Preparation and Evaluation of Dental Resin with Antibacterial and Radio-Opaque Functions
Abstract
:1. Introduction
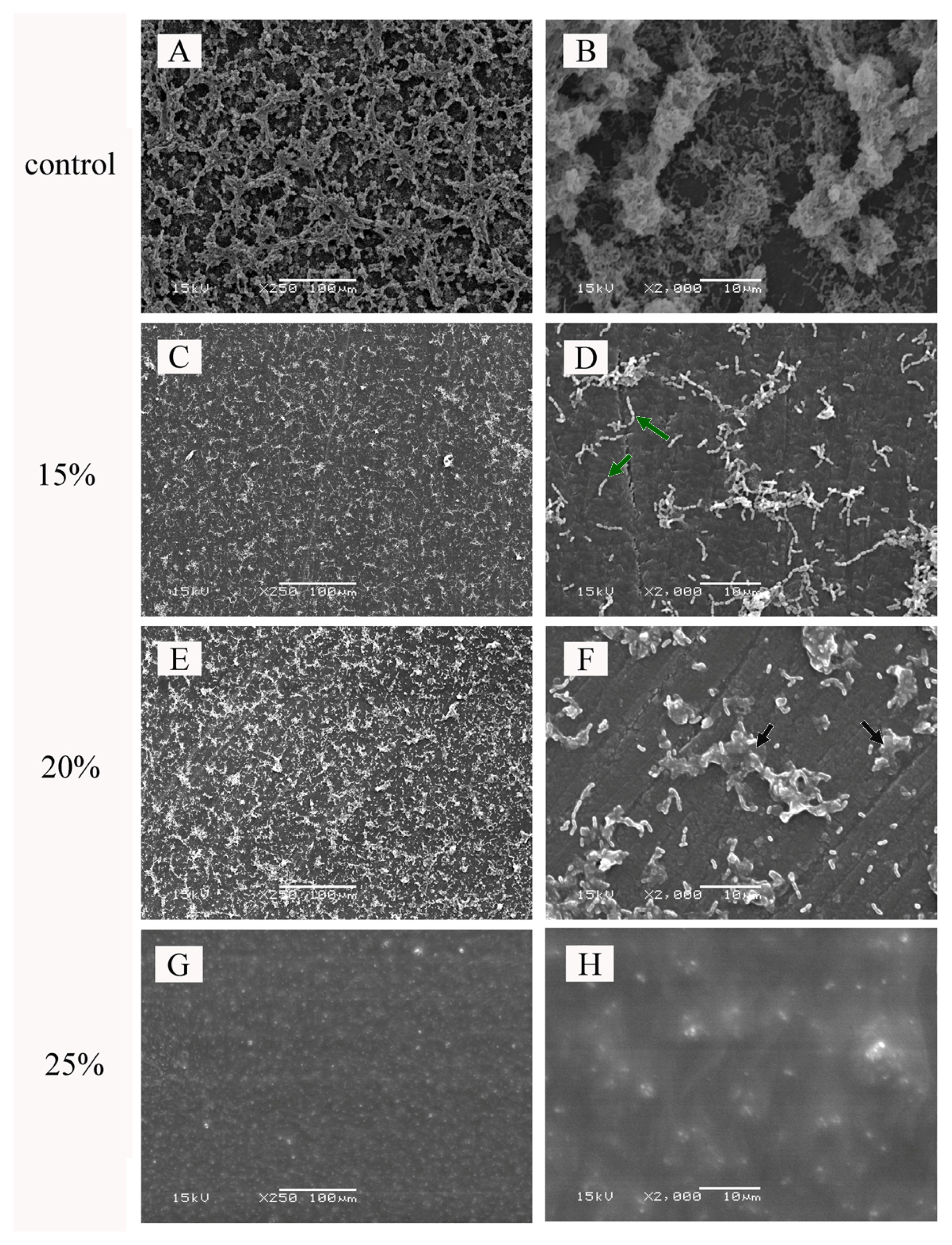
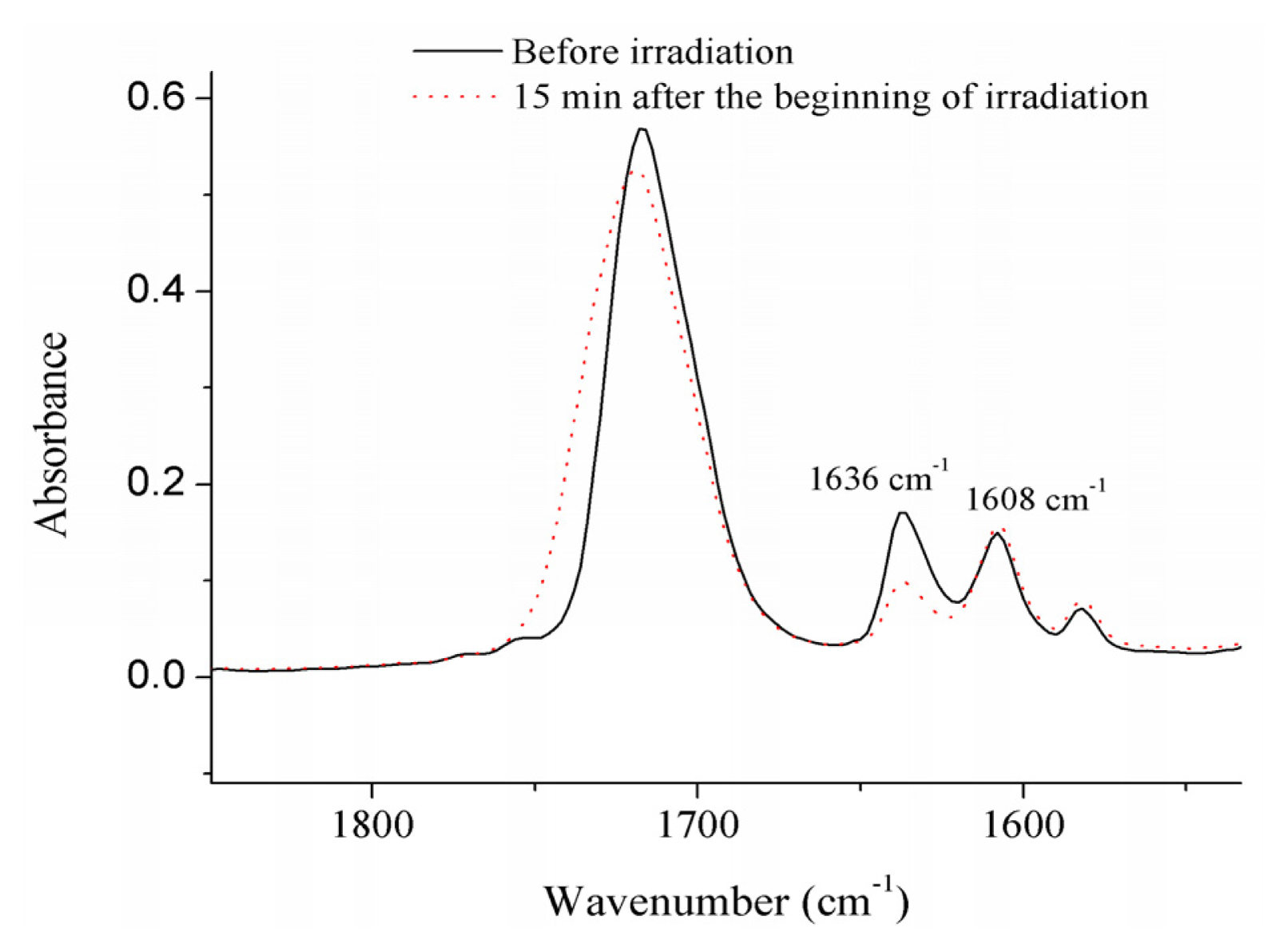
2. Results
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Methods
4.2.1. Monomer (Double-Bond) Conversion
4.2.2. Flexural Strength and Modulus
4.2.3. Water Sorption and Solubility
4.2.4. Radio-Opacity
4.2.5. Biofilm Inhibition Test
4.2.6. Scanning Electron Microscope (SEM)
4.2.7. Statistical Analysis
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Atai, M.; Nekoomanesh, M.; Hashemi, S.A.; Amani, S. Physical and mechanical properties of an experimental dental composite based on a new monomer. Dent. Mater 2004, 20, 663–668. [Google Scholar]
- Pereira, S.G.; Osorio, R.; Toledano, M.; Nunes, T.G. Evaluation of two Bis-GMA analogues as potential monomer diluents to improve the mechanical properties of light-cured composite resins. Dent. Mater 2005, 21, 823–830. [Google Scholar]
- Lee, V.A.; Cardenas, H.L.; Rawls, H.R. Rubber-toughening of dimethacrylate dental composite resin. J. Biomed. Mater. Res. B 2010, 94, 447–454. [Google Scholar]
- Kurata, S.; Yamazaki, N. Synthesis of dimethacryloxy ethyl-1,1,6,6-tetrahydro-perfluorohexamethylene- 1,6-dicarbamate as dental base monomers and the mechanical properties of the copolymers of the monomer and methy methacrylate. Dent. Mater. J 2011, 30, 103–108. [Google Scholar]
- Khatri, C.A.; Stansbury, J.W.; Schultheisz, C.R.; Antonucci, J.M. Synthesis, characterization and evaluation of urethane derivatives of Bis-GMA. Dent. Mater 2003, 19, 584–588. [Google Scholar]
- Sideridou, I.D.; Achilias, D.S.; Spyoudi, C.; Karabela, M. Water sorption characteristics of light-cured dental resins and composites based on Bis-EMA/PCDMA. Biomaterials 2004, 25, 367–376. [Google Scholar]
- Kim, J.G.; Chung, C.M. Trifunctional methacrylate monomers and their photocured composites with reduced curing shrinkage, water sorption, and water solubility. Biomaterials 2003, 24, 3845–3851. [Google Scholar]
- He, J.W.; Liu, F.; Luo, Y.F.; Jia, D.M. Synthesis and characterization of a dimethacrylates monomer with low shrinkage and water sorption for dental application. J. Appl. Polym. Sci 2012, 125, 114–120. [Google Scholar]
- He, J.W.; Liao, L.L.; Liu, F.; Luo, Y.F.; Jia, D.M. Synthesis and characterization of a new dimethacrylate monomer based on 5,5-bis(4-hydroxylphenyl)-hexahydro-4,7-methanoindan for root canal sealer application. J. Mater. Sci. Mater. Med 2010, 21, 1135–1142. [Google Scholar]
- He, J.W.; Luo, Y.F.; Liu, F.; Jia, D.M. Synthesis, characterization and photopolymerization of a new dimethacrylate monomer based on (alpha-methyl-benzylidene)bisphenol used as root canal sealer. J. Biomater Sci. Polym. Ed 2010, 21, 1191–1205. [Google Scholar]
- He, J.W.; Luo, Y.F.; Liu, F.; Jia, D.M. Synthesis and characterization of a new trimethacrylate monomer with low polymerization shrinkage and its application in dental restoration materials. J. Biomater. Appl 2010, 25, 235–249. [Google Scholar]
- Viljanen, E.K.; Skrifvars, M.; Vallittu, P.K. Degree of conversion of an experimental monomer and methyl methacrylate copolymer for dental applications. J. Appl. Polym. Sci 2004, 93, 1908–1912. [Google Scholar]
- Viljanen, E.K.; Skrifvars, M.; Vallittu, P.K. Dendrimer/methyl methacrylate copolymers: residual methyl methacrylate and degree of conversion. J. Biomater. Sci. Polym. Ed 2005, 16, 1219–1231. [Google Scholar]
- Viljanen, E.K.; langer, S.; Skrifvars, M.; Vallittu, P.K. Analysis of residual monomers by HPLC and HS-GC/MS. Dent. Mater 2006, 22, 845–851. [Google Scholar]
- Imazato, S.; Chen, J.H.; Ma, S.; Izutani, N.; Li, F. Antibacterial resin monomers based on quaternary ammonium and their benefits in restorative dentistry. Jpn. Dent. Sci. Rev 2012, 48, 115–125. [Google Scholar]
- Shay, D.E.; Allen, T.J.; Mantz, R.F. The antibacterial effects of some dental restorative materials. J. Dent. Res 1956, 35, 25–32. [Google Scholar]
- Ehara, A.; Torii, M.; Imazato, S.; Ebisu, S. Antibacterial activities and release kinetics of a newly developed recoverable controlled agent-release system. J. Dent. Res 2000, 79, 824–828. [Google Scholar]
- Leung, D.; Spratt, D.A.; Pratten, J.; Gulabivala, K.; Mordan, N.J.; Young, A.M. Chlorhexidine-releasing methacrylate dental composite materials. Biomaterials 2005, 26, 7145–7153. [Google Scholar]
- Al-Musallam, T.A.; Evans, C.A.; Drummond, J.L.; Matasa, C.; Wu, C.D. Antimicrobial properties of an orthodontic adhesive combined with cetylpyridinium chloride. Am. J. Orthod. Dentofacial. Orthop 2006, 129, 245–251. [Google Scholar]
- Nganga, S.; Travan, A.; Donati, I.; Crosera, M.; Paoletti, S.; Vallittu, P.K. Degradation of silver-polysaccharide nanocomposite in solution and as coating on fiber-reinforced composites by lysozyme and hydrogen peroxide. Biomacromolecules 2012, 13, 2605–2608. [Google Scholar]
- Jedrychowski, J.R.; Caputo, A.A.; Kerper, S. Antibacterial and mechanical properties of restorative materials combined with chlorhexidines. J. Oral. Rehabil 1983, 10, 373–381. [Google Scholar]
- Wilson, S.J.; Wilson, H.J. The release of chlorhexidine from modified dental acrylic resin. J. Oral. Rehabil 1993, 20, 311–319. [Google Scholar]
- Waltimo, T.; Luo, G.; Samaranayake, L.P.; Vallittu, P.K. Glass fibre-reinforced composite laced with chlorhexidine diglucoonate and yeast adhesion. J. Mater. Sci. Mater. Med 2004, 15, 117–121. [Google Scholar]
- Lahdenperä, M.S.; Puska, M.A.; Alander, P.M.; Waltimo, T.; Vallittu, P.K. Release of chlorhexidine digluconate and flexural properties of glass fibre reinforced provisional fixed partial denture polymer. J. Mater. Sci. Mater. Med 2004, 15, 1349–1353. [Google Scholar]
- Imazato, S.; Torii, M.; Tsuchitani, Y. Immobilization of an antibacterial component in composite resin. Dent. Jpn 1993, 30, 63–68. [Google Scholar]
- Imazato, S.; Torii, M.; Tsuchitani, Y.; McCabe, J.F.; Russell, R.R.B. Incorporation of bacterial inhibitor into resin composite. J. Dent. Res 1994, 73, 1437–1443. [Google Scholar]
- Imazato, S.; Russell, R.R.B.; McCabe, J.F. Antibacterial activity of MDPB polymer incorporated in dental resin. J. Dent 1995, 23, 177–181. [Google Scholar]
- Imazato, S. Antibacterial properties of resin composites and dentin bonding systems. Dent. Mater 2003, 19, 449–57. [Google Scholar]
- Xiao, Y.H.; Ma, S.; Chen, J.H.; Chai, Z.G.; Li, F.; Wang, Y.J. Antibacterial activity and bonding ability of an adhesive incorporating an antibacterial monomer DMA-CB. J. Biomed. Mater. Res. B 2009, 90, 813–817. [Google Scholar]
- Huang, L.; Xiao, Y.H.; Xing, X.D.; Li, F.; Ma, S.; Qi, L.L.; Chen, J.H. Antibacterial activity and cytotoxicity of two novel cross-linking antibacterial monomers on oral pathogens. Arch. Oral Biol 2011, 56, 367–373. [Google Scholar]
- Xie, D.; Weng, Y.M.; Guo, X.; Zhao, J.; Gregory, R.L.; Zheng, C. Preparation and evaluation of a novel glass-ionomer cement with antibacterial functions. Dent. Mater 2011, 27, 487–496. [Google Scholar]
- He, J.W.; Söderling, E.; Österblad, M.; Vallittu, P.K.; Lassila, L.V.J. Synthesis of methacrylate monomers with antibacterial effects against S. Mutans. Molecules 2011, 6, 9755–9763. [Google Scholar]
- Antonucci, J.M.; Zeiger, D.N.; Tang, K.; Lin-Gibson, S.; Fowler, B.O.; Lin, N.J. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent. Mater 2012, 28, 219–228. [Google Scholar]
- Xu, X.; Wang, Y.; Liao, S.; Wen, Z.T.; Fan, Y. Synthesis and characterization of antibacterial dental monomers and composites. J. Biomed. Mater. Res. B 2012, 100B, 1151–1162. [Google Scholar]
- Davy, K.W.M.; Anseau, M.R.; Odlyha, M.; Foster, G.M. X-ray opaque methacrylate polymers for biomedical applications. Polym. Int 1997, 43, 143–154. [Google Scholar]
- Davy, K.W.M.; Anseau, M.R.; Berry, C. Iodinated methacrylate copolymers as X-ray opaque denture base acrylics. J. Dent 1997, 25, 499–505. [Google Scholar]
- Galperin, A.; Margel, S. Synthesis and characterization of new micrometer-sized radio-opaque polymeric particles of narrow size distribution by a single-step swelling of uniform polystyrene template microspheres for X-ray imaging applications. Biomacromolecules 2006, 7, 2650–2660. [Google Scholar]
- Galperin, A.; Margel, D.; Margel, S. Synthesis and characterization of uniform radio-opaque polystyrene microspheres for X-ray imaging by a single-step swelling process. J. Biomed. Mater. Res. A 2006, 79, 544–551. [Google Scholar]
- He, J.; Söderling, E.; Lassila, L.V.J.; Vallittu, P.K. Incorporation of an antibacterial and radio-opaque monomer into dental resin system. Dent. Mater 2012, 28, e110–e117. [Google Scholar]
- Kanazawa, A.; Ikeda, T.; Endo, T. Polymeric phosphonium salts as novel class of cationic biocides. III. Immobilization of phosphonium salts by surface photografting and antibacterial activity of the surface-treated polymer-films. J. Polym. Sci. Pol. Chem 1993, 31, 1467–1472. [Google Scholar]
- Worley, S.D.; Sun, G. Biocidal polymers. Trends Polym. Sci 1996, 4, 364–370. [Google Scholar]
- Kenawy, E.R.; Worley, S.D.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar]
- Moran, J.; Addy, M.; Jackson, R.; Newcombe, R.G. Comparative effects of quaternary ammonium mouthrinses on 4-day plaque regrowth. J. Clin. Periodontol 2000, 27, 37–40. [Google Scholar]
- Simoncic, B.; Tomsic, B. Structure of novel antimicrobial agents for textile—A review. Text. Res. J 2010, 80, 1721–1737. [Google Scholar]
- Goncalves, F.; Kawano, Y.; Pfeifer, C.; Stansbury, J.W.; Braga, R.R. Influence of Bis-GMA, TEGDMA, and BisEMA contents on viscosity, conversion, and flexural strength of experimental resins and composites. Eur. J. Oral. Sci 2009, 117, 442–446. [Google Scholar]
- Lu, H.; Stansbury, J.W.; Nie, J.; Berchtold, K.A.; Bowman, C.N. Development of highly reactive mono-(meth)acrylates as reactive diluents for dimethacrylate-based dental resin systems. Biomaterials 2005, 26, 1329–1336. [Google Scholar]
- He, J.; Söderling, E.; Vallittu, P.K.; Lassila, L.V.J. Investigation of double bond conversion, mechanical properties, and antibacterial activity of dental resins with different alkyl chain length quaternary ammonium methacrylate monomers (QAM). J. Biomater. Sci. Polym. E 699709. [CrossRef]
- Weng, Y.; Guo, X.; Chong, V.J.; Howard, L.; Gregory, R.L.; Xie, D. Synthesis and evaluation of a novel antibacterial dental resin composite with quaternary ammonium salts. J. Biomed. Sci. Eng 2011, 4, 147–157. [Google Scholar]
- Weng, Y.; Howard, L.; Guo, X.; Gong, Y.J.; Gregory, R.L.; Xie, D. A novel antibacterial resin composite for improved dental restoratives. J. Mater. Sci.Mater. Med 2012, 23, 1553–1561. [Google Scholar]
- Dermaut, W.; van den Kerkhof, T.; van der Veken, B.J.; Mertens, R.; Geise, H.J. Cold stretching of PPV with water as a plasticizer. Macromolecules 2000, 33, 5634–5637. [Google Scholar]
- Sideridou, I.D.; Karabela, M.M.; Voucoudi, E.C. Dynamic thermomechanical properties and sorption characteristics of two commercial light cured dental resin composites. Dent. Mater 2008, 24, 737–743. [Google Scholar]
- Wang, G.; Weng, Y.; Chu, D.; Xie, D.; Chen, R. Preparation of alkaline anion exchange membranes based on functional poly(ether-imide) polymers for potential fuel cell applications. J. Membr. Sci 2009, 326, 4–8. [Google Scholar]
- Kasapoglu, F.; Aydin, M.; Arsu, N.; Yagci, Y. Photoinitiated polymerization of methyl methacrylate by phenacyl type salts. J. Photochem. Photobiol. A 2003, 159, 151–159. [Google Scholar]
- Kiskan, B.; Zhang, J.; Wang, X.; Antonietti, M.; Yagc, I.Y. Mescoporous graphitic carbon nitride as a heterogeneous visible light photoinitiator for radical polymerization. ACS Macro. Lett. 2012, 1, 546–549. [Google Scholar]
- Lassila, L.V.J.; Nohrström, T.; Vallittu, P.K. The influence of short-term water storage on the flexural properties of unidirectional glass fiber-reinforced composite. Biomaterials 2002, 23, 2221–2229. [Google Scholar]
- Tanzer, J.M.; Livingston, J.; Thompson, A.M. The microbiology of primary dental caries in humans. J. Dent. Educ 2001, 65, 1028–1037. [Google Scholar]
- Rupf, S.; Balkenhol, M.; Sahrhage, T.O.; Baum, A.; Chromik, J.N.; Ruppert, K.; Wissenbachc, D.K.; Maurerc, H.H.; Hanniga, M. Biofilm inhibition by an experimental dental resin composite containing octenidine dihydrochloride. Dent. Mater 2012, 28, 974–984. [Google Scholar]
- Balamurugan, A.; Balossier, G.; Laurent-Maquin, D.; Pina, S.; Rebelo, A.H.; Faure, J.; Ferreira, J.M. An in vitro biological and anti-bacterial study on a sol-gel derived silver-incorporated bioglass system. Dent. Mater 2008, 24, 1343–1351. [Google Scholar]
- Türkün, L.S.; Türkün, M.; Ertuğrul, F.; Ateş, M.; Brugger, S. Long-term antibacterial effect and physical properties of a chlorhexidine-containing glass ionomer cement. J. Esthet. Restor. Dent 2008, 20, 29–44. [Google Scholar]
- Giammanco, G.M.; Cumbo, E.M.; Luciani, A.; Gallina, G.; Mammina, C.; Pizzo, G. In vitro evaluation of the antibacterial activity of cured dentin/enamel adhesive incorporating the antimicrobial agent MDPB. New Microbiol 2009, 32, 385–390. [Google Scholar]
- Imazato, S.; Imai, T.; Russell, R.R.; Torii, M.; Ebisu, S. Antibacterial activity of cured dental resin incorporating the antibacterial monomer MDPB and an adhesion-promoting monomer. J. Biomed. Mater. Res 1998, 39, 511–515. [Google Scholar]
- Geurtsen, W.; Leyhausen, G. Concise review biomaterials & bioengineering: chemical-biological interactions of the resin monomer triethyleneglycol-dimethacrylate (TEGDMA). J. Dent. Res 2001, 80, 2046–2050. [Google Scholar]
- Thonemann, B.; Schmalz, G.; Hiller, K.A.; Schweikl, H. Response of L929 mouse fibroblasts, primary and immortalized bovine dental papilla-derived cell lines to dental resin components. Dent. Mater 2002, 18, 318–323. [Google Scholar]
- Ebi, N.; Imazato, S.; Noiri, Y.; Ebisu, S. Inhibitory effects of resin composite containing bactericide-immobilized filler on plaque accumulation. Dent. Mater 2001, 17, 485–491. [Google Scholar]
- Tanner, J.; Robinson, C.; Soderling, E.; Vallittu, P.K. Early plaque formation on fibre-reinforced composites in vivo. Clin. Oral Investig 2005, 9, 154–160. [Google Scholar]
- Lassila, L.V.; Garoushi, S.; Tanner, J.; Vallittu, P.K.; Soderling, E. Adherence of Streptococcus mutans to fiber-reinforced filling composite and conventional restorative materials. Open Dent. J 2009, 3, 227–232. [Google Scholar]
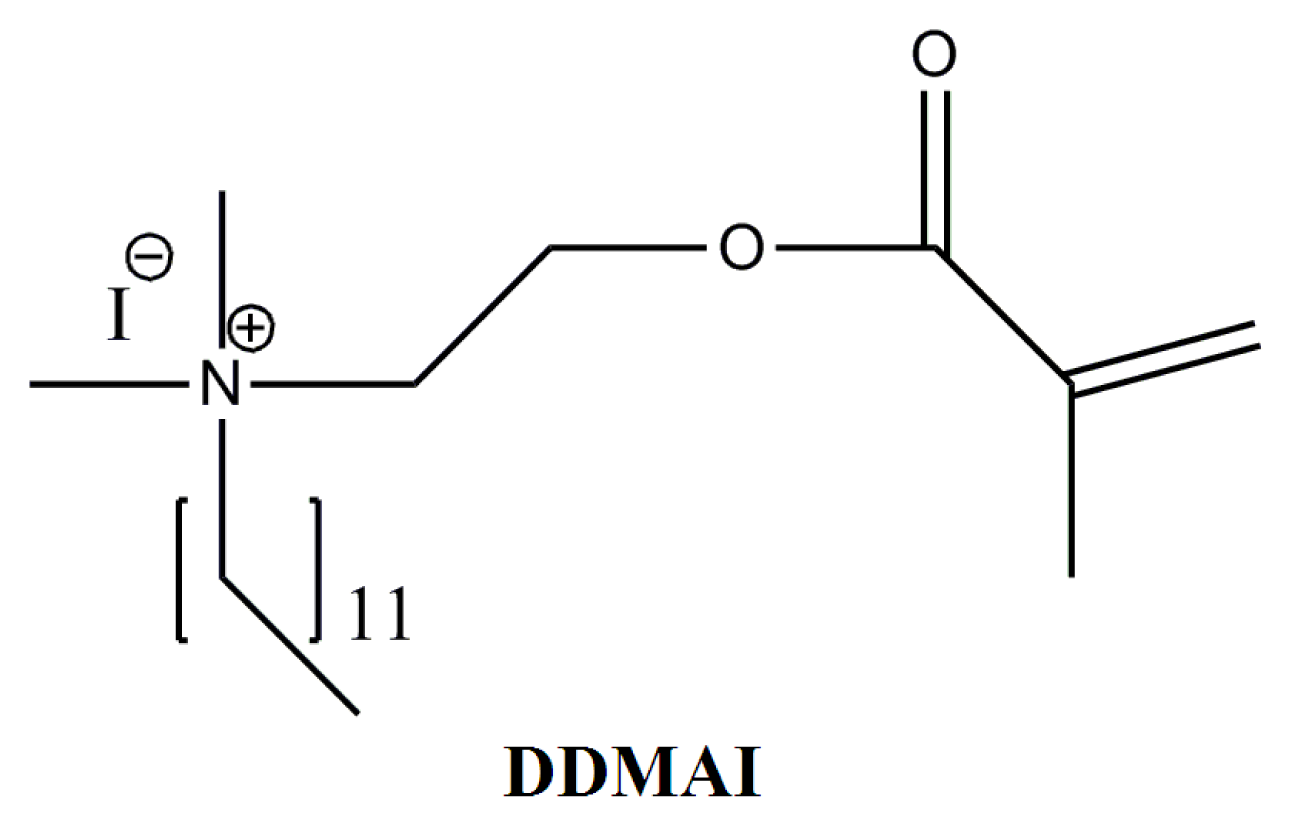
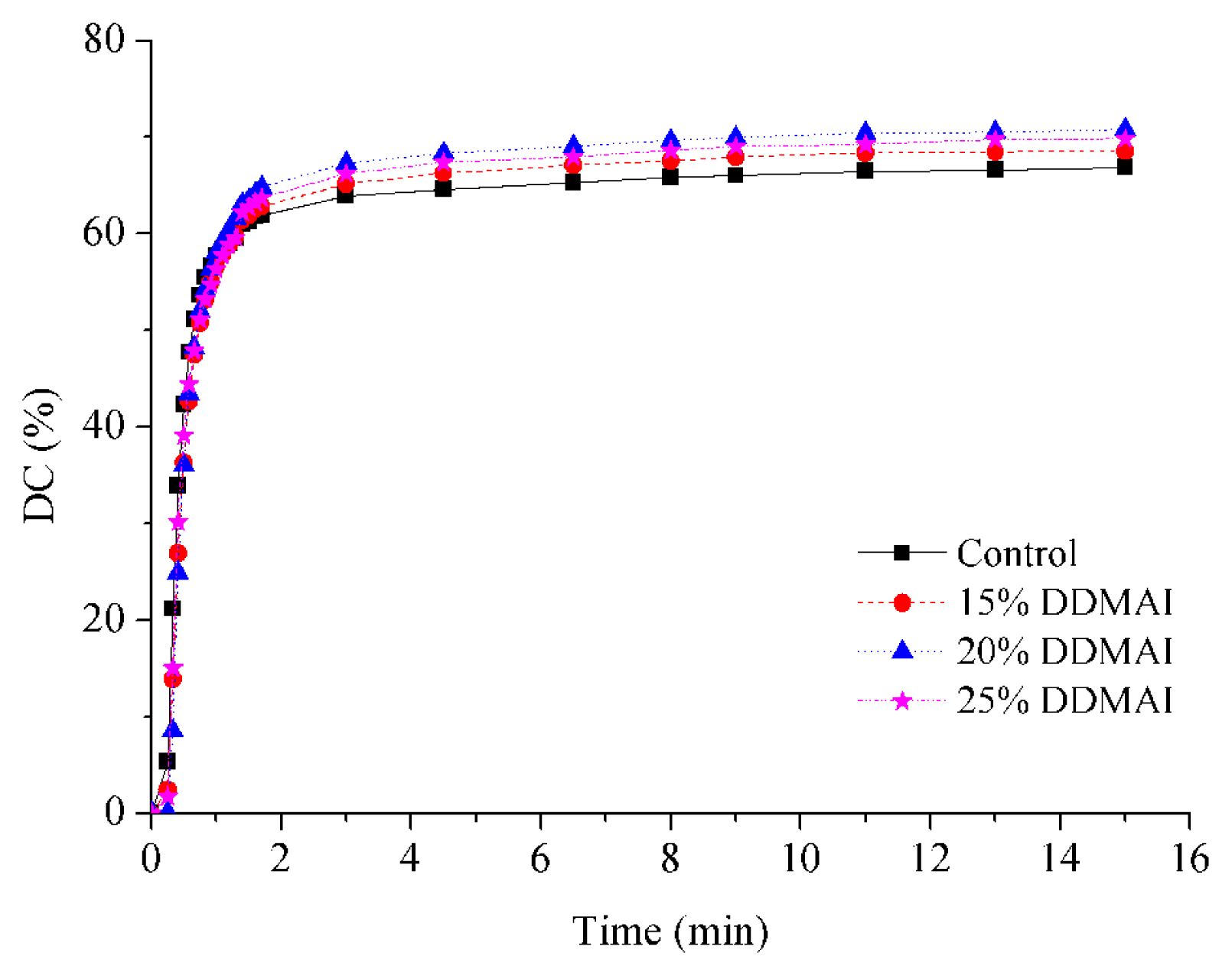
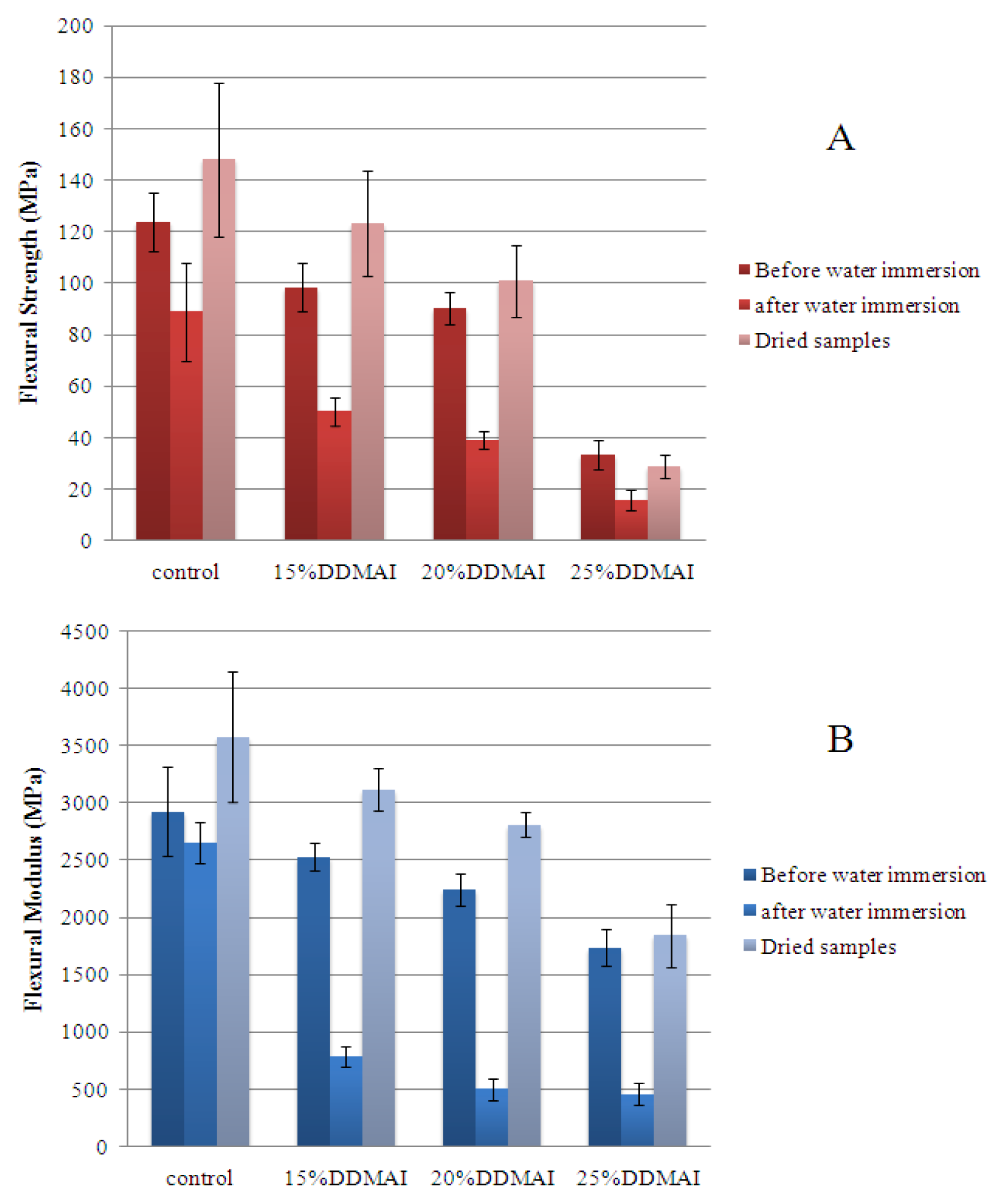
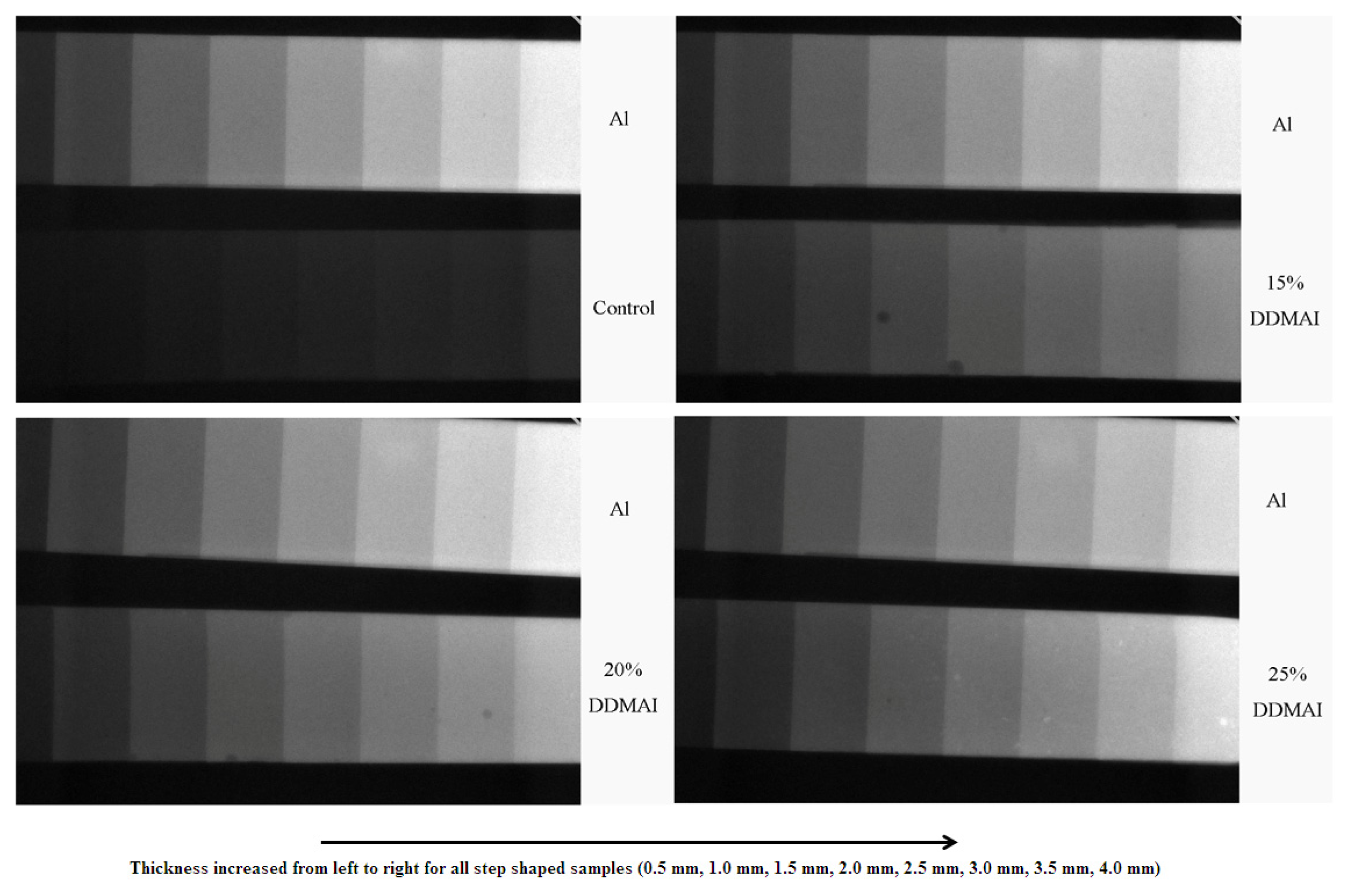
| Resin system | DC (%) | WS (%) | WSL (%) | ROX (%) | Streptococcus mutans colonies (Log CFU/mm2 × 102) | |
|---|---|---|---|---|---|---|
| Before water immersion | After water immersion | |||||
| Control | 66.8 ± 1.3 a | 3.47 ± 0.20 a | 0.60 ± 0.05 a | 11.4 | 13.61 ± 0.39 a | 14.94 ± 0.25 a |
| 15% DDMAI | 68.5 ± 1.0 b | 4.06 ± 0.17 b | 1.13 ± 0.11 b | 51.2 | 2.17 ± 4.35 b | 12.37 ± 0.43 b |
| 20% DDMAI | 70.8 ± 0.7 c | 4.37 ± 0.08 b | 1.34 ± 0.06 b | 67.7 | 1.62 ± 3.24 b | 12.56 ± 0.20 b |
| 25% DDMAI | 69.8 ± 1.1 b,c | 8.38 ± 0.98 c | 3.18 ± 0.64 c | 85.9 | 0.00 ± 0.00 b | 10.30 ± 0.21 c |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
He, J.; Söderling, E.; Vallittu, P.K.; Lassila, L.V.J. Preparation and Evaluation of Dental Resin with Antibacterial and Radio-Opaque Functions. Int. J. Mol. Sci. 2013, 14, 5445-5460. https://doi.org/10.3390/ijms14035445
He J, Söderling E, Vallittu PK, Lassila LVJ. Preparation and Evaluation of Dental Resin with Antibacterial and Radio-Opaque Functions. International Journal of Molecular Sciences. 2013; 14(3):5445-5460. https://doi.org/10.3390/ijms14035445
Chicago/Turabian StyleHe, Jingwei, Eva Söderling, Pekka K. Vallittu, and Lippo V. J. Lassila. 2013. "Preparation and Evaluation of Dental Resin with Antibacterial and Radio-Opaque Functions" International Journal of Molecular Sciences 14, no. 3: 5445-5460. https://doi.org/10.3390/ijms14035445
APA StyleHe, J., Söderling, E., Vallittu, P. K., & Lassila, L. V. J. (2013). Preparation and Evaluation of Dental Resin with Antibacterial and Radio-Opaque Functions. International Journal of Molecular Sciences, 14(3), 5445-5460. https://doi.org/10.3390/ijms14035445





