Different Forms of Selenoprotein M Differentially Affect Aβ Aggregation and ROS Generation
Abstract
:1. Introduction
2. Results and Discussion
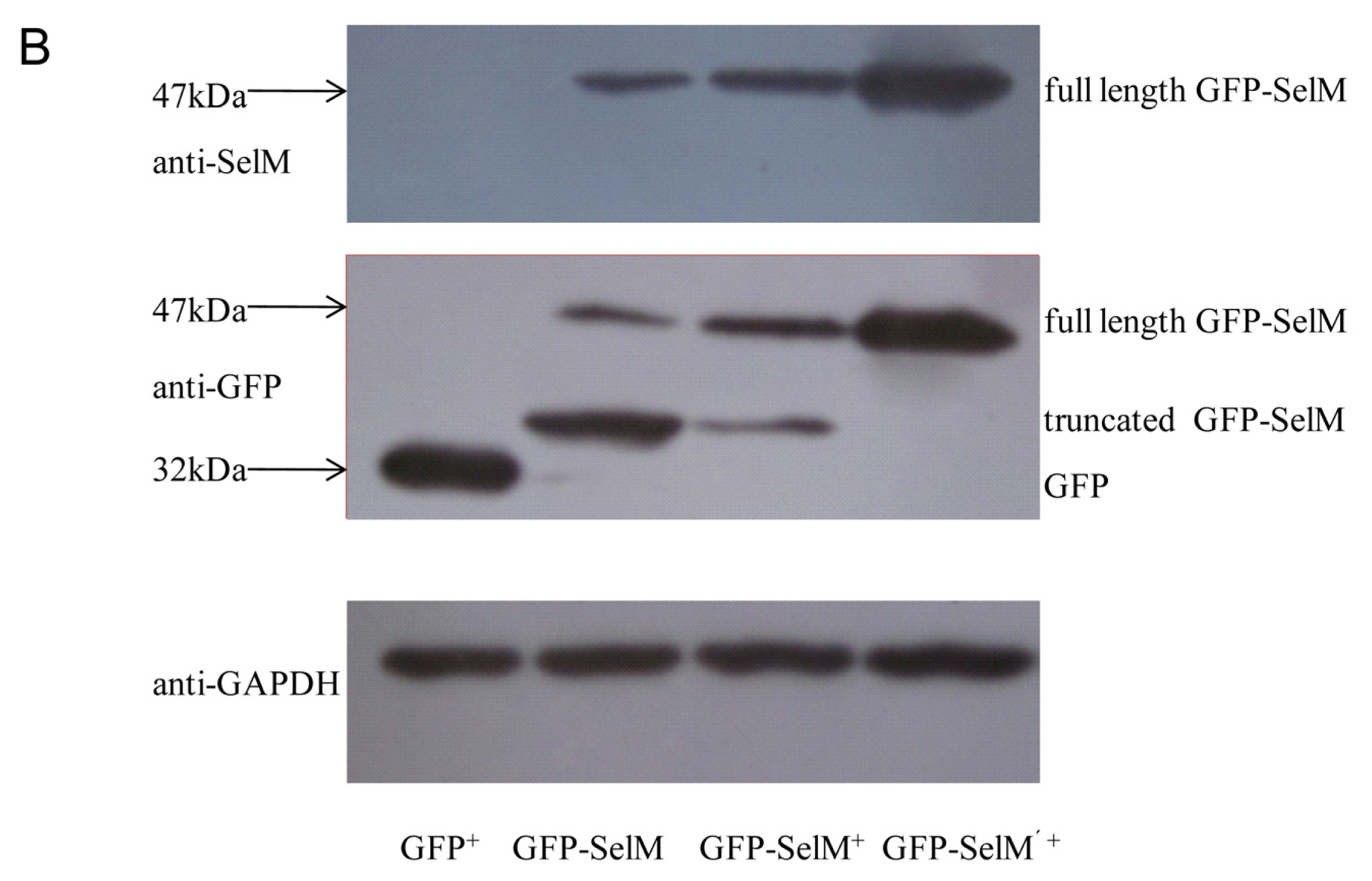
2.1. Effect of Sodium Selenite on the Expression Form of SelM
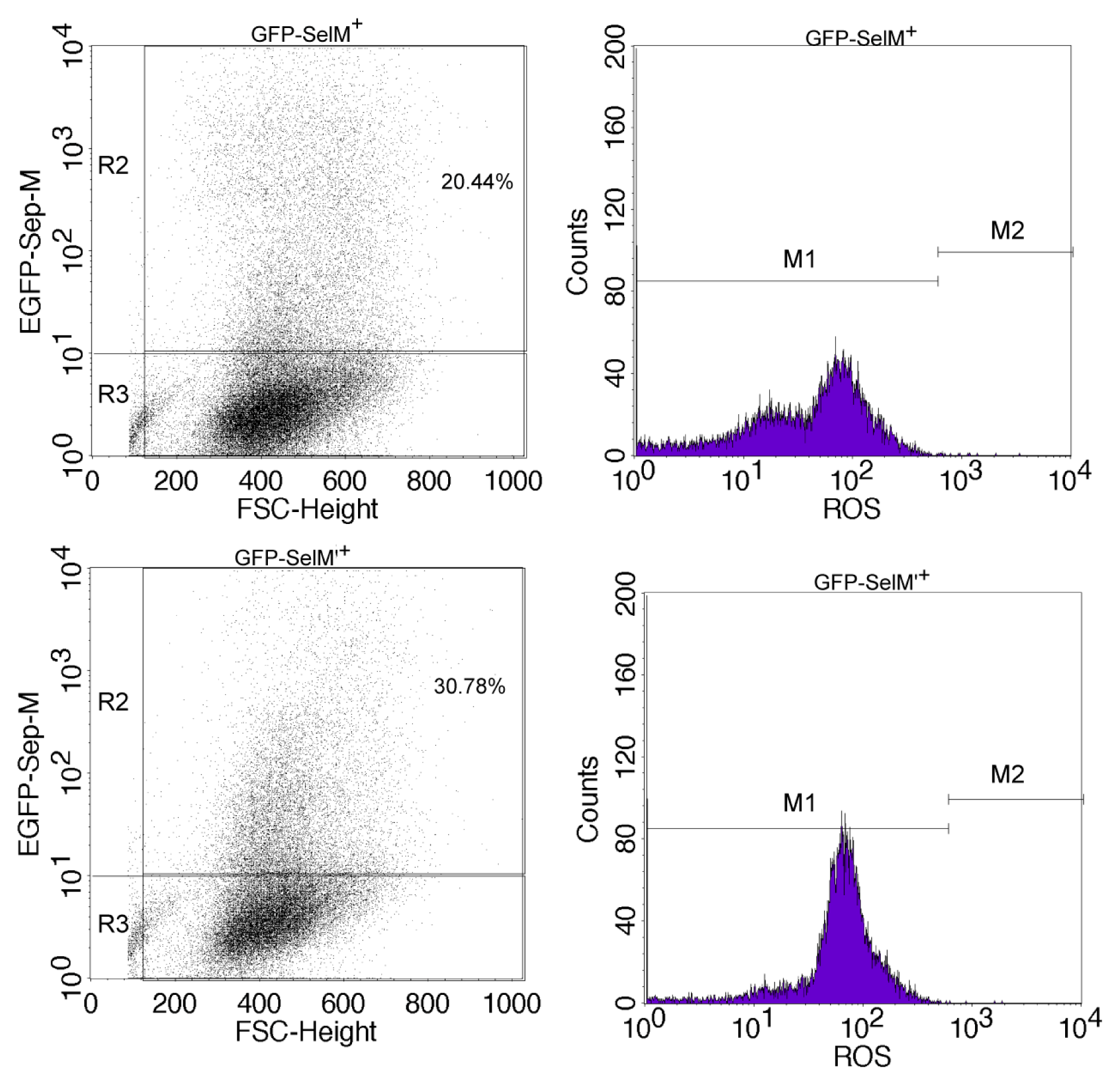
2.2. Effect of Different Forms of SelM on Intracellular ROS and Redox-Regulating Proteins
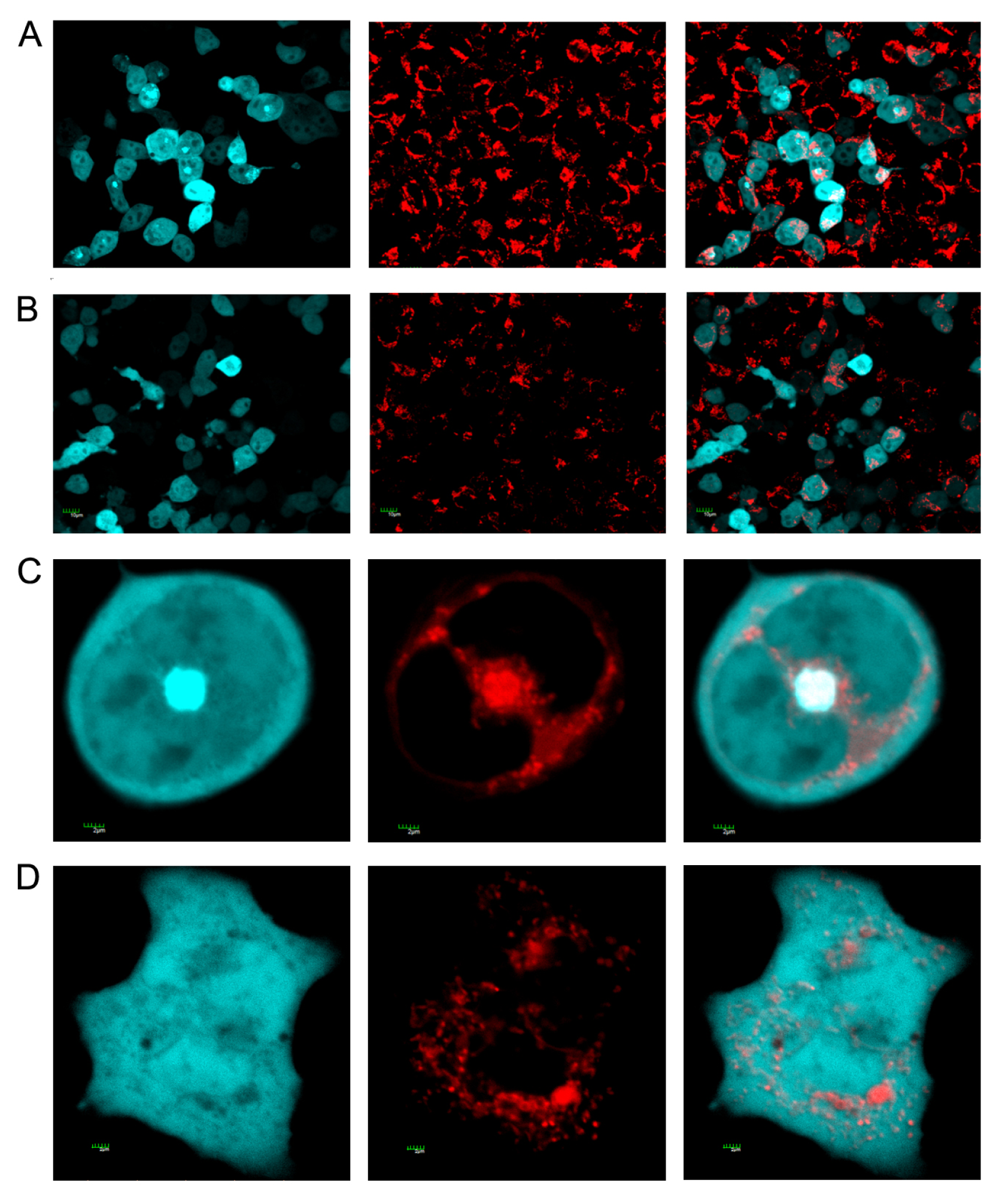
2.3. Effect of Different Forms of SelM on Aβ Aggregation
3. Experimental Section
3.1. Plasmid Construction
3.2. Cell Culture and Transfection
3.3. Cell Viability Assay
3.4. Staining of Mitochondria
3.5. Western Blot Analysis
3.6. Measurement of Intracellular ROS Level
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact 2006, 160, 1–40. [Google Scholar]
- Van Schooten, F.J.; Knaapen, A.M.; Izzotti, A. DNA damage, mutagenesis and cardiovascular disease. Mutat. Res 2007, 621, 1–4. [Google Scholar]
- Melo, A.; Monteiro, L.; Lima, R.M.; Oliveira, D.M.; Cerqueira, M.D.; El-Bacha, R.S. Oxidative stress in neurodegenerative diseases: Mechanisms and therapeutic perspectives. Oxid. Med. Cell Longev 2011, 2011, 467180. [Google Scholar]
- Kryukov, G.V.; Castellano, S.; Novoselov, S.V.; Lobanov, A.V.; Zehtab, O.; Guigo, R.; Gladyshev, V.N. Characterization of mammalian selenoproteomes. Science 2003, 300, 1439–1443. [Google Scholar]
- Bellinger, F.P.; Raman, A.V.; Reeves, M.A.; Berry, M.J. Regulation and function of selenoproteins in human disease. Biochem. J 2009, 422, 11–22. [Google Scholar]
- Loef, M.; Schrauzer, G.N.; Walach, H. Selenium and Alzheimer’s disease: A systematic review. J. Alzheimers Dis 2011, 26, 81–104. [Google Scholar]
- Korotkov, K.V.; Novoselov, S.V.; Hatfield, D.L.; Gladyshev, V.N. Mammalian selenoprotein in which selenocysteine (Sec) incorporation is supported by a new form of Sec insertion sequence element. Mol. Cell. Biol 2002, 22, 1402–1411. [Google Scholar]
- Ferguson, A.D.; Labunskyy, V.M.; Fomenko, D.E.; Arac, D.; Chelliah, Y.; Amezcua, C.A.; Rizo, J.; Gladyshev, V.N.; Deisenhofer, J. NMR structures of the selenoproteins Sep15 and SelM reveal redox activity of a new thioredoxin-like family. J. Biol. Chem 2006, 281, 3536–3543. [Google Scholar]
- Dikiy, A.; Novoselov, S.V.; Fomenko, D.E.; Sengupta, A.; Carlson, B.A.; Cerny, R.L.; Ginalski, K.; Grishin, N.V.; Hatfield, D.L.; Gladyshev, V.N. SelT, SelW, SelH, and Rdx12: Genomics and molecular insights into the functions of selenoproteins of a novel thioredoxin-like family. Biochemistry 2007, 46, 6871–6882. [Google Scholar]
- Hwang, D.Y.; Sin, J.S.; Kim, M.S.; Yim, S.Y.; Kim, Y.K.; Kim, C.K.; Kim, B.G.; Shim, S.B.; Jee, S.W.; Lee, S.H.; et al. Overexpression of human selenoprotein M differentially regulates the concentrations of antioxidants and H2O2, the activity of antioxidant enzymes, and the composition of white blood cells in a transgenic rat. Int. J. Mol. Med. 2008, 21, 169–179. [Google Scholar]
- Wirth, E.K.; Conrad, M.; Winterer, J.; Wozny, C.; Carlson, B.A.; Roth, S.; Schmitz, D.; Bornkamm, G.W.; Coppola, V.; Tessarollo, L.; et al. Neuronal selenoprotein expression is required for interneuron development and prevents seizures and neurodegeneration. FASEB J. 2010, 24, 844–852. [Google Scholar]
- Hwang, D.Y.; Cho, J.S.; Oh, J.H.; Shim, S.B.; Jee, S.W.; Lee, S.H.; Seo, S.J.; Lee, S.K.; Kim, Y.K. Differentially expressed genes in transgenic mice carrying human mutant presenilin-2 (N141I): Correlation of selenoprotein M with Alzheimer’s disease. Neurochem. Res 2005, 30, 1009–1019. [Google Scholar]
- Yim, S.Y.; Chae, K.R.; Shim, S.B.; Hong, J.T.; Park, J.Y.; Lee, C.Y.; Son, H.J.; Sheen, Y.Y.; Hwang, D.Y. ERK activation induced by selenium treatment significantly downregulates beta/gamma-secretase activity and Tau phosphorylation in the transgenic rat overexpressing human selenoprotein M. Int. J. Mol. Med 2009, 24, 91–96. [Google Scholar]
- Reeves, M.A.; Bellinger, F.P.; Berry, M.J. The neuroprotective functions of selenoprotein M and its role in cytosolic calcium regulation. Antioxid. Redox Signal 2010, 12, 809–818. [Google Scholar]
- Driscoll, D.M.; Copeland, P.R. Mechanism and regulation of selenoprotein synthesis. Annu. Rev. Nutr 2003, 23, 17–40. [Google Scholar]
- Arner, E.S.; Sarioglu, H.; Lottspeich, F.; Holmgren, A.; Bock, A. High-level expression in Escherichia coli of selenocysteine-containing rat thioredoxin reductase utilizing gene fusions with engineered bacterial-type SECIS elements and co-expression with the selA, selB and selC genes. J. Mol. Biol 1999, 292, 1003–1016. [Google Scholar]
- Eckenroth, B.; Harris, K.; Turanov, A.A.; Gladyshev, V.N.; Raines, R.T.; Hondal, R.J. Semisynthesis and characterization of mammalian thioredoxin reductase. Biochemistry 2006, 45, 5158–5170. [Google Scholar]
- Rengby, O.; Johansson, L.; Carlson, L.A.; Serini, E.; Vlamis-Gardikas, A.; Karsnas, P.; Arner, E.S. Assessment of production conditions for efficient use of Escherichia coli in high-yield heterologous recombinant selenoprotein synthesis. Appl. Environ. Microbiol 2004, 70, 5159–5167. [Google Scholar]
- Novoselov, S.V.; Lobanov, A.V.; Hua, D.; Kasaikina, M.V.; Hatfield, D.L.; Gladyshev, V.N. A highly efficient form of the selenocysteine insertion sequence element in protozoan parasites and its use in mammalian cells. Proc. Natl. Acad. Sci. USA 2007, 104, 7857–7862. [Google Scholar]
- Madeja, Z.; Sroka, J.; Nystrom, C.; Bjorkhem-Bergman, L.; Nordman, T.; Damdimopoulos, A.; Nalvarte, I.; Eriksson, L.C.; Spyrou, G.; Olsson, J.M.; et al. The role of thioredoxin reductase activity in selenium-induced cytotoxicity. Biochem. Pharmacol. 2005, 69, 1765–1772. [Google Scholar]
- Sunde, R.A.; Raines, A.M.; Barnes, K.M.; Evenson, J.K. Selenium status highly regulates selenoprotein mRNA levels for only a subset of the selenoproteins in the selenoproteome. Biosci. Rep 2009, 29, 329–338. [Google Scholar]
- Jameson, R.R.; Diamond, A.M. A regulatory role for Sec tRNA[Ser]Sec in selenoprotein synthesis. RNA 2004, 10, 1142–1152. [Google Scholar]
- Mitsui, A.; Hirakawa, T.; Yodoi, J. Reactive oxygen-reducing and protein-refolding activities of adult T cell leukemia-derived factor/human thioredoxin. Biochem. Biophys. Res. Commun 1992, 186, 1220–1226. [Google Scholar]
- Zhong, L.; Arner, E.S.; Holmgren, A. Structure and mechanism of mammalian thioredoxin reductase: The active site is a redox-active selenolthiol/selenenylsulfide formed from the conserved cysteine-selenocysteine sequence. Proc. Natl. Acad. Sci. USA 2000, 97, 5854–5859. [Google Scholar]
- Anestal, K.; Arner, E.S. Rapid induction of cell death by selenium-compromised thioredoxin reductase 1 but not by the fully active enzyme containing selenocysteine. J. Biol. Chem 2003, 278, 15966–15972. [Google Scholar]
- Onyango, I.G.; Khan, S.M. Oxidative stress, mitochondrial dysfunction, and stress signaling in Alzheimer’s disease. Curr. Alzheimer Res 2006, 3, 339–349. [Google Scholar]
- Chakrabortee, S.; Boschetti, C.; Walton, L.J.; Sarkar, S.; Rubinsztein, D.C.; Tunnacliffe, A. Hydrophilic protein associated with desiccation tolerance exhibits broad protein stabilization function. Proc. Natl. Acad. Sci. USA 2007, 104, 18073–18078. [Google Scholar]
- Beal, M.F. Energetics in the pathogenesis of neurodegenerative diseases. Trends Neurosci 2000, 23, 298–304. [Google Scholar]
- Sultana, R.; Butterfield, D.A. Oxidatively modified, mitochondria-relevant brain proteins in subjects with Alzheimer disease and mild cognitive impairment. J. Bioenerg. Biomembr 2009, 41, 441–446. [Google Scholar]
- Du, H.; Yan, S.S. Mitochondrial permeability transition pore in Alzheimer’s disease: Cyclophilin D and amyloid beta. Biochim. Biophys. Acta 2010, 1802, 198–204. [Google Scholar]
- Heckman, K.L.; Pease, L.R. Gene splicing and mutagenesis by PCR-driven overlap extension. Nat. Protoc 2007, 2, 924–932. [Google Scholar]
- Wang, H.; Kwok, D.T.; Wang, W.; Wu, Z.; Tong, L.; Zhang, Y.; Chu, P.K. Osteoblast behavior on polytetrafluoroethylene modified by long pulse, high frequency oxygen plasma immersion ion implantation. Biomaterials 2010, 31, 413–419. [Google Scholar]






| Primer | Sequence | Restriction site | Gene fragment | Plasmid constructed |
|---|---|---|---|---|
| F1 | 5′-GCCACTGCCTACCGGCCGGAC-3′ | SelM ORF cutting off the first 69 bp sequence coding for a signal peptide | pMD18T-SelM | |
| R1 | 5′-CTACAGGTCAGCGTGGTCCGAAG-3′ | |||
| F1 | 5′-GCCACTGCCTACCGGCCGGAC-3′ | SelM ORF cutting off the first 69 bp sequence coding for a signal peptide and creating the mutation site from TGA to TGC | pMD18T-SelM’ | |
| R1 | 5′-CTACAGGTCAGCGTGGTCCGAAG-3′ | |||
| F2 | 5′-GCGGGGGATGCCAGCTGAAC-3′ | |||
| R2 | 5′-GTTCAGCTGGCATCCCCCGC-3′ | |||
| F3 | 5′-ACGCGTCGACATGGTGAGCAAGGG-3′ | SalI | GFP ORF (following a stop codon) fused with SelM ORF mentioned above | pSelExpress1-GFP |
| R3 | 5′-CGGAATTCTTACTTGTACAGCTCGTCCATG-3′ | ECoRI | ||
| F4 | 5′-CGGAATTCGCCACTGCCTACCGG-3′ | ECoRI | ||
| R4 | 5′-GCTCTAGACTACAGGTCAGCGTGG-3′ | XbaI | ||
| F3 | 5′-ACGCGTCGACATGGTGAGCAAGGG-3′ | SalI | GFP ORF fused with SelM ORF or its mutant mentioned above | pSelExpress1-GFP-SelM or pSelExpress1-GFP-SelM’ |
| R5 | 5′-CGGAATTCCTTGTACAGCTCGTCCATG-3′ | ECoRI | ||
| F4 | 5′-CGGAATTCGCCACTGCCTACCGG-3′ | ECoRI | ||
| R4 | 5′-GCTCTAGACTACAGGTCAGCGTGG-3′ | XbaI | ||
| F5 | 5′-ACGCGTCGACGCCACTGCCTACCGG-3′ | SalI | SelM or its mutant mentioned above | pSelExpress1-SelM-myc or pSelExpress1-SelM’-myc |
| R6 | 5′-GCTCTAGACTACAGATCCTCTTCAGAGAT GAGTTTCTGCTCCAGGTCAGCGTGGTCCG-3′ | XbaI | ||
| F6 | 5′-CTAGCTAGCGCCACCATGGATGCGGAAT TTCGCCAT-3′ | NheI | Aβ42 fused CFP | pCDNA3.1(+)-Aβ42-CFP |
| R7 | 5′-GGAATTCCATATGGGATTCGCCAG-3′ | NdeI | ||
| F7 | 5′-GGAATTCCATATGGTGAGCAAGGGCGAG-3′ | NdeI | ||
| R8 | 5′-CCGGATATCTTACTTGTACAGCTCGT-3′ | EcoRV | ||
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, P.; Wang, R.-R.; Ma, X.-J.; Liu, Q.; Ni, J.-Z. Different Forms of Selenoprotein M Differentially Affect Aβ Aggregation and ROS Generation. Int. J. Mol. Sci. 2013, 14, 4385-4399. https://doi.org/10.3390/ijms14034385
Chen P, Wang R-R, Ma X-J, Liu Q, Ni J-Z. Different Forms of Selenoprotein M Differentially Affect Aβ Aggregation and ROS Generation. International Journal of Molecular Sciences. 2013; 14(3):4385-4399. https://doi.org/10.3390/ijms14034385
Chicago/Turabian StyleChen, Ping, Ruo-Ran Wang, Xiao-Jie Ma, Qiong Liu, and Jia-Zuan Ni. 2013. "Different Forms of Selenoprotein M Differentially Affect Aβ Aggregation and ROS Generation" International Journal of Molecular Sciences 14, no. 3: 4385-4399. https://doi.org/10.3390/ijms14034385





