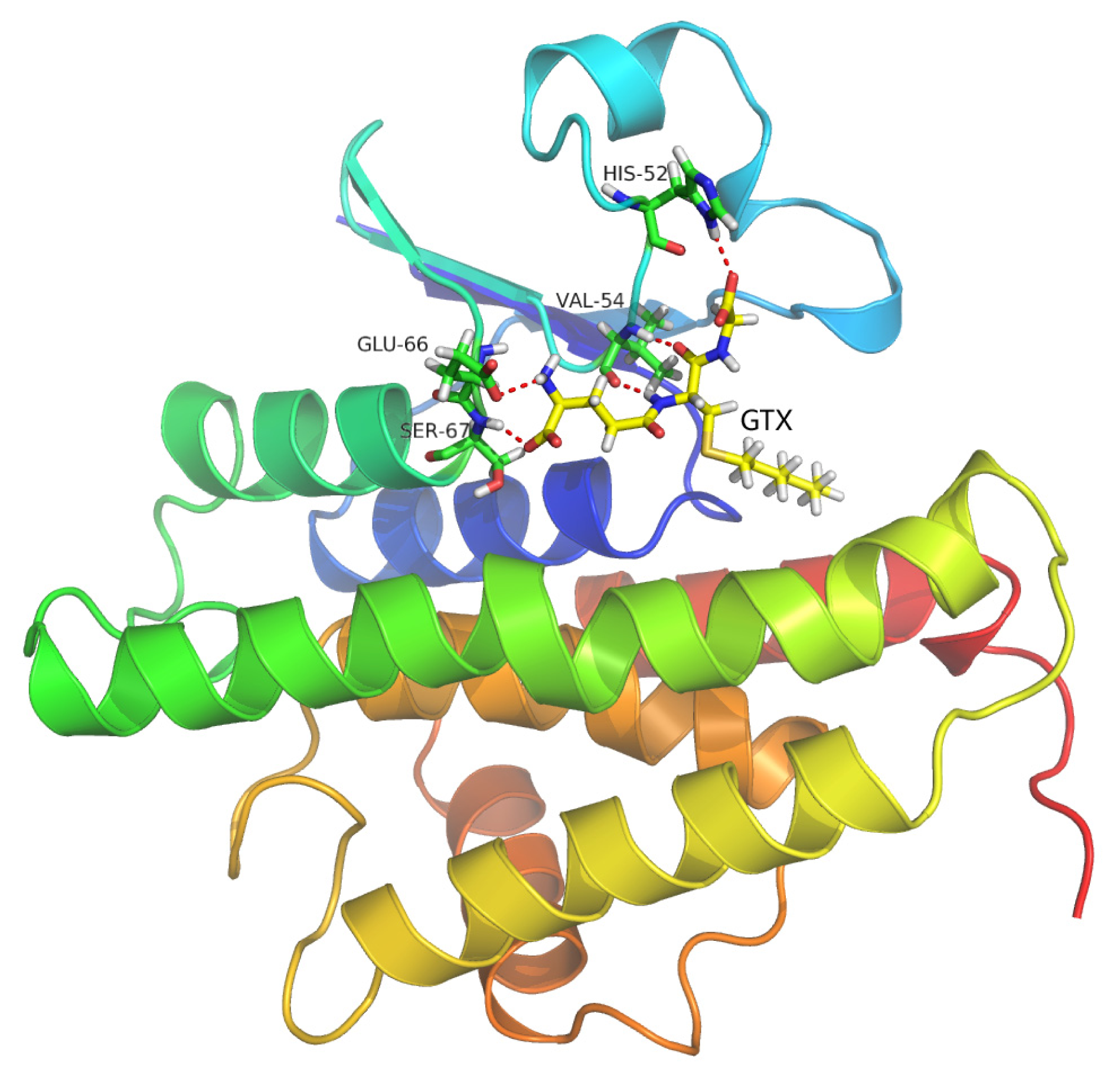
2.9. Molecular Docking Simulations
2.10. Binding Energy Calculations
3. Experimental Section
3.1. Insects
3.2. Bioassay
3.3. Larval Treatment
3.4. Total RNA Preparation and Synthesis of cDNAs Template for Real-Time qPCR
3.5. Cloning of Glutathione S-Transferase Gene from H. armigera
3.6. Construction of Recombinant Expression Plasmid
3.7. SDS-PAGE Analysis of Recombinant Protein and Immunoblotting
3.8. Prokaryotic Expression and Purification of the Soluble Recombinant Protein, rHaGST
3.9. Enzyme Extract Preparation
3.10. GST Activity Determination Assay
3.11. Kinetic Properties of GST
3.12. Kinetic Properties of Purified Soluble rHaGST
3.13. Determination of IC50
3.14. Real-Time qPCR Analysis of Gene Expression
3.15. Homology Modeling of HaGST
3.16. Molecular Docking Simulations
3.17. Binding Energy Calculations
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Hayes, J.D.; Pulford, D.J. The glutathione S-transferase supergene family-regulation of GST and the contribution of isozymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol 1995, 30, 445–600. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. J. Biol. Chem 1974, 249, 7130–7141. [Google Scholar]
- Grant, D.F.; Dietze, E.C.; Hammock, B.D. Glutathione S-transferase isozymes in Aedes aegypti: Purification, characterization and isozyme specific regulation. Insect Biochem 1991, 21, 421–433. [Google Scholar]
- Huang, H.S.; Hu, N.T.; Yao, Y.E.; Wu, C.Y.; Chiang, S.W.; Sun, C.N. Molecular cloning and heterologous expression of glutathione S-transferase involved in insecticide resistance from the diamondback moth, Plutella xylostella. Insect Biochem. Mol. Biol 1998, 28, 651–658. [Google Scholar]
- Armes, N.J.; Jadhav, D.R.; Bond, G.S.; King, A.B.S. Insecticide resistance in Helicoverpa armigera in South India. Pestic. Sci 1992, 34, 355–364. [Google Scholar]
- Yu, S.J. Molecular Aspects of Insect—Plant Association; Brattsten, L.B., Ahmad, S., Eds.; Plenum: New York, NY, USA, 1986; p. 153. [Google Scholar]
- Motoyama, N.; Dauterman, W.C. Glutathione S-transferases: Their role in the metabolism of organophosphorus insecticides. Rev. Biochem. Toxicol 1980, 20, 49–70. [Google Scholar]
- Motoyama, N.; Dauterman, W.C. Inter-strain comparison of glutathione dependent reactions in susceptible and resistant houseflies. Pestic. Biochem. Physiol 1975, 5, 489–493. [Google Scholar]
- Clark, A.G.; Shamaan, N.A.; Dauterman, W.C.; Hayaoka, T. Characterization of multiple glutathione transferases from the housefly, Musca domestica. Pestic. Biochem. Physiol 1984, 22, 51–59. [Google Scholar]
- Hemingway, J.; Malcolm, C.A.; Kissoon, K.E.; Boddington, R.G.; Curtis, C.F.; Hill, N. The biochemistry of insecticide resistance in Anopheles sacharovi: Comparative studies with a range of insecticide susceptible and resistance Anopheles and Culex species. Pestic. Biochem. Physiol 1985, 24, 68–71. [Google Scholar]
- Grant, D.F.; Matsumura, F. Glutathione S-transferase 1 and 2 in susceptible and insecticide resistant Aedes aegypti. Pestic. Biochem. Physiol 1989, 33, 132–140. [Google Scholar]
- Fournier, D.; Bride, J.M.; Poire, M.; Berge, J.B.; Plapp, F.W. Insect glutathione S-transferases: Biochemical characteristics of the major forms from houseflies susceptible and resistant to insecticides. J. Biol. Chem 1992, 267, 1840–1845. [Google Scholar]
- Legadic, L.; Cuany, A.; Berge, J.B.; Echaubard, M. Purification and partial characterization of glutathione S-transferases from insecticide resistant and lindane-induced susceptible Spodoptera littoralis (Boisd.) larvae. Insect Biochem. Mol. Biol 1993, 23, 467–474. [Google Scholar]
- Zibaee, A.; Jalali, S.J.; Alinia, F.; Ghadamyari, M. Diazinon resistance in different selected strains of Chilo suppressalis Walker (Lepidoptera: Pyralidae), rice striped stem borer, in the north of Iran. J. Econ. Entomol 2008, 102, 1189–1196. [Google Scholar]
- Wang, G.S. Medical uses of Mylabris in ancient China and recent studies. J. Ethnoparmacol 1989, 26, 147–162. [Google Scholar]
- Oaks, W.W.; diTunno, J.F.; Magnam, T.; Levy, H.A.; Mills, L.C. Cantharidin Poisoning. Arch. Intern. Med 1960, 105, 574–582. [Google Scholar]
- Robiquet, P.J. Expériences sur les cantharides. Ann. Chim 1810, 76, 302–322. [Google Scholar]
- Cui, F.L.; Li, X.; Ma, Z.Q.; Zhang, Y.L. Safety evaluation of animal-origin pesticide cantharidin against some non-target organisms. J. Environ. Entomol 2009, 31, 143–149. [Google Scholar]
- Zhang, Y.L.; Zhou, Y.; Zhang, Z.Y. Effect of cantharidin on the midgut of oriental armyworm (Methimna. seperata) and diamond moth Plutella xylostella. Acta Entomol. Sin 2003, 46, 272–276. [Google Scholar]
- Ma, Y.; Liu, R.R.; Ma, Z.Q.; Zhang, Y.L. Effects of cantharidin on four metabolizing enzymes and PPO in Mythimna separata (Walker) (Lepidoptera Noctuidae). Acta Entomol. Sin 2010, 53, 870–875. [Google Scholar]
- Mao, YB.; Cai, W.J.; Wang, J.W.; Hong, G.J.; Tao, X.Y.; Wang, L.J.; Huang, Y.P.; Chen, X.Y. Silencing a cotton bollworm P450 monooxygenase gene by plant mediated RNAi impairs larval tolerance to gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar]
- Chen, L.; Hall, P.R.; Zhou, X.E.; Ranson, H.; Hemingway, J.; Meehan, E.J. Structure of an insect delta-class glutathione S-transferase from a DDT-resistant strain of the malaria vector Anopheles gambiae. Acta Crystallogr. D 2003, D59, 2211–2217. [Google Scholar]
- Furlong, M.J.; Wright, D.J. Examination of stability of resistance and cross resistance patterns to acylurea insect growth regulators in field populations of diamond back moth, Plutella xylostella from Malaysia. J. Pestic. Sci 1994, 42, 315–326. [Google Scholar]
- Mohan, M.; Gujar, G.T. Local variation in susceptibility of diamondback moth, Plutella xylostella (L.) to insecticides and role of detoxifying enzymes. Crop Prot 2003, 2, 495–504. [Google Scholar]
- Balabaskaran, S.; Chuen, S.S.; Muniandy, S. Glutathione S-transferase from the diamondback moth. Insect Biochem 1989, 19, 435–443. [Google Scholar]
- Kao, C.H.; Hung, C.F.; Sun, C.N. Parathion and methyl parathion resistance in diamond back moth (Lepidoptera: Plutellidae) larvae. J. Econ. Entomol 1989, 82, 1299–1300. [Google Scholar]
- Yu, S.J.; Nguyen, S.N. Detection and biochemical characterization of insecticide resistance in the diamond back moth. Pestic. Biochem. Physiol 1992, 44, 74–81. [Google Scholar]
- Wu, Y.; Shen, D.J. Character of Fenvalerate resistance in Helicoverpa arraigera (Hubner) from China. Resistant Pest Manag. Newsl 1996, 8, 25–27. [Google Scholar]
- Kostaropoulos, I.; Papadopoulos, A.I.; Metaxakis, A.; Boukouvala, E.; Papadopoulou-Maurkidou, E. Glutathione S-transferase in the defense against pyrethroids in insects. Insect Biochem. Mol. Biol 2001, 31, 313–319. [Google Scholar]
- Soderlund, D.M.; Hessney, C.W.; Helmuth, D.W. Pharmacokinetics of cis- and trans-substituted pyrethroids in the American cockroach. Pestic. Biochem. Physiol 1983, 20, 161–168. [Google Scholar]
- Hayes, J.D.; Wolf, C.R. Role of Glutathione Transferases in Drug Resistance. In Glutathione Conjugation: Mechanisms and Biological Significance; Sites, H., Ketter, B., Eds.; Academic Press: London, UK, 1988; pp. 3150–355. [Google Scholar]
- Harold, J.A.; Ottea, J.A. Toxicological significance of enzyme activities in profenofos-resistant tobacco budworms, Heliothis virescens (F.). Pestic. Biochem. Physiol 1997, 58, 23–33. [Google Scholar]
- Vontas, J.G.; Small, G.J.; Hemingway, J. Glutathione S-transferases as antioxidant defense agents confer pyrethroid resistance in Nilaparvata lugens. Biochem. J 2001, 357, 65–72. [Google Scholar]
- Ahmed, M.; McCaffery, A.R. Elucidation of detoxification mechanisms involved in resistance to insecticides against third instar larvae of a field selected strain of Helicoverpa armigera with the use of synergists. Pestic. Biochem. Physiol 1991, 41, 41–52. [Google Scholar]
- Sambrook, J.; Russel, D.W. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989; pp. 18:1–18:85. [Google Scholar]
- Booth, G.M.; Connor, J.; Metcalf, R.A.; Larsen, J.R. A comparative study of the effects of selective inhibitors on esterase isozymes from the mosquito Anopheles punctipennis. Comp. Biochem. Physiol. B 1973, 44, 1185–1195. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001, 29, 2002–2007. [Google Scholar]
- Sali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol 1993, 234, 779–815. [Google Scholar]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The ClustalX windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 1997, 24, 4876–4882. [Google Scholar]
- Chen, V.B.; Arendall, W.B., 3rd; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 2010, D66, 12–21. [Google Scholar]
- GOLD, version 5.1; The Cambridge Crystallographic Data Centre (CCDC): Cambridge, UK, 2012.
- DeLano, W.L. The PyMol Molecular Graphics System; DeLano Scientific: Palo Alto, CA, USA, 2010. [Google Scholar]
- Ivetic, T.V.; Hrvacic, B.; Bosnar, M.; Cuzic, S.; Bosnjak, B.; Erakovic, H.V.; Glojnaric, I. Cantharidin-induced inflammation in mouse rar model for translational research of novel anti-inflammatories. Tranl. Res 2012, 160, 137–145. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Van de streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Cryst 2008, 41, 466–470. [Google Scholar]

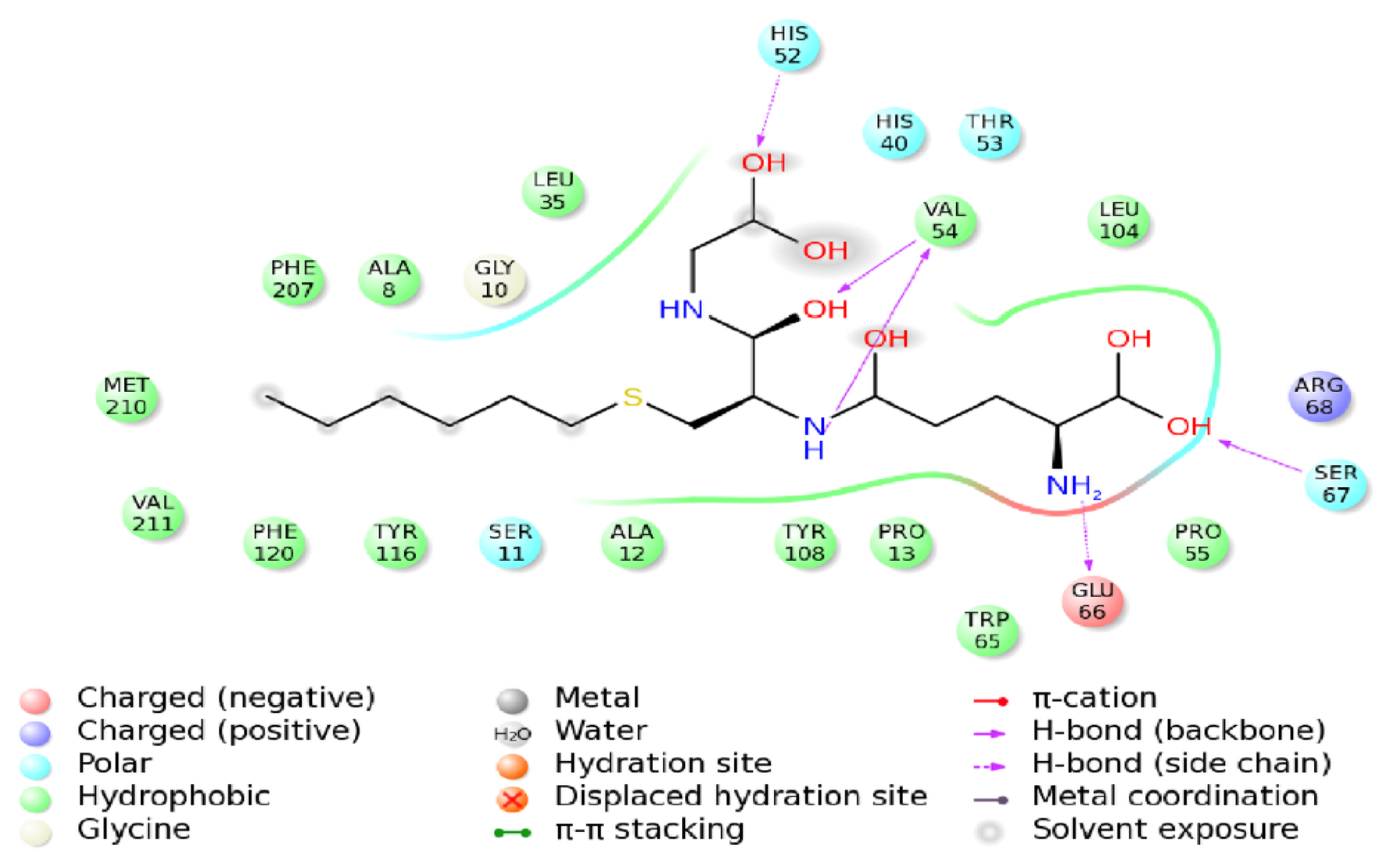
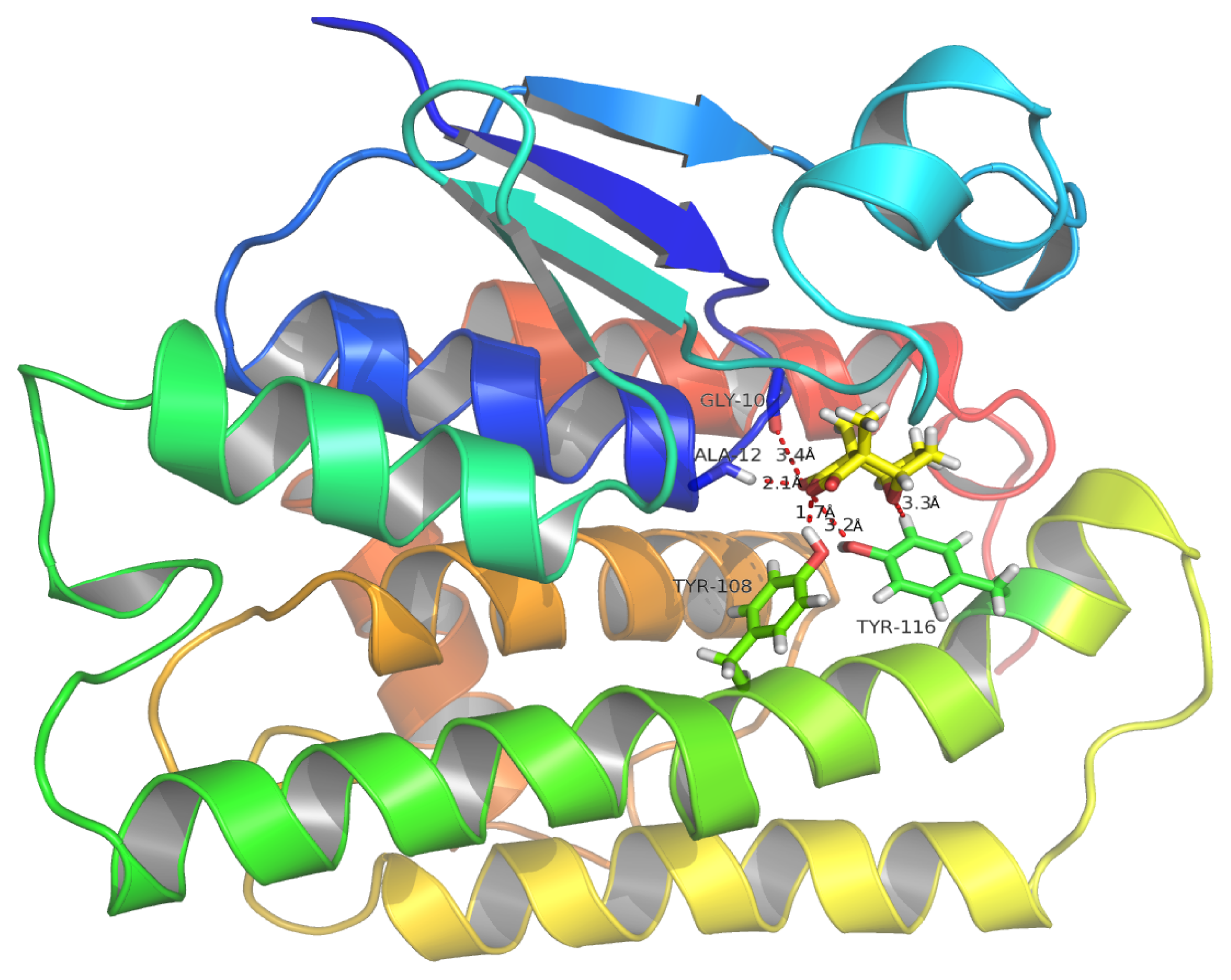
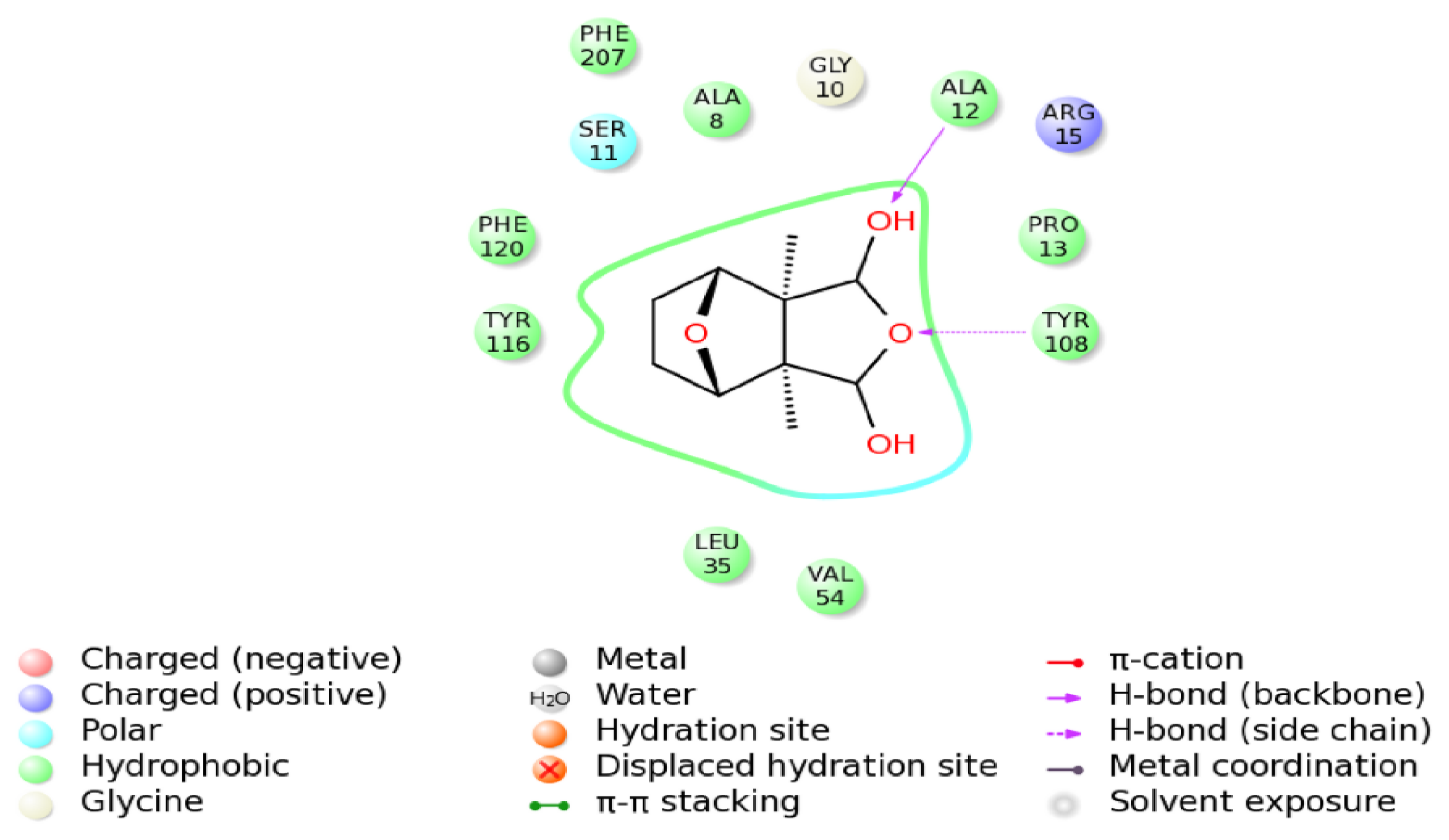
| Molecule | Score | DG | S (hbond) | S (lipo) | H (rot) | DE (clash) | DE (int) |
|---|---|---|---|---|---|---|---|
| GTX | 16.76 | −19.65 | 3.87 | 142.65 | 6.04 | 0.10 | 2.79 |
| Cantharidin | 21.58 | −21.86 | 1.80 | 88.77 | 0.00 | 0.28 | 0.00 |
| Name (bp) | Sequence (5-3) | Usage | Tma | Corresponding cDNA region |
|---|---|---|---|---|
| HaGST-F (29) | CGGATCCATGTCCTTAGACTTGTATTACG | RT | 59.2 | 43–705 |
| HaGST-R (30) | CGAAGCTTTTACAATTCAGTTTTAGCTTTT | RT | 56.6 | |
| HaGST-Fq (19) | ACGCTTTACCCAAGATTTG | Real-time qPCR | 60 | 316–430 |
| HaGST-Rq (19) | GGAATGTGTTGAGGAAGTG | Real-time qPCR | 60 | |
| HaBA-Fq (18) | GTATTGCTGACCGTATGC | Real-time qPCR | 59.7 | 11–s152 |
| HaBA-Rq (18) | ATCTGTTGGAAGGTGGAG | Real-time qPCR | 60 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Khan, R.A.; Liu, J.Y.; Rashid, M.; Wang, D.; Zhang, Y.L. Cantharidin Impedes Activity of Glutathione S-Transferase in the Midgut of Helicoverpa armigera Hübner. Int. J. Mol. Sci. 2013, 14, 5482-5500. https://doi.org/10.3390/ijms14035482
Khan RA, Liu JY, Rashid M, Wang D, Zhang YL. Cantharidin Impedes Activity of Glutathione S-Transferase in the Midgut of Helicoverpa armigera Hübner. International Journal of Molecular Sciences. 2013; 14(3):5482-5500. https://doi.org/10.3390/ijms14035482
Chicago/Turabian StyleKhan, Rashid Ahmed, Ji Yuan Liu, Maryam Rashid, Dun Wang, and Ya Lin Zhang. 2013. "Cantharidin Impedes Activity of Glutathione S-Transferase in the Midgut of Helicoverpa armigera Hübner" International Journal of Molecular Sciences 14, no. 3: 5482-5500. https://doi.org/10.3390/ijms14035482






