Lipid Nanotechnology
Abstract
:1. Introduction
2. Experimental Lipid Nanotechnology
(1) Solid-supported lipid bilayer
(2) Polymer-supported lipid bilayer
(3) Pore-spanning lipid bilayer
(4) Protein incorporation into membranes
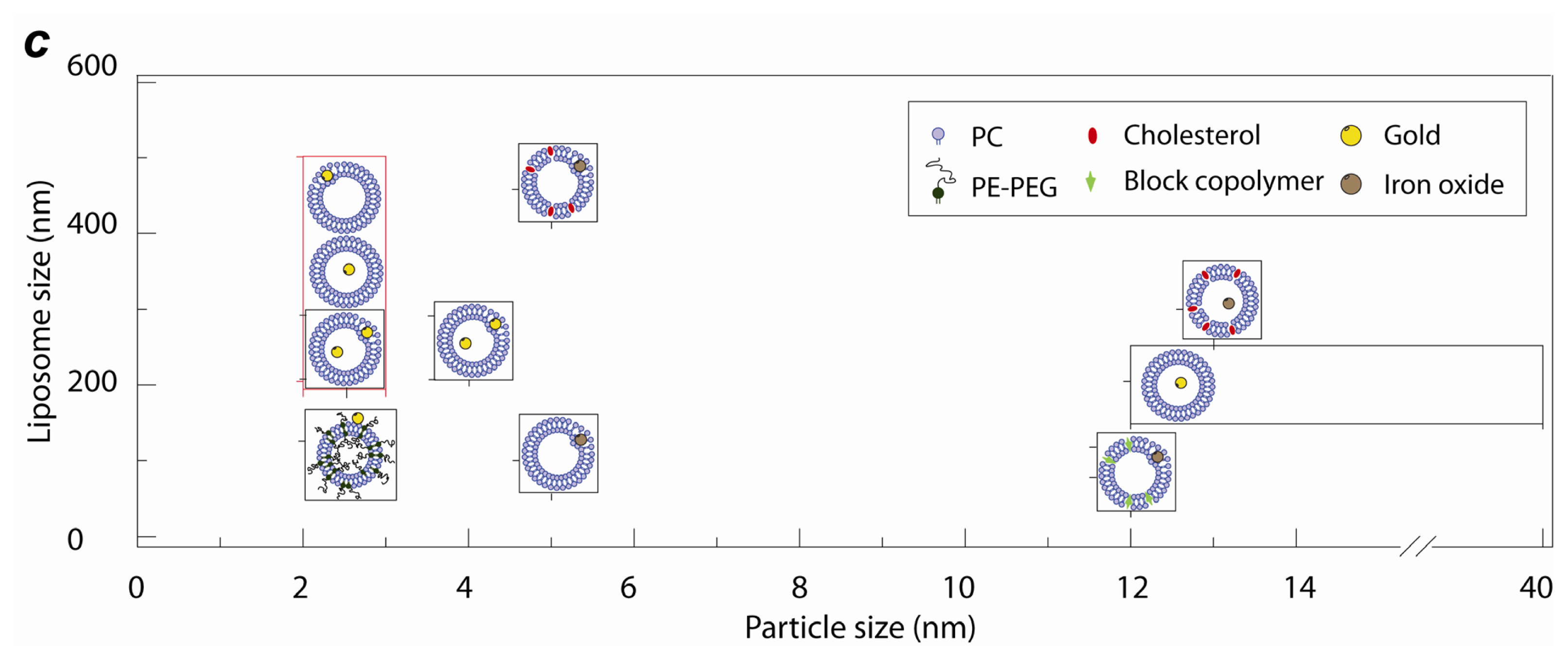
2.1. Lipid Nanomedicine
2.2. Lipid Nanofluidics
2.3. Lipid Nanoassemblies
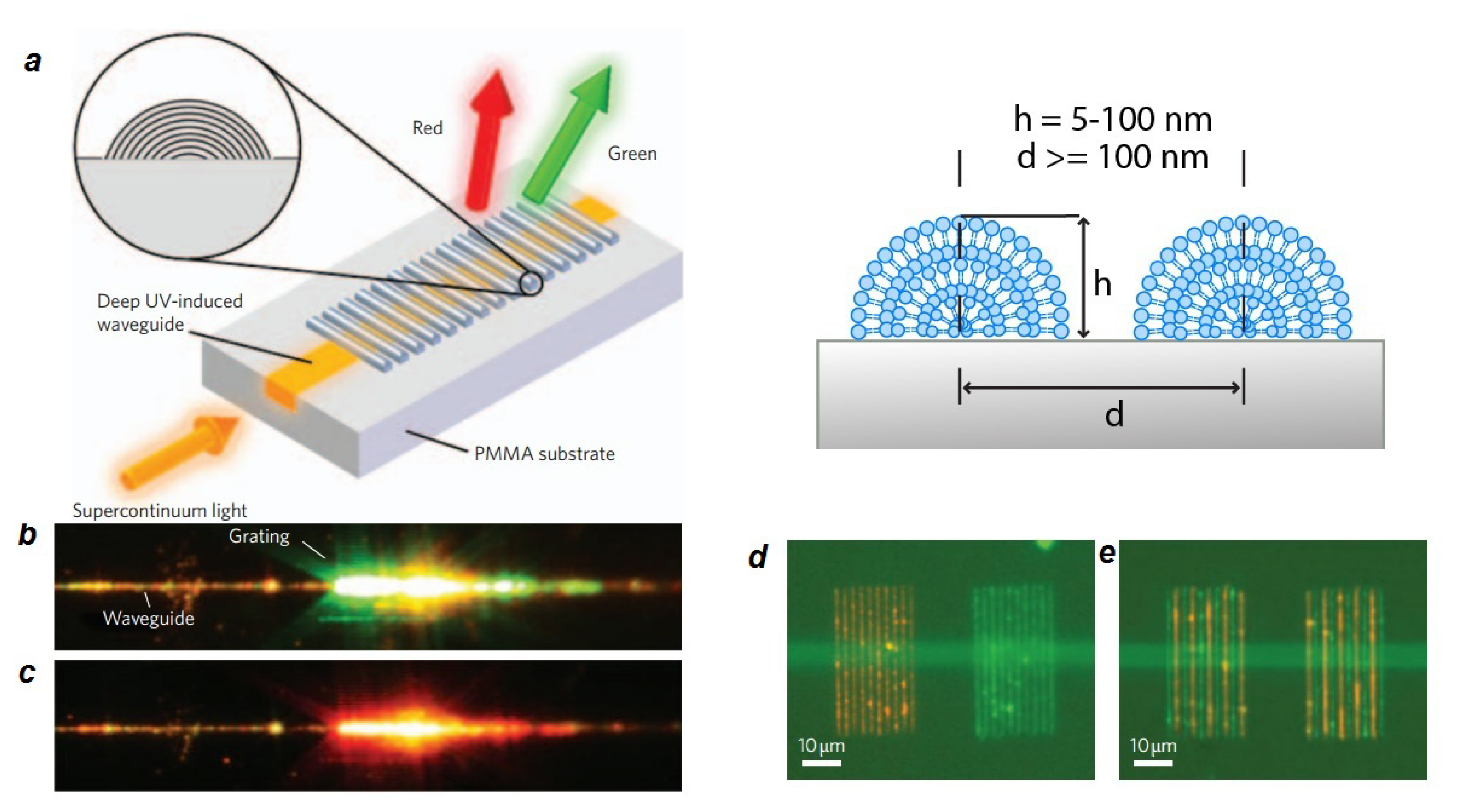
2.4. Lipid Nanoelectronics and Photonics
3. Computational Lipid Nanotechnology
(1) Force-fields
(2) Atomistic force fields
(3) Coarse-grained force fields
3.1. Lipid-Based Nano-Structures in Silico
Flat and Curved Bilayers: Nano-Vesicles and Tubes
3.2. Membrane-Mediated Transport at the Nano Scale
3.2.1. Lipidic Nano-Pores and Pore-Induced Transport in Lipid Membranes
- Electric field poration. One of the best-known ways to create pores is by exerting electric potential differences to the membrane of a cell. Experimentally, electric pulses produced by high-energy lasers are applied to the cell membrane. Computationally, the proper potential difference across a membrane is produced either by applying a constant electric field [219–227], or by creating an ionic imbalance around the membrane, which causes an electric field responsible for pore formation [227–230]. As a result of the electric field, water molecules can penetrate into the membrane. The process of opening a pore usually starts by spreading of water molecules throughout the membrane. When water molecules permeate the membrane, some small water defects form around the lipid head groups. A pore will be later stabilized when the head groups move towards this initial pore. The pore then enlarges and becomes lined with lipid head groups and the head groups distribute evenly over the pore “surface” [218].
- Pore formation induced by mechanical stress. Mechanical stress such as osmotic swelling can also induce pore formation in lipid bilayer membranes. In this case pores are formed as a response of the membrane to the surface tension. To simulate a membrane under different levels of surface tension, one possibility is to change the surface density of lipids. Forming a pore requires a critical threshold surface strain, which depends on the lipid composition and environmental condition [189,218]. By estimating the free energy of the system, the stability of pores can be investigated. Several ways have been proposed to calculate free energy differences in membrane systems caused by pore formation. One possibility is to use constraint forces to calculate the free energy profile for pore formation as a function of pore radius [231–233]. Another option is to calculate the free energy of pore evolution by pushing a lipid molecule into the bilayer interior in a reversible manner [233]. During this process, a small water pore forms spontaneously.
- Pore formation in lipid membranes by peptides. One of the main pharmacological mechanisms for killing microbial cells is by antimicrobial peptides, which form pores in the bacterial cell membrane, leading to ion influx and cell death. Typically, amphipathic peptides containing lysine and arginine residues are able to induce pores. Pore formation occurs when the hydrophobic parts of the peptide are in contact with the lipid membrane and the polar residues face the water. Usually, formation of a toroidal membrane-spanning pore is the result of the cooperative self-assembly of five to six magainin (an antimicrobial peptide) molecules [163,218]. The first computational study of peptide-induced pores suggests that pores can be formed in a few nanoseconds [234,235]. Recent molecular dynamics simulation studies reported also a time scale between 10 to 100 nanoseconds for pore formation in the presence of enough peptide [236].
3.2.2. Fusion and Fission of Lipid Membranes
3.3. Lipid-Nano Device Interaction Studies
4. Concluding Remarks
Acknowledgments
Conflict of Interest
References
- Whitesides, G.M.; Lipomi, D.J. Soft nanotechnology: “structure” vs. “function”. Faraday Discuss 2009, 143, 373–384. [Google Scholar]
- Hamley, I.W. Nanotechnology with soft materials. Angew Chem. Int. Edit 2003, 42, 1692–1712. [Google Scholar]
- Willson, C.G. A Decade of Step and Flash Imprint Lithography. J. Photopolym. Sci. Tech 2009, 22, 147–153. [Google Scholar]
- Shir, D.J.; Jeon, S.; Liao, H.; Highland, M.; Cahill, D.G.; Su, M.F.; El-Kady, I.F.; Christodoulou, C.G.; Bogart, G.R.; Hamza, A.V.; et al. Three-dimensional nanofabrication with elastomeric phase masks. J. Phys. Chem. B 2007, 111, 12945–12958. [Google Scholar]
- Zantye, P.B.; Kumar, A.; Sikder, A.K. Chemical mechanical planarization for microelectronics applications. Mat. Sci. Eng. R 2004, 45, 89–220. [Google Scholar]
- Cheng, C.J.; Saltzman, W.M. NANOMEDICINE Downsizing tumour therapeutics. Nat. Nanotechnol 2012, 7, 346–347. [Google Scholar]
- Lobatto, M.E.; Fuster, V.; Fayad, Z.A.; Mulder, W.J.M. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat. Rev. Drug Discov 2011, 10, 963–963. [Google Scholar]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed; Academic Press: Burlington, MA, 2011; p. 674. [Google Scholar]
- Peer, D.; Karp, J.M.; Hong, S.; FaroKHzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol 2007, 2, 751–760. [Google Scholar]
- Srivastava, D.; Atluri, S.N. Computational nanotechnology: A current perspective. Cmes-Comp. Model. Eng 2002, 3, 531–538. [Google Scholar]
- Essmann, U.; Perera, L.; Berkowitz, M.L. The Origin of the Hydration Interaction of Lipid Bilayers from Md Simulation of Dipalmitoylphosphatidylcholine Membranes in Gel and Liquid-Crystalline Phases. Langmuir 1995, 11, 4519–4531. [Google Scholar]
- Wilson, E.K. Computational nanotechnology - Modeling and theory are becoming vital to designing and improving nanodevices. Chem. Eng. News 2003, 81, 27–29. [Google Scholar]
- Barnard, A.S. How can ab initio simulations address risks in nanotech? Nat. Nanotechnol 2009, 4, 332–335. [Google Scholar]
- Damodaran, K.V.; Merz, K.M.; Gaber, B.P. Structure and Dynamics of the Dilauroylphosphatidylethanolamine Lipid Bilayer. Biochemistry 1992, 31, 7656–7664. [Google Scholar]
- Schulz, M.; Olubummo, A.; Binder, W.H. Beyond the lipid-bilayer: interaction of polymers and nanoparticles with membranes. Soft Matter 2012, 8, 4849–4864. [Google Scholar]
- Mashaghi, A.; Partovi-Azar, P.; Jadidi, T.; Nafari, N.; Maass, P.; Tabar, M.R.R.; Bonn, M.; Bakker, H.J. Hydration strongly affects the molecular and electronic structure of membrane phospholipids. J. Chem. Phys 2012, 136, 114709–114714. [Google Scholar]
- Elezgaray, J.; Laguerre, M. A systematic method to derive force fields for coarse-grained simulations of phospholipids. Comput. Phys. Commun 2006, 175, 264–268. [Google Scholar]
- Heimburg, T. Thermal Biophysics of Membrane; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; p. 378. [Google Scholar]
- Michalet, X.; Bensimon, D. Observation of Stable Shapes and Conformal Diffusion in Genus-2 Vesicles. Science 1995, 269, 666–668. [Google Scholar]
- Mashaghi, A.; Partovi-Azar, P.; Jadidi, T.; Nafari, N.; Esfarjani, K.; Maass, P.; Tabar, M.R.R.; Bakker, H.J.; Bonn, M. Interfacial Water Facilitates Energy Transfer by Inducing Extended Vibrations in Membrane Lipids. J. Phys. Chem. B 2012, 116, 6455–6460. [Google Scholar]
- Teissie, J.; Prats, M.; Soucaille, P.; Tocanne, J.F. Evidence for conduction of protons along the interface between water and a polar lipid monolayer. Proc. Nat. Acad. Sci. USA 1985, 82, 3217–3221. [Google Scholar]
- Nakano, T.; Kikugawa, G.; Ohara, T. A molecular dynamics study on heat conduction characteristics in DPPC lipid bilayer. J. Chem. Phys 2010, 133, 154705–154714. [Google Scholar]
- Michel Prats, J.T.; Tocanne, J.F. Lateral proton conduction at lipid–water interfaces and its implications for the chemiosmotic-coupling hypothesis. Nature 1986, 322, 756–758. [Google Scholar]
- Heim, M.; Cevc, G.; Guckenberger, R.; Knapp, H.F.; Wiegrabe, W. Lateral Electrical-Conductivity of Mica-Supported Lipid Bilayer-Membranes Measured by Scanning-Tunneling-Microscopy. Biophys. J 1995, 69, 489–497. [Google Scholar]
- Garcia-Fandino, R.; Sansom, M.S.P. Designing biomimetic pores based on carbon nanotubes. Proc. Nat. Acad. Sci. USA 2012, 109, 6939–6944. [Google Scholar]
- Shen, S.; Henry, A.; Tong, J.; Zheng, R.T.; Chen, G. Polyethylene nanofibres with very high thermal conductivities. Nat. Nanotechnol 2010, 5, 251–255. [Google Scholar]
- Smits, M.; Ghosh, A.; Bredenbeck, J.; Yamamoto, S.; Muller, M.; Bonn, M. Ultrafast energy flow in model biological membranes. New J. Phys 2007, 9, 390–410. [Google Scholar]
- Mashaghi, A.; Swann, M.; Popplewell, J.; Textor, M.; Reimhult, E. Optical anisotropy of supported lipid structures probed by waveguide spectroscopy and its application to study of supported lipid bilayer formation kinetics. Anal. Chem 2008, 80, 5276–5276. [Google Scholar]
- Mashaghi, A.; Partovi-Azar, P.; Jadidi, T.; Anvari, M.; Panahian, J.S.; Nafari, N.; Rahimi Tabar, M.R.; Maass, P.; Bakker, H.J.; Bonn, M. Enhanced Autoionization of Water at Phospholipid Interfaces. J. Phys. Chem. C 2013, 117, 510–514. [Google Scholar]
- Bothun, G.D.; Preiss, M.R. Bilayer heating in magnetite nanoparticle-liposome dispersions via fluorescence anisotropy. J. Colloid Interf. Sci 2011, 357, 70–74. [Google Scholar]
- Katagiri, K.; Imai, Y.; Koumoto, K.; Kaiden, T.; Kono, K.; Aoshima, S. Magnetoresponsive On-Demand Release of Hybrid Liposomes Formed from Fe3O4 Nanoparticles and Thermosensitive Block Copolymers. Small 2011, 7, 1683–1689. [Google Scholar]
- Troutman, T.S.; Leung, S.J.; Romanowski, M. Light-Induced Content Release from Plasmon-Resonant Liposomes. Adv. Mater 2009, 21, 2334. [Google Scholar]
- Amstad, E.; Kohlbrecher, J.; Muller, E.; Schweizer, T.; Textor, M.; Reimhult, E. Triggered Release from Liposomes through Magnetic Actuation of Iron Oxide Nanoparticle Containing Membranes. Nano Lett 2011, 11, 1664–1670. [Google Scholar]
- Paasonen, L.; Laaksonen, T.; Johans, C.; Yliperttula, M.; Kontturi, K.; Urth, A. Gold nanoparticles enable selective light-induced contents release from liposomes. J. Control. Release 2007, 122, 86–93. [Google Scholar]
- Nappini, S.; Bombelli, F.B.; Bonini, M.; Norden, B.; Baglioni, P. Magnetoliposomes for controlled drug release in the presence of low-frequency magnetic field. Soft Matter 2010, 6, 154–162. [Google Scholar]
- Paasonen, L.; Sipila, T.; Subrizi, A.; Laurinmaki, P.; Butcher, S.J.; Rappolt, M.; Yaghmur, A.; Urtti, A.; Yliperttula, M. Gold-embedded photosensitive liposomes for drug delivery: Triggering mechanism and intracellular release. J.Control. Release 2010, 147, 136–143. [Google Scholar]
- Oberle, V.; Bakowsky, U.; Zuhorn, I.S.; Hoekstra, D. Lipoplex formation under equilibrium conditions reveals a three-step mechanism. Biophys. J 2000, 79, 1447–1454. [Google Scholar]
- Tabatt, K.; Kneuer, C.; Sameti, M.; Olbrich, C.; Muller, R.H.; Lehr, C.M.; Bakowsky, U. Transfection with different colloidal systems: comparison of solid lipid nanoparticles and liposomes. J. Control. Release 2004, 97, 321–332. [Google Scholar]
- Xu, Y.; Szoka, F.C., Jr. Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry 1996, 35, 5616–5623. [Google Scholar]
- Szoka, F.C., Jr. The future of liposomal drug delivery. Biotechnol. Appl. Biochem. 1990, 12, 496–500. [Google Scholar]
- Li, Y.; Gu, N. Thermodynamics of Charged Nanoparticle Adsorption on Charge-Neutral Membranes: A Simulation Study. J. Phys. Chem. B 2010, 114, 2749–2754. [Google Scholar]
- Li, Y.; Chen, X.; Gu, N. Computational Investigation of Interaction between Nanoparticles and Membranes: Hydrophobic/Hydrophilic Effect. J. Phys. Chem. B 2008, 112, 16647–16653. [Google Scholar]
- Ashley, C.E.; Carnes, E.C.; Phillips, G.K.; Padilla, D.; Durfee, P.N.; Brown, P.A.; Hanna, T.N.; Liu, J.W.; Phillips, B.; Carter, M.B.; et al. The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nat. Mater 2011, 10, 476–476. [Google Scholar]
- Irvine, D.J. DRUG DELIVERY One nanoparticle, one kill. Nat. Mater 2011, 10, 342–343. [Google Scholar]
- Kobayashi, E.; Iyer, A.K.; Hornicek, F.J.; Amiji, M.M.; Duan, Z. Lipid-functionalized Dextran Nanosystems to Overcome Multidrug Resistance in Cancer: A Pilot Study. Clin. Orthop. Relat. Res 2012, 471, 915–925. [Google Scholar]
- Holme, M.N.; Fedotenko, I.A.; Abegg, D.; Althaus, J.; Babel, L.; Favarger, F.; Reiter, R.; Tanasescu, R.; Zaffalon, P.L.; Ziegler, A.; et al. Shear-stress sensitive lenticular vesicles for targeted drug delivery. Nat. Nanotechnol 2012, 7, 536–543. [Google Scholar]
- Olton, D.; Li, J.H.; Wilson, M.E.; Rogers, T.; Close, J.; Huang, L.; Kumta, P.N.; Sfeir, C. Nanostructured calcium phosphates (NanoCaPs) for non-viral gene delivery: Influence of the synthesis parameters on transfection efficiency. Biomaterials 2007, 28, 1267–1279. [Google Scholar]
- Zhou, C.; Yu, B.; Yang, X.; Huo, T.; Lee, L.J.; Barth, R.F.; Lee, R.J. Lipid-coated nano-calcium-phosphate (LNCP) for gene delivery. Int. J. Pharm 2010, 392, 201–208. [Google Scholar]
- Schafer, J.; Hobel, S.; Bakowsky, U.; Aigner, A. Liposome-polyethylenimine complexes for enhanced DNA and siRNA delivery. Biomaterials 2010, 31, 6892–6900. [Google Scholar]
- Cauda, V.; Engelke, H.; Sauer, A.; Arcizet, D.; Brauchle, C.; Radler, J.; Bein, T. Colchicine-Loaded Lipid Bilayer-Coated 50 nm Mesoporous Nanoparticles Efficiently Induce Microtubule Depolymerization upon Cell Uptake. Nano Lett 2010, 10, 2484–2492. [Google Scholar]
- Cui, H.S.; Lyman, E.; Voth, G.A. Mechanism of Membrane Curvature Sensing by Amphipathic Helix Containing Proteins. Biophys. J 2011, 100, 1271–1279. [Google Scholar]
- DeMuth, P.C.; Moon, J.J.; Suh, H.; Hammond, P.T.; Irvine, D.J. Releasable Layer-by-Layer Assembly of Stabilized Lipid Nanocapsules on Microneedles for Enhanced Transcutaneous Vaccine Delivery. ACS Nano 2012, 6, 8041–8051. [Google Scholar]
- Petty, M.C. Possible Applications for Langmuir-Blodgett-Films. Thin Solid Films 1992, 210, 417–426. [Google Scholar]
- Richter, R.P.; Berat, R.; Brisson, A.R. Formation of solid-supported lipid bilayers: An integrated view. Langmuir 2006, 22, 3497–3505. [Google Scholar]
- Mennicke, U.; Salditt, T. Preparation of solid-supported lipid bilayers by spin-coating. Langmuir 2002, 18, 8172–8177. [Google Scholar]
- Simonsen, A.C.; Bagatolli, L.A. Structure of spin-coated lipid films and domain formation in supported membranes formed by hydration. Langmuir 2004, 20, 9720–9728. [Google Scholar]
- Tamm, L.K.; McConnell, H.M. Supported phospholipid bilayers. Biophys. J 1985, 47, 105–113. [Google Scholar]
- Sackmann, E. Supported membranes: Scientific and practical applications. Science 1996, 271, 43–48. [Google Scholar]
- Cremer, P.S.; Boxer, S.G. Formation and spreading of lipid bilayers on planar glass supports. J. Phys. Chem. B 1999, 103, 2554–2559. [Google Scholar]
- Reviakine, I.; Brisson, A. Formation of supported phospholipid bilayers from unilamellar vesicles investigated by atomic force microscopy. Langmuir 2000, 16, 1806–1815. [Google Scholar]
- Khan, T.R.; Grandin, H.M.; Mashaghi, A.; Textor, M.; Reimhult, E.; Reviakine, I. Lipid redistribution in phosphatidylserine-containing vesicles adsorbing on titania. Biointerphases 2008, 3, Fa90–Fa95. [Google Scholar]
- Silin, V.I.; Wieder, H.; Woodward, J.T.; Valincius, G.; Offenhausser, A.; Plant, A.L. The role of surface free energy on the formation of hybrid bilayer membranes. J. Am. Chem. Soc 2002, 124, 14676–14683. [Google Scholar]
- Baumann, M.K.; Amstad, E.; Mashaghi, A.; Textor, M.; Reimhult, E. Characterization of supported lipid bilayers incorporating the phosphoinositides phosphatidylinositol 4,5-biphosphate and phosphoinositol-3,4,5-triphosphate by complementary techniques. Biointerphases 2010, 5, 114–119. [Google Scholar]
- Kaufmann, S.; Ilg, K.; Mashaghi, A.; Textor, M.; Priem, B.; Aebi, M.; Reimhult, E. Supported Lipopolysaccharide Bilayers. Langmuir 2012, 28, 12199–12208. [Google Scholar]
- Kalb, E.; Frey, S.; Tamm, L.K. Formation of Supported Planar Bilayers by Fusion of Vesicles to Supported Phospholipid Monolayers. Biochim. Biophys. Acta 1992, 1103, 307–316. [Google Scholar]
- Crane, J.M.; Kiessling, V.; Tamm, L.K. Measuring lipid asymmetry in planar supported bilayers by fluorescence interference contrast microscopy. Langmuir 2005, 21, 1377–1388. [Google Scholar]
- Hennesthal, C.; Drexler, J.; Steinem, C. Membrane-suspended nanocompartments based on ordered pores in alumina. Chemphyschem 2002, 3, 885–889. [Google Scholar]
- Purrucker, O.; Fortig, A.; Jordan, R.; Tanaka, M. Supported membranes with well-defined polymer tethers-incorporation of cell receptors. Chemphyschem 2004, 5, 327–335. [Google Scholar]
- Naumann, R.; Schmidt, E.K.; Jonczyk, A.; Fendler, K.; Kadenbach, B.; Liebermann, T.; Offenhausser, A.; Knoll, W. The peptide-tethered lipid membrane as a biomimetic system to incorporate cytochrome c oxidase in a functionally active form. Biosens. Bioelectron 1999, 14, 651–662. [Google Scholar]
- Tanaka, M.; Sackmann, E. Polymer-supported membranes as models of the cell surface. Nature 2005, 437, 656–663. [Google Scholar]
- Shen, W.W.; Boxer, S.G.; Knoll, W.; Frank, C.W. Polymer-supported lipid bilayers on benzophenone-modified substrates. Biomacromolecules 2001, 2, 70–79. [Google Scholar]
- Elender, G.; Kuhner, M.; Sackmann, E. Functionalisation of Si/SiO2 and glass surfaces with ultrathin dextran films and deposition of lipid bilayers. Biosens. Bioelectron 1996, 11, 565–577. [Google Scholar]
- Baumgart, T.; Offenhausser, A. Polysaccharide-supported planar bilayer lipid model membranes. Langmuir 2003, 19, 1730–1737. [Google Scholar]
- Munro, J.C.; Frank, C.W. In situ formation and characterization of poly(ethylene glycol)-supported lipid bilayers on gold surfaces. Langmuir 2004, 20, 10567–10575. [Google Scholar]
- Nissen, J.; Gritsch, S.; Wiegand, G.; Radler, J.O. Wetting of phospholipid membranes on hydrophilic surfaces - Concepts towards self-healing membranes. Eur. Phys. J. B 1999, 10, 335–344. [Google Scholar]
- Tanaka, M.; Rehfeldt, F.; Schneider, M.F.; Mathe, G.; Albersdorfer, A.; Neumaier, K.R.; Purrucker, O.; Sackmann, E. Wetting and dewetting of extracellular matrix and glycocalix models. J. Phys.Condens. Mat 2005, 17, S649–S663. [Google Scholar]
- Plant, A.L. Self-Assembled Phospholipid Alkanethiol Biomimetic Bilayers on Gold. Langmuir 1993, 9, 2764–2767. [Google Scholar]
- Plant, A.L. Supported hybrid bilayer membranes as rugged cell membrane mimics. Langmuir 1999, 15, 5128–5135. [Google Scholar]
- Proux-Delrouyre, V.; Elie, C.; Laval, J.M.; Moiroux, J.; Bourdillon, C. Formation of tethered and streptavidin-supported lipid bilayers on a microporous electrode for the reconstitution of membranes of large surface area. Langmuir 2002, 18, 3263–3272. [Google Scholar]
- Berquand, A.; Mazeran, P.E.; Pantigny, J.; Proux-Delrouyre, V.; Laval, J.M.; Bourdillon, C. Two-step formation of streptavidin-supported lipid bilayers by PEG-triggered vesicle fusion. Fluorescence and atomic force microscopy characterization. Langmuir 2003, 19, 1700–1707. [Google Scholar]
- Schuster, B.; Pum, D.; Sara, M.; Braha, O.; Bayley, H.; Sleytr, U.B. S-layer ultrafiltration membranes: A new support for stabilizing functionalized lipid membranes. Langmuir 2001, 17, 499–503. [Google Scholar]
- Bayley, H. Self-assembling biomolecular materials in medicine. J. Cell Biochem 1994, 56, 168–170. [Google Scholar]
- Wetzer, B.; Pum, D.; Sleytr, U.B. S-layer stabilized solid supported lipid bilayers. J. Struct. Biol 1997, 119, 123–128. [Google Scholar]
- Naumann, C.A.; Prucker, O.; Lehmann, T.; Ruhe, J.; Knoll, W.; Frank, C.W. The polymersupported phospholipid bilayer: Tethering as a new approach to substrate-membrane stabilization. Biomacromolecules 2002, 3, 27–35. [Google Scholar]
- Seitz, M.; Ter-Ovanesyan, E.; Hausch, M.; Park, C.K.; Zasadzinski, J.A.; Zentel, R.; Israelachvili, J.N. Formation of tethered supported bilayers by vesicle fusion onto lipopolymer monolayers promoted by osmotic stress. Langmuir 2000, 16, 6067–6070. [Google Scholar]
- Reich, C.; Andruzzi, L. Preparation of fluid tethered lipid bilayers on poly(ethylene glycol) by spin-coating. Soft Matter 2010, 6, 493–500. [Google Scholar]
- Diaz, A.J.; Albertorio, F.; Daniel, S.; Cremer, P.S. Double cushions preserve transmembrane protein mobility in supported bilayer systems. Langmuir 2008, 24, 6820–6826. [Google Scholar]
- Albertorio, F.; Diaz, A.J.; Yang, T.L.; Chapa, V.A.; Kataoka, S.; Castellana, E.T.; Cremer, P.S. Fluid and air-stable lipopolymer membranes for biosensor applications. Langmuir 2005, 21, 7476–7482. [Google Scholar]
- Kaufmann, S.; Papastavrou, G.; Kumar, K.; Textor, M.; Reimhult, E. A detailed investigation of the formation kinetics and layer structure of poly(ethylene glycol) tether supported lipid bilayers. Soft Matter 2009, 5, 2804–2814. [Google Scholar]
- Mueller, P.; Rudin, D.; Tien, H.; Wescott, W. Methods for the formation of single bimolecular lipid membranes in aqueous solution. J. Phys. Chem. B 1963, 67, 534–535. [Google Scholar]
- Han, X.J.; Studer, A.; Sehr, H.; Geissbuhler, I.; Di Berardino, M.; Winkler, F.K.; Tiefenauer, L.X. Nanopore arrays for stable and functional free-standing lipid bilayers. Adv. Mater 2007, 19, 4466. [Google Scholar]
- Romer, W.; Steinem, C. Impedance analysis and single-channel recordings on nano-black lipid membranes based on porous alumina. Biophys. J 2004, 86, 955–965. [Google Scholar]
- Kepplinger, C.; Hofer, I.; Steinem, C. Impedance analysis of valinomycin activity in nano-BLMs. Chem. Phys. Lipids 2009, 160, 109–113. [Google Scholar]
- Kresak, S.; Hianik, T.; Naumann, R.L.C. Giga-seal solvent-free bilayer lipid membranes: from single nanopores to nanopore arrays. Soft Matter 2009, 5, 4021–4032. [Google Scholar]
- Mey, I.; Stephan, M.; Schmitt, E.K.; Muller, M.M.; Ben Amar, M.; Steinem, C.; Janshoff, A. Local Membrane Mechanics of Pore-Spanning Bilayers. J. Am. Chem. Soc 2009, 131, 7031–7039. [Google Scholar]
- Kumar, K.; Isa, L.; Egner, A.; Schmidt, R.; Textor, M.; Reimhult, E. Formation of Nanopore-Spanning Lipid Bilayers through Liposome Fusion. Langmuir 2011, 27, 10920–10928. [Google Scholar]
- Karatekin, E.; Rothman, J.E. Fusion of single proteoliposomes with planar, cushioned bilayers in microfluidic flow cells. Nat. Protoc 2012, 7, 903–920. [Google Scholar]
- Milhiet, P.E.; Gubellini, F.; Berquand, A.; Dosset, P.; Rigaud, J.L.; Le Grimellec, C.; Levy, D. High-resolution AFM of membrane proteins directly incorporated at high density in planar lipid bilayer. Biophys. J 2006, 91, 3268–3275. [Google Scholar]
- Neumann, J.; Hennig, M.; Wixforth, A.; Manus, S.; Radler, J.O.; Schneider, M.F. Transport, Separation, and Accumulation of Proteins on Supported Lipid Bilayers. Nano Lett 2010, 10, 2903–2908. [Google Scholar]
- Shum, H.C.; Lee, D.; Yoon, I.; Kodger, T.; Weitz, D.A. Double emulsion templated monodisperse phospholipid vesicles. Langmuir 2008, 24, 7651–7653. [Google Scholar]
- Seth, A.; Bealle, G.; Santanach-Carreras, E.; Abou-Hassan, A.; Menager, C. Design of Vesicles Using Capillary Microfluidic Devices: From Magnetic to Multifunctional Vesicles. Adv. Mater 2012, 24, 3544–3548. [Google Scholar]
- Ota, S.; Yoshizawa, S.; Takeuchi, S. Microfluidic Formation of Monodisperse, Cell-Sized, and Unilamellar Vesicles. Angew Chem. Int. Ed 2009, 48, 6533–6537. [Google Scholar]
- Stachowiak, J.C.; Richmond, D.L.; Li, T.H.; Brochard-Wyart, F.; Fletcher, D.A. Inkjet formation of unilamellar lipid vesicles for cell-like encapsulation. Lab Chip 2009, 9, 2003–2009. [Google Scholar]
- Jahn, A.; Stavis, S.M.; Hong, J.S.; Vreeland, W.N.; Devoe, D.L.; Gaitan, M. Microfluidic Mixing and the Formation of Nanoscale Lipid Vesicles. ACS Nano 2010, 4, 2077–2087. [Google Scholar]
- Persson, F.; Fritzsche, J.; Mir, K.U.; Modesti, M.; Westerlund, F.; Tegenfeldt, J.O. Lipid-Based Passivation in Nanofluidics. Nano Lett 2012, 12, 2260–2265. [Google Scholar]
- Vasdekis, A.E.; Laporte, G.P.J. Enhancing Single Molecule Imaging in Optofluidics and Microfluidics. Int. J. Mol. Sci 2011, 12, 5135–5156. [Google Scholar]
- Hiyama, S.; Moritani, Y.; Gojo, R.; Takeuchi, S.; Sutoh, K. Biomolecular-motor-based autonomous delivery of lipid vesicles as nano- or microscale reactors on a chip. Lab Chip 2010, 10, 2741–2748. [Google Scholar]
- Christensen, S.M.; Bolinger, P.Y.; Hatzakis, N.S.; Mortensen, M.W.; Stamou, D. Mixing subattolitre volumes in a quantitative and highly parallel manner with soft matter nanofluidics. Nat. Nanotechnol 2012, 7, 51–55. [Google Scholar]
- Teh, S.Y.; Lin, R.; Hung, L.H.; Lee, A.P. Droplet microfluidics. Lab Chip 2008, 8, 198–220. [Google Scholar]
- Guo, M.T.; Rotem, A.; Heyman, J.A.; Weitz, D.A. Droplet microfluidics for high-throughput biological assays. Lab Chip 2012, 12, 2146–2155. [Google Scholar]
- Theberge, A.B.; Courtois, F.; Schaerli, Y.; Fischlechner, M.; Abell, C.; Hollfelder, F.; Huck, W.T.S. Microdroplets in Microfluidics: An Evolving Platform for Discoveries in Chemistry and Biology. Angew Chem. Int. Ed 2010, 49, 5846–5868. [Google Scholar]
- Villar, G.; Heron, A.J.; Bayley, H. Formation of droplet networks that function in aqueous environments. Nat. Nanotechnol 2011, 6, 803–808. [Google Scholar]
- Punnamaraju, S.; You, H.; Steckl, A.J. Triggered Release of Molecules across Droplet Interface Bilayer Lipid Membranes Using Photopolymerizable Lipids. Langmuir 2012, 28, 7657–7664. [Google Scholar]
- Takegami, S.; Kitamura, K.; Kawada, H.; Matsumoto, Y.; Kitade, T.; Ishida, H.; Nagata, C. Preparation and characterization of a new lipid nano-emulsion containing two cosurfactants, sodium palmitate for droplet size reduction and sucrose palmitate for stability enhancement. Chem. Pharm. Bull 2008, 56, 1097–1102. [Google Scholar]
- Son, S.; Kim, G.; Singha, K.; Park, S.; Ree, M.; Kim, W.J. Artificial Cell Membrane-Mimicking Nanostructure Facilitates Efficient Gene Delivery through Fusogenic Interaction with the Plasma Membrane of Living Cells. Small 2011, 7, 2991–2997. [Google Scholar]
- Pang, S.P.; Zhu, C.S.; Xu, F.M.; Chen, C.J.; Ji, J. Layer by layer self-assembly of poly[2-(methacryloyloxy) ethyl phosphorylcholine] multilayer via the ionic complexation with zirconium. Colloid Surface B 2012, 94, 22–26. [Google Scholar]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling Coatings: Recent Developments in the Design of Surfaces That Prevent Fouling by Proteins, Bacteria, and Marine Organisms. Adv. Mater 2011, 23, 690–718. [Google Scholar]
- He, Y.; Hower, J.; Chen, S.F.; Bernards, M.T.; Chang, Y.; Jiang, S.Y. Molecular simulation studies of protein interactions with zwitterionic phosphorylcholine self-assembled monolayers in the presence of water. Langmuir 2008, 24, 10358–10364. [Google Scholar]
- Alberts, B.; Wilson, J.H.; Hunt, T. Molecular Biology of the Cell, 5th ed; Garland Science: New York, NY, USA, 2008; p. 1601. [Google Scholar]
- Jonsson, P.; Beech, J.P.; Tegenfeldt, J.O.; Hook, F. Shear-Driven Motion of Supported Lipid Bilayers in Microfluidic Channels. J. Am. Chem. Soc 2009, 131, 5294–5297. [Google Scholar]
- Graneli, A.; Yeykal, C.C.; Robertson, R.B.; Greene, E.C. Long-distance lateral diffusion of human Rad51 on double-stranded DNA. Proc. Nat. Acad. Sci. USA 2006, 103, 1221–1226. [Google Scholar]
- Albet-Torres, N.; Gunnarsson, A.; Persson, M.; Balaz, M.; Hook, F.; Mansson, A. Molecular motors on lipid bilayers and silicon dioxide: different driving forces for adsorption. Soft Matter 2010, 6, 3211–3219. [Google Scholar]
- Zhou, Y. Lipid Nanotubes as Scaffold Toward Construction of One-Dimensional Nanostructures. Sci. Adv. Mater 2010, 2, 359–364. [Google Scholar]
- Woods, D.M.; Li, Z.; Rosenblatt, C.; Yager, P.; Schoen, P.E. Electric-Field Manipulation of Phospholipid Tubules - Optical Birefringence Measurements. Mol. Cryst. Liq. Cryst 1989, 167, 1–6. [Google Scholar]
- Krishnan, M.; Mojarad, N.; Kukura, P.; Sandoghdar, V. Geometry-induced electrostatic trapping of nanometric objects in a fluid. Nature 2010, 467, U692–U675. [Google Scholar]
- Yu, X.F.; Liu, Z.H.; Janzen, J.; Chafeeva, I.; Horte, S.; Chen, W.; Kainthan, R.K.; Kizhakkedathu, J.N.; Brooks, D.E. Polyvalent choline phosphate as a universal biomembrane adhesive. Nat. Mater 2012, 11, 468–476. [Google Scholar]
- Dogterom, M.; Koenderink, G. Cell-Membrane Mechanics Vesicles in and Tubes Out. Nat. Mater 2011, 10, 561–562. [Google Scholar]
- Jonsson, P.; Jonsson, M.P.; Hook, F. Sealing of Submicrometer Wells by a Shear-Driven Lipid Bilayer. Nano Lett 2010, 10, 1900–1906. [Google Scholar]
- Yusko, E.C.; Johnson, J.M.; Majd, S.; Prangkio, P.; Rollings, R.C.; Li, J.L.; Yang, J.; Mayer, M. Controlling protein translocation through nanopores with bio-inspired fluid walls. Nat. Nanotechnol 2011, 6, 253–260. [Google Scholar]
- Hernandez-Ainsa, S.; Muus, C.; Bell, N.A.; Steinbock, L.J.; Thacker, V.V.; Keyser, U.F. Lipid-coated nanocapillaries for DNA sensing. Analyst 2012, 138, 104–106. [Google Scholar]
- Rasch, M.R.; Rossinyol, E.; Hueso, J.L.; Goodfellow, B.W.; Arbiol, J.; Korgel, B.A. Hydrophobic gold nanoparticle self-assembly with phosphatidylcholine lipid: membrane-loaded and janus vesicles. Nano Lett 2010, 10, 3733–3739. [Google Scholar]
- Gornall, J.L.; Mahendran, K.R.; Pambos, O.J.; Steinbock, L.J.; Otto, O.; Chimerel, C.; Winterhalter, M.; Keyser, U.F. Simple reconstitution of protein pores in nano lipid bilayers. Nano Lett 2011, 11, 3334–3340. [Google Scholar]
- Wu, Y.; Hudson, J.S.; Lu, Q.; Moore, J.M.; Mount, A.S.; Rao, A.M.; Alexov, E.; Ke, P.C. Coating single-walled carbon nanotubes with phospholipids. J. Phys. Chem. B 2006, 110, 2475–2478. [Google Scholar]
- Qiao, R.; Ke, P.C. Lipid-carbon nanotube self-assembly in aqueous solution. J. Am. Chem. Soc 2006, 128, 13656–13657. [Google Scholar]
- Richard, C.; Balavoine, F.; Schultz, P.; Ebbesen, T.W.; Mioskowski, C. Supramolecular self-assembly of lipid derivatives on carbon nanotubes. Science 2003, 300, 775–778. [Google Scholar]
- Thauvin, C.; Rickling, S.; Schultz, P.; Celia, H.; Meunier, S.; Mioskowski, C. Carbon nanotubes as templates for polymerized lipid assemblies. Nat. Nanotechnol 2008, 3, 743–748. [Google Scholar]
- Borch, J.; Hamann, T. The nanodisc: a novel tool for membrane protein studies. Biol. Chem 2009, 390, 805–814. [Google Scholar]
- Frauenfeld, J.; Gumbart, J.; van der Sluis, E.O.; Funes, S.; Gartmann, M.; Beatrix, B.; Mielke, T.; Berninghausen, O.; Becker, T.; Schulten, K.; et al. Cryo-EM structure of the ribosome-SecYE complex in the membrane environment. Nat. Struct. Mol. Biol 2011, 18, U 614–U127. [Google Scholar]
- Katayama, H.; Wang, J.; Tama, F.; Chollet, L.; Gogol, E.P.; Collier, R.J.; Fisher, M.T. Three-dimensional structure of the anthrax toxin pore inserted into lipid nanodiscs and lipid vesicles. Proc. Nat. Acad. Sci. USA 2010, 107, 3453–3457. [Google Scholar]
- Marty, M.T.; Das, A.; Sligar, S.G. Ultra-thin layer MALDI mass spectrometry of membrane proteins in nanodiscs. Anal. Bioanal. Chem 2012, 402, 721–729. [Google Scholar]
- Marty, M.T.; Zhang, H.; Cui, W.D.; Blankenship, R.E.; Gross, M.L.; Sligar, S.G. Native Mass Spectrometry Characterization of Intact Nanodisc Lipoprotein Complexes. Anal. Chem 2012, 84, 8957–8960. [Google Scholar]
- Shi, L.; Shen, Q.T.; Kiel, A.; Wang, J.; Wang, H.W.; Melia, T.J.; Rothman, J.E.; Pincet, F. SNARE Proteins: One to Fuse and Three to Keep the Nascent Fusion Pore Open. Science 2012, 335, 1355–1359. [Google Scholar]
- Orwick-Rydmark, M.; Lovett, J.E.; Graziadei, A.; Lindholm, L.; Hicks, M.R.; Watts, A. Detergent-Free Incorporation of a Seven-Transmembrane Receptor Protein into Nanosized Bilayer Lipodisq Particles for Functional and Biophysical Studies. Nano Lett 2012, 12, 4687–4692. [Google Scholar]
- Ryan, R.O. Nanobiotechnology applications of reconstituted high density lipoprotein. J. Nanobiotechnology 2010, 8, 28. [Google Scholar]
- Murakami, T. Phospholipid nanodisc engineering for drug delivery systems. Biotechnol. J 2012, 7, 762–767. [Google Scholar]
- Ranaghan, M.J.; Schwall, C.T.; Alder, N.N.; Birge, R.R. Green Proteorhodopsin Reconstituted into Nanoscale Phospholipid Bilayers (Nanodiscs) as Photoactive Monomers. J. Am. Chem. Soc 2011, 133, 18318–18327. [Google Scholar]
- Lu, W.; Lieber, C.M. Nanoelectronics from the bottom up. Nat. Mater 2007, 6, 841–850. [Google Scholar]
- Vladimir, V; Mitin, V.A.K.; Stroscio Michael, A. Introduction to Nanoelectronics: Science, Nanotechnology, Engineering, and Applications; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Noy, A.; Artyukhin, A.B.; Huang, S.C.; Martinez, J.A.; Misra, N. Functional integration of membrane proteins with nanotube and nanowire transistor devices. Methods Mol. Biol 2011, 751, 533–552. [Google Scholar]
- Holden, M.A.; Needham, D.; Bayley, H. Functional bionetworks from nanoliter water droplets. J. Am. Chem. Soc 2007, 129, 8650–8655. [Google Scholar]
- Maglia, G.; Heron, A.J.; Hwang, W.L.; Holden, M.A.; Mikhailova, E.; Li, Q.H.; Cheley, S.; Bayley, H. Droplet networks with incorporated protein diodes show collective properties. Nat. Nanotechnol 2009, 4, 437–440. [Google Scholar] [Green Version]
- Sapra, K.T.; Bayley, H. Lipid-coated hydrogel shapes as components of electrical circuits and mechanical devices. Sci. Rep 2012, 2, 848–857. [Google Scholar]
- Weiss, N.O.; Zhou, H.; Liao, L.; Liu, Y.; Jiang, S.; Huang, Y.; Duan, X. Graphene: an emerging electronic material. Adv. Mater 2012, 24, 5782–5825. [Google Scholar]
- Ang, P.K.; Jaiswal, M.; Lim, C.H.Y.X.; Wang, Y.; Sankaran, J.; Li, A.; Lim, C.T.; Wohland, T.; Barbaros, O.; Loh, K.P. A Bioelectronic Platform Using a Graphene-Lipid Bilayer Interface. ACS Nano 2010, 4, 7387–7394. [Google Scholar]
- Liu, J.Y.; Guo, S.J.; Han, L.; Wang, T.S.; Hong, W.; Liu, Y.Q.; Wang, E.K. Synthesis of phospholipid monolayer membrane functionalized graphene for drug delivery. J. Mater. Chem 2012, 22, 20634–20640. [Google Scholar]
- Frost, R.; Jonsson, G.E.; Chakarov, D.; Svedhem, S.; Kasemo, B. Graphene Oxide and Lipid Membranes: Interactions and Nanocomposite Structures. Nano Lett 2012, 12, 3356–3362. [Google Scholar]
- Lenhert, S.; Brinkmann, F.; Laue, T.; Walheim, S.; Vannahme, C.; Klinkhammer, S.; Xu, M.; Sekula, S.; Mappes, T.; Schimmel, T.; et al. Lipid multilayer gratings. Nat. Nanotechnol 2010, 5, 275–279. [Google Scholar]
- Letellier, D.; Sandre, O.; Menager, C.; Cabuil, V.; Lavergne, M. Magnetic tubules. Mat. Sci. Eng. C 1997, 5, 153–162. [Google Scholar]
- Yang, B.; Kamiya, S.; Shimizu, Y.; Koshizaki, N.; Shimizu, T. Glycolipid nanotube hollow cylinders as substrates: Fabrication of one-dimensional metallic-organic nanocomposites and metal nanowires. Chem. Mater 2004, 16, 2826–2831. [Google Scholar]
- Zhao, Y.; Mahaja, N.; Fang, J.Y. Self-assembled cylindrical lipid tubules with a birefringent core. Small 2006, 2, 364–367. [Google Scholar]
- Nafday, O.A.; Lowry, T.W.; Lenhert, S. Multifunctional Lipid Multilayer Stamping. Small 2012, 8, 1021–1028. [Google Scholar]
- Lyubartsev, A.P.; Rabinovich, A.L. Recent development in computer simulations of lipid bilayers. Soft Matter 2011, 7, 25–39. [Google Scholar]
- Vattulainen, I.; Rog, T. Lipid Simulations: A Perspective on Lipids in Action. Csh Perspect Biol 2011, 3, a004655. [Google Scholar]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem..Soc. Faraday Trans 1976, 2, 1525–1566. [Google Scholar]
- Cooke, I.R.; Deserno, M. Coupling between lipid shape and membrane curvature. Biophys. J 2006, 91, 487–495. [Google Scholar]
- Nishizawa, M.; Nishizawa, K. Curvature-driven lipid sorting: coarse-grained dynamics simulations of a membrane mimicking a hemifusion intermediate. J. BioPhys. Chem 2010, 1, 86–95. [Google Scholar]
- Tian, A.; Baumgart, T. Sorting of lipids and proteins in membrane curvature gradients. Biophys. J 2009, 96, 2676–2688. [Google Scholar]
- Tian, A.; Capraro, B.R.; Esposito, C.; Baumgart, T. Bending stiffness depends on curvature of ternary lipid mixture tubular membranes. Biophys. J 2009, 97, 1636–1646. [Google Scholar]
- Mannock, D.A.; Lewis, R.N.; McMullen, T.P.; McElhaney, R.N. The effect of variations in phospholipid and sterol structure on the nature of lipid-sterol interactions in lipid bilayer model membranes. Chem. Phys. Lipids 2010, 163, 403–448. [Google Scholar]
- Radhakrishnan, A.; McConnell, H. Condensed complexes in vesicles containing cholesterol and phospholipids. Proc. Natl Acad Sci. USA 2005, 102, 12662–12666. [Google Scholar]
- Wang, W.; Yang, L.; Huang, H.W. Evidence of cholesterol accumulated in high curvature regions: implication to the curvature elastic energy for lipid mixtures. Biophys. J 2007, 92, 2819–2830. [Google Scholar]
- Klauda, J.B.; Venable, R.M.; MacKerell, A.D.; Pastor, R.W. Considerations for lipid force field development. Curr. Top. Membr 2008, 60, 1–48. [Google Scholar]
- Feller, S.E.; Yin, D.X.; Pastor, R.W.; MacKerell, A.D. Molecular dynamics simulation of unsaturated lipid bilayers at low hydration: Parameterization and comparison with diffraction studies. Biophys. J 1997, 73, 2269–2279. [Google Scholar]
- Feller, S.E.; MacKerell, A.D. An improved empirical potential energy function for molecular simulations of phospholipids. J. Phys. Chem. B 2000, 104, 7510–7515. [Google Scholar]
- Klauda, J.B.; Brooks, B.R.; MacKerell, A.D.; Venable, R.M.; Pastor, R.W. An ab initio study on the torsional surface of alkanes and its effect on molecular simulations of alkanes and a DPPC bilayer. J. Phys. Chem. B 2005, 109, 5300–5311. [Google Scholar]
- Klauda, J.B.; Venable, R.M.; Freites, J.A.; O’Connor, J.W.; Tobias, D.J.; Mondragon-Ramirez, C.; Vorobyov, I.; MacKerell, A.D.; Pastor, R.W. Update of the CHARMM All-Atom Additive Force Field for Lipids: Validation on Six Lipid Types. J. Phys. Chem. B 2010, 114, 7830–7843. [Google Scholar]
- Jensen, M.O.; Mouritsen, O.G.; Peters, G.H. Simulations of a membrane-anchored peptide: Structure, dynamics, and influence on bilayer properties. Biophys. J 2004, 86, 3556–3575. [Google Scholar]
- Hyvonen, M.T.; Kovanen, P.T. Molecular dynamics simulations of unsaturated lipid bilayers: effects of varying the numbers of double bonds. Eur. Biophys. J 2005, 34, 294–305. [Google Scholar]
- Sonne, J.; Jensen, M.O.; Hansen, F.Y.; Hemmingsen, L.; Peters, G.H. Reparameterization of all-atom dipalmitoylphosphatidylcholine lipid parameters enables simulation of fluid bilayers at zero tension. Biophys. J 2007, 92, 4157–4167. [Google Scholar]
- Hogberg, C.J.; Nikitin, A.M.; Lyubartsev, A.P. Modification of the CHARMM force field for DMPC lipid bilayer. J. Comput. Chem 2008, 29, 2359–2369. [Google Scholar]
- Tieleman, D.P.; MacCallum, J.L.; Ash, W.L.; Kandt, C.; Xu, Z.T.; Monticelli, L. Membrane protein simulations with a united-atom lipid and all-atom protein model: lipid-protein interactions, side chain transfer free energies and model proteins. J. Phys.Condens Mat 2006, 18, S1221–S1234. [Google Scholar]
- Chandrasekhar, I.; Kastenholz, M.; Lins, R.D.; Oostenbrink, C.; Schuler, L.D.; Tieleman, D.P.; van Gunsteren, W.F. A consistent potential energy parameter set for lipids: dipalmitoylphosphatidylcholine as a benchmark of the GROMOS96 45A3 force field. Eur. Biophys. J 2003, 32, 67–77. [Google Scholar]
- Lindahl, E.; Hess, B.; van der Spoel, D. GROMACS 3.0: a package for molecular simulation and trajectory analysis. J. Mol. Model 2001, 7, 306–317. [Google Scholar]
- Moore, P.B.; Lopez, C.F.; Klein, M.L. Dynamical properties of a hydrated lipid bilayer from a multinanosecond molecular dynamics simulation. Biophys. J 2001, 81, 2484–2494. [Google Scholar]
- Lopez, C.F.; Nielsen, S.O.; Klein, M.L.; Moore, P.B. Hydrogen bonding structure and dynamics of water at the dimyristoylphosphatidylcholine lipid bilayer surface from a molecular dynamics simulation. J. Phys. Chem. B 2004, 108, 6603–6610. [Google Scholar]
- Siu, S.W.I.; Vacha, R.; Jungwirth, P.; Bockmann, R.A. Biomolecular simulations of membranes: Physical properties from different force fields. J. Chem. Phys 2008, 128, 125103–125115. [Google Scholar]
- Lyubartsev, A.P.; Laaksonen, A. Calculation of Effective Interaction Potentials from Radial-Distribution Functions - a Reverse Monte-Carlo Approach. Phys. Rev. E 1995, 52, 3730–3737. [Google Scholar]
- Reith, D.; Putz, M.; Muller-Plathe, F. Deriving effective mesoscale potentials from atomistic simulations. J. Comput. Chem 2003, 24, 1624–1636. [Google Scholar]
- Marrink, S.J.; de Vries, A.H.; Mark, A.E. Coarse grained model for semiquantitative lipid simulations. J. Phys. Chem. B 2004, 108, 750–760. [Google Scholar]
- Marrink, S.J.; Risselada, H.J.; Yefimov, S.; Tieleman, D.P.; de Vries, A.H. The MARTINI force field: Coarse grained model for biomolecular simulations. J. Phys. Chem. B 2007, 111, 7812–7824. [Google Scholar]
- Risselada, H.J.; Marrink, S.J. The molecular face of lipid rafts in model membranes. Proc. Nat. Acad. Sci. USA 2008, 105, 17367–17372. [Google Scholar]
- Baoukina, S.; Monticelli, L.; Risselada, H.J.; Marrink, S.J.; Tieleman, D.P. The molecular mechanism of lipid monolayer collapse. Proc. Nat. Acad. Sci. USA 2008, 105, 10803–10808. [Google Scholar]
- Wang, Z.L.; He, X.H. Dynamics of vesicle formation from lipid droplets: Mechanism and controllability. J. Chem. Phys 2009, 130, 094905–094912. [Google Scholar]
- Bennett, W.F.; MacCallum, J.L.; Tieleman, D.P. Thermodynamic analysis of the effect of cholesterol on dipalmitoylphosphatidylcholine lipid membranes. J. Am. Chem. Soc 2009, 131, 1972–1978. [Google Scholar]
- Monticelli, L.; Salonen, E.; Ke, P.C.; Vattulainen, I. Effects of carbon nanoparticles on lipid membranes: a molecular simulation perspective. Soft Matter 2009, 5, 4433–4445. [Google Scholar]
- Esteban-Martin, S.; Risselada, H.J.; Salgado, J.; Marrink, S.J. Stability of Asymmetric Lipid Bilayers Assessed by Molecular Dynamics Simulations. J. Am. Chem. Soc 2009, 131, 15194–15202. [Google Scholar]
- Risselada, H.J.; Marrink, S.J. Curvature effects on lipid packing and dynamics in liposomes revealed by coarse grained molecular dynamics simulations. Phys. Chem. Chem. Phys 2009, 11, 2056–2067. [Google Scholar]
- Hinner, M.J.; Marrink, S.J.; de Vries, A.H. Location, Tilt, and Binding: A Molecular Dynamics Study of Voltage-Sensitive Dyes in Biomembranes. J. Phys. Chem. B 2009, 113, 15807–15819. [Google Scholar]
- Reimhult, E.; Kumar, K. Membrane biosensor platforms using nano- and microporous supports. Trends Biotechnol 2008, 26, 82–89. [Google Scholar]
- Ahmad, M. Lipids in Nanotechnology; AOCS Press: Urbana, IL, USA, 2012; p. 294. [Google Scholar]
- Orsi, M.; Haubertin, D.Y.; Sanderson, W.E.; Essex, J.W. A quantitative coarse-grain model for lipid bilayers. J. Phys. Chem. B 2008, 112, 802–815. [Google Scholar]
- Orsi, M.; Michel, J.; Essex, J.W. Coarse-grain modelling of DMPC and DOPC lipid bilayers. J. Phys.Condens Mater 2010, 22, 155106–155121. [Google Scholar]
- Gao, L.H.; Shillcock, J.; Lipowsky, R. Improved dissipative particle dynamics simulations of lipid bilayers. J. Chem. Phys 2007, 126, 015101–015109. [Google Scholar]
- Shillcock, J.C.; Lipowsky, R. Tension-induced fusion of bilayer membranes and vesicles. Nat. Mater 2005, 4, 225–228. [Google Scholar]
- Hoogerbrugge, P.J.; Koelman, J.M.V.A. Simulating Microscopic Hydrodynamic Phenomena with Dissipative Particle Dynamics. Eur.Ophys. Lett 1992, 19, 155–160. [Google Scholar]
- Shillcock, J.C.; Lipowsky, R. Equilibrium structure and lateral stress distribution of amphiphilic bilayers from dissipative particle dynamics simulations. J. Chem. Phys 2002, 117, 5048–5061. [Google Scholar]
- Kwok, R.; Evans, E. Thermoelasticity of large lecithin bilayer vesicles. Biophys. J 1981, 35, 637–652. [Google Scholar]
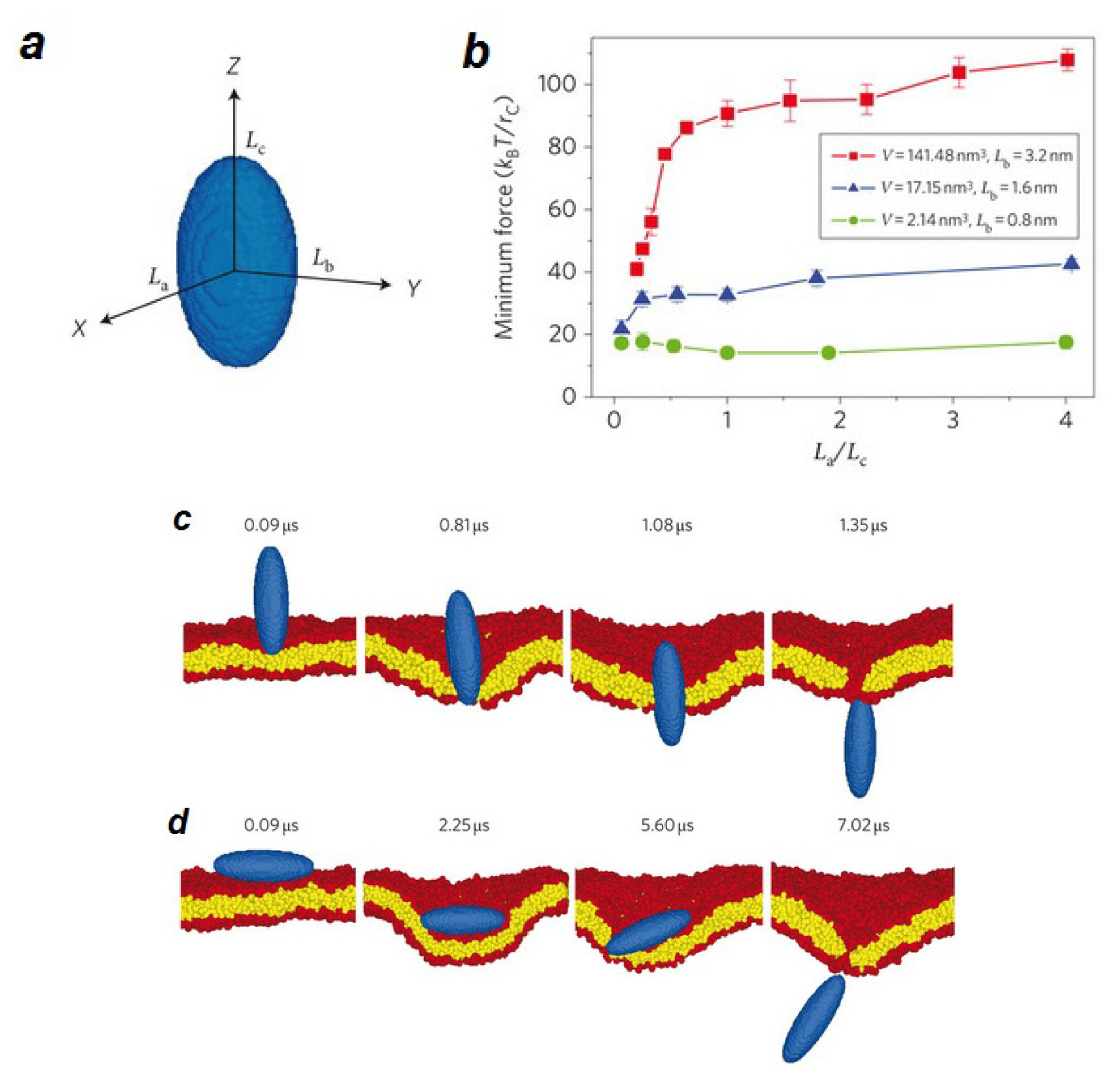
- Ding, H.M.; Tian, W.D.; Ma, Y.Q. Designing Nanoparticle Translocation through Membranes by Computer Simulations. ACS Nano 2012, 6, 1230–1238. [Google Scholar]
- Ding, H.M.; Ma, Y.Q. Role of physicochemical properties of coating ligands in receptor-mediated endocytosis of nanoparticles. Biomaterials 2012, 33, 5798–5802. [Google Scholar]
- Li, Y.; Yue, T.T.; Yang, K.; Zhang, X.R. Molecular modeling of the relationship between nanoparticle shape anisotropy and endocytosis kinetics. Biomaterials 2012, 33, 4965–4973. [Google Scholar]
- Shi, X.H.; von dem Bussche, A.; Hurt, R.H.; Kane, A.B.; Gao, H.J. Cell entry of one-dimensional nanomaterials occurs by tip recognition and rotation. Nat. Nanotechnol 2011, 6, 714–719. [Google Scholar]
- Nielsen, S.O.; Ensing, B.; Ortiz, V.; Moore, P.B.; Klein, M.L. Lipid bilayer perturbations around a transmembrane nanotube: A coarse grain molecular dynamics study. Biophys. J 2005, 88, 3822–3828. [Google Scholar]
- Venturoli, M.; Smit, B.; Sperotto, M.M. Simulation studies of protein-induced bilayer deformations, and lipid-induced protein tilting, on a mesoscopic model for lipid bilayers with embedded proteins. Biophys. J 2005, 88, 1778–1798. [Google Scholar]
- Yang, K.; Ma, Y.Q. Computer simulation of the translocation of nanoparticles with different shapes across a lipid bilayer. Nat. Nanotechnol 2010, 5, 579–583. [Google Scholar]
- Gurtovenko, A.A.; Anwar, J. Ion transport through chemically induced pores in protein-free phospholipid membranes. J. Phys. Chem. B 2007, 111, 13379–13382. [Google Scholar]
- Gurtovenko, A.A.; Onike, O.I.; Anwar, J. Chemically induced phospholipid translocation across biological membranes. Langmuir 2008, 24, 9656–9660. [Google Scholar]
- Marrink, S.J.; Lindahl, E.; Edholm, O.; Mark, A.E. Simulation of the spontaneous aggregation of phospholipids into bilayers. J. Am. Chem. Soc 2001, 123, 8638–8639. [Google Scholar]
- Marrink, S.J.; de Vries, A.H.; Tieleman, D.P. Lipids on the move: Simulations of membrane pores, domains, stalks and curves. Bba-Biomembranes 2009, 1788, 149–168. [Google Scholar]
- Tieleman, D.P. The molecular basis of electroporation. Biophys. J 2004, 86, 371a–372a. [Google Scholar]
- Bockmann, R.A.; de Groot, B.L.; Kakorin, S.; Neumann, E.; Grubmuller, H. Kinetics, statistics, and energetics of lipid membrane electroporation studied by molecular dynamics simulations. Biophys. J 2008, 95, 1837–1850. [Google Scholar]
- Tieleman, D.P.; Leontiadou, H.; Mark, A.E.; Marrink, S.J. Simulation of pore formation in lipid bilayers by mechanical stress and electric fields. J. Am. Chem. Soc 2003, 125, 6382–6383. [Google Scholar]
- Tarek, M. Membrane electroporation: A molecular dynamics simulation. Biophys. J 2005, 88, 4045–4053. [Google Scholar]
- Vernier, P.T.; Ziegler, M.J. Nanosecond field alignment of head group and water dipoles in electroporating phospholipid bilayers. J. Phys. Chem. B 2007, 111, 12993–12996. [Google Scholar]
- Vernier, P.T.; Ziegler, M.J.; Sun, Y.H.; Chang, W.V.; Gundersen, M.A.; Tieleman, D.P. Nanopore formation and phosphatidylserine externalization in a phospholipid bilayer at high transmembrane potential. J. Am. Chem. Soc 2006, 128, 6288–6289. [Google Scholar]
- Hu, Q.; Joshi, R.P.; Schoenbach, K.H. Simulations of nanopore formation and phosphatidylserine externalization in lipid membranes subjected to a high-intensity, ultrashort electric pulse. Phys. Rev. E 2005, 72, 031902–031912. [Google Scholar]
- Ziegler, M.J.; Vernier, P.T. Interface water dynamics and porating electric fields for phospholipid bilayers. J. Phys. Chem. B 2008, 112, 13588–13596. [Google Scholar]
- Vernier, P.T.; Ziegler, M.J.; Sun, Y.H.; Gundersen, M.A.; Tieleman, D.P. Nanopore-facilitated, voltage-driven phosphatidylserine translocation in lipid bilayers–in cells and in silico. Phys. Biol 2006, 3, 233–247. [Google Scholar]
- Gurtovenko, A.A.; Vattulainen, I. Pore formation coupled to ion transport through lipid membranes as induced by transmembrane ionic charge imbalance: Atomistic molecular dynamics study. J. Am. Chem. Soc 2005, 127, 17570–17571. [Google Scholar]
- Gurtovenko, A.A.; Vattulainen, I. Ion leakage through transient water pores in protein-free lipid membranes driven by transmembrane ionic charge imbalance. Biophys. J 2007, 92, 1878–1890. [Google Scholar]
- Kandasamy, S.K.; Larson, R.G. Cation and anion transport through hydrophilic pores in lipid bilayers. J. Chem. Phys 2006, 125, 074901–074910. [Google Scholar]
- Tolpekina, T.V.; den Otter, W.K.; Briels, W.J. Nucleation free energy of pore formation in an amphiphilic bilayer studied by molecular dynamics simulations. J. Chem. Phys 2004, 121, 12060–12066. [Google Scholar]
- Tolpekina, T.V.; den Otter, W.K.; Briels, W.J. Simulations of stable pores in membranes: System size dependence and line tension. J. Chem. Phys 2004, 121, 8014–8020. [Google Scholar]
- Tieleman, D.P.; Marrink, S.J. Lipids out of equilibrium: Energetics of desorption and pore mediated flip-flop. J. Am. Chem. Soc 2006, 128, 12462–12467. [Google Scholar]
- Pasenkiewicz-Gierula, M.; Murzyn, K.; Rog, T.; Czaplewski, C. Molecular dynamics simulation studies of lipid bilayer systems. Acta Biochim. Pol 2000, 47, 601–611. [Google Scholar]
- Murzyn, K.; Rog, T.; Pasenkiewicz-Gierula, M. Interactions of magainin-2 amide with membrane lipids. Comput. Sci 2004, 3037, 325–331. [Google Scholar]
- Leontiadou, H.; Mark, A.E.; Marrink, S.J. Antimicrobial peptides in action. J. Am. Chem. Soc 2006, 128, 12156–12161. [Google Scholar]
- Leontiadou, H.; Mark, A.E.; Marrink, S.J. Ion transport across transmembrane pores. Biophys. J 2007, 92, 4209–4215. [Google Scholar]
- de Vries, A.H.; Mark, A.E.; Marrink, S.J. Molecular dynamics simulation of the spontaneous formation of a small DPPC vesicle in water in atomistic detail. J. Am. Chem. Soc 2004, 126, 4488–4489. [Google Scholar]
- Gurtovenko, A.A.; Vattulainen, I. Molecular mechanism for lipid flip-flops. J. Phys. Chem. B 2007, 111, 13554–13559. [Google Scholar]
- Bennett, W.F.D.; MacCallum, J.L.; Hinner, M.J.; Marrink, S.J.; Tieleman, D.P. Molecular View of Cholesterol Flip-Flop and Chemical Potential in Different Membrane Environments. J. Am. Chem. Soc 2009, 131, 12714–12720. [Google Scholar]
- Yang, K.; Ma, Y.Q. Computer simulations of fusion, fission and shape deformation in lipid membranes. Soft Matter 2012, 8, 606–618. [Google Scholar]
- Kozlovsky, Y.; Chernomordik, L.V.; Kozlov, M.M. Lipid intermediates in membrane fusion: Formation, structure, and decay of hemifusion diaphragm. Biophys. J 2002, 83, 2634–2651. [Google Scholar]
- Marrink, S.J.; Mark, A.E. The mechanism of vesicle fusion as revealed by molecular dynamics simulations. J. Am. Chem. Soc 2003, 125, 11144–11145. [Google Scholar]
- Safran, S.A. Statistical thermodynamics of surfaces, interfaces, and membranes; Addison-Wesley Pub.: Boston, MA, USA, 1994; p. 270. [Google Scholar]
- Shillcock, J.C.; Lipowsky, R. The computational route from bilayer membranes to vesicle fusion. J. Phys. Condens Matter 2006, 18, S1191–S1219. [Google Scholar]
- Smeijers, A.F.; Markvoort, A.J.; Pieterse, K.; Hilbers, P.A.J. A detailed look at vesicle fusion. J. Phys. Chem. B 2006, 110, 13212–13219. [Google Scholar]
- Stevens, M.J.; Hoh, J.H.; Woolf, T.B. Insights into the molecular mechanism of membrane fusion from simulation: Evidence for the association of splayed tails. Phys. Rev. Lett. 2003, 91. [Google Scholar]
- Mirjanian, D.; Dickey, A.N.; Hoh, J.H.; Woolf, T.B.; Stevens, M.J. Splaying of Aliphatic Tails Plays a Central Role in Barrier Crossing During Liposome Fusion. J. Phys. Chem. B 2010, 114, 11061–11068. [Google Scholar]
- Li, D.W.; Liu, X.Y. Examination of membrane fusion by dissipative particle dynamics simulation and comparison with continuum elastic models. J. Chem. Phys 2005, 122, 174909–174917. [Google Scholar]
- Noguchi, H.; Takasu, M. Fusion pathways of vesicles: A Brownian dynamics simulation. J. Chem. Phys 2001, 115, 9547–9551. [Google Scholar]
- Kasson, P.M.; Kelley, N.W.; Singhal, N.; Vrljic, M.; Brunger, A.T.; Pande, V.S. Ensemble molecular dynamics yields submillisecond kinetics and intermediates of membrane fusion. Proc. Nat. Acad. Sci. USA 2006, 103, 11916–11921. [Google Scholar]
- Grafmuller, A.; Shillcock, J.; Lipowsky, R. Dissipative particle dynamics of tension-induced membrane fusion. Mol. Simulat 2009, 35, 554–560. [Google Scholar]
- Smirnova, Y.G.; Marrink, S.J.; Lipowsky, R.; Knecht, V. Solvent-Exposed Tails as Prestalk Transition States for Membrane Fusion at Low Hydration. J. Am. Chem. Soc 2010, 132, 6710–6718. [Google Scholar]
- Knecht, V.; Marrink, S.J. Molecular dynamics simulations of lipid vesicle fusion in atomic detail. Biophys. J 2007, 92, 4254–4261. [Google Scholar]
- Grafmuller, A.; Shillcock, J.; Lipowsky, R. The Fusion of Membranes and Vesicles: Pathway and Energy Barriers from Dissipative Particle Dynamics. Biophys. J 2009, 96, 2658–2675. [Google Scholar]
- Gao, L.H.; Lipowsky, R.; Shillcock, J. Tension-induced vesicle fusion: pathways and pore dynamics. Soft Matter 2008, 4, 1208–1214. [Google Scholar]
- Veatch, S.L.; Keller, S.L. Organization in lipid membranes containing cholesterol. Phys. Rev. Lett 2002, 89, 268101. [Google Scholar]
- Baumgart, T.; Hess, S.T.; Webb, W.W. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature 2003, 425, 821–824. [Google Scholar]
- Bacia, K.; Schwille, P.; Kurzchalia, T. Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes. Proc. Natl Acad Sci. USA 2005, 102, 3272–3277. [Google Scholar]
- Li, L.; Liang, X.; Lin, M.; Qiu, F.; Yang, Y. Budding dynamics of multicomponent tubular vesicles. J. Am. Chem. Soc 2005, 127, 17996–17997. [Google Scholar]
- Hamada, T.; Miura, Y.; Ishii, K.; Araki, S.; Yoshikawa, K.; Vestergaard, M.; Takagi, M. Dynamic processes in endocytic transformation of a raft-exhibiting giant liposome. J. Phys. Chem. B 2007, 111, 10853–10857. [Google Scholar]
- Cooke, I.R.; Kremer, K.; Deserno, M. Tunable generic model for fluid bilayer membranes. Phys. Rev. E 2005, 72, 011506. [Google Scholar]
- Laradji, M.; Sunil Kumar, P.B. Dynamics of domain growth in self-assembled fluid vesicles. Phys. Rev. Lett 2004, 93, 198105. [Google Scholar]
- Yamamoto, S.; Hyodo, S. Budding and fission dynamics of two-component vesicles. J. Chem. Phys 2003, 118, 7937–7943. [Google Scholar]
- Hong, B.B.; Qiu, F.; Zhang, H.D.; Yang, Y.L. Budding dynamics of individual domains in multicomponent membranes simulated by N-varied dissipative particle dynamics. J. Phys. Chem. B 2007, 111, 5837–5849. [Google Scholar]
- Devaux, P.F.; Lopez-Montero, I.; Bryde, S. Proteins involved in lipid translocation in eukaryotic cells. Chem. Phys. Lipids 2006, 141, 119–132. [Google Scholar]
- Ramachandran, S.; Kumar, P.B.; Laradji, M. Lipid flip-flop driven mechanical and morphological changes in model membranes. J. Chem. Phys 2008, 129, 125104. [Google Scholar]
- Seifert, U. Configurations of fluid membranes and vesicles. Adv. Phys 1997, 46, 13–137. [Google Scholar]
- Miao, L.; Seifert, U.; Wortis, M.; Dobereiner, H.G. Budding Transitions of Fluid-Bilayer Vesicles - the Effect of Area-Difference Elasticity. Phys. Rev. E 1994, 49, 5389–5407. [Google Scholar]
- Inaoka, Y.; Yamazaki, M. Vesicle fission of giant unilamellar vesicles of liquid-ordered-phase membranes induced by amphiphiles with a single long hydrocarbon chain. Langmuir 2007, 23, 720–728. [Google Scholar]
- Yue, T.T.; Li, S.Y.; Zhang, X.R.; Wang, W.C. The relationship between membrane curvature generation and clustering of anchored proteins: a computer simulation study. Soft Matter 2010, 6, 6109–6118. [Google Scholar]
- Andes-Koback, M.; Keating, C.D. Complete Budding and Asymmetric Division of Primitive Model Cells To Produce Daughter Vesicles with Different Interior and Membrane Compositions. J. Am. Chem. Soc 2011, 133, 9545–9555. [Google Scholar]
- Noguchi, H.; Takasu, M. Adhesion of nanoparticles to vesicles: A Brownian dynamics simulation. Biophys. J 2002, 83, 299–308. [Google Scholar]
- Fakhri, N.; Tsyboulski, D.A.; Cognet, L.; Weisman, R.B.; Pasquali, M. Diameter-dependent bending dynamics of single-walled carbon nanotubes in liquids. Proc. Nat. Acad. Sci. USA 2009, 106, 14219–14223. [Google Scholar]
- Liu, Z.; Chen, K.; Davis, C.; Sherlock, S.; Cao, Q.Z.; Chen, X.Y.; Dai, H.J. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res 2008, 68, 6652–6660. [Google Scholar]
- Kraszewski, S.; Bianco, A.; Tarek, M.; Ramseyer, C. Insertion of Short Amino-Functionalized Single-Walled Carbon Nanotubes into Phospholipid Bilayer Occurs by Passive Diffusion. PLoS One 2012, 7, e40703. [Google Scholar]
- Wallace, E.J.; Sansom, M.S.P. Blocking of carbon nanotube based nanoinjectors by lipids: A simulation study. Nano Lett 2008, 8, 2751–2756. [Google Scholar]
- Gangupomu, V.K.; Capaldi, F.M. Interactions of Carbon Nanotube with Lipid Bilayer Membranes. J. Nanomater 2011, 830436, 1–6. [Google Scholar]
- Shityakov, S.; Dandekar, T. Molecular Dynamics Simulation of Popc and Pope Lipid Membrane Bilayers Enforced by an Intercalated Single-Wall Carbon Nanotube. Nano 2011, 6, 19–29. [Google Scholar]
- Bakry, R.; Vallant, R.M.; Najam-Ul-Haq, M.; Rainer, M.; Szabo, Z.; Huck, C.W.; Bonn, G.K. Medicinal applications of fullerenes. Int. J. Nanomed 2007, 2, 639–649. [Google Scholar]
- Kraszewski, S.; Tarek, M.; Ramseyer, C. Uptake and translocation mechanisms of cationic amino derivatives functionalized on pristine C60 by lipid membranes: a molecular dynamics simulation study. ACS Nano 2011, 5, 8571–8578. [Google Scholar]
- Jusufi, A.; DeVane, R.H.; Shinoda, W.; Klein, M.L. Nanoscale carbon particles and the stability of lipid bilayers. Soft Matter 2011, 7, 1139–1146. [Google Scholar]
- Lin, X.B.; Li, Y.; Gu, N. Nanoparticle’s Size Effect on Its Translocation Across a Lipid Bilayer: A Molecular Dynamics Simulation. J. Comput. Theor. Nanos 2010, 7, 269–276. [Google Scholar]
- Titov, A.V.; Kral, P.; Pearson, R. Sandwiched graphene--membrane superstructures. ACS Nano 2010, 4, 229–234. [Google Scholar]
- Yamamoto, S.; Maruyama, Y.; Hyodo, S. Dissipative particle dynamics study of spontaneous vesicle formation of amphiphilic molecules. J. Chem. Phys 2002, 117, 2990. [Google Scholar]
- Lai, K.; Wang, B.; Zhang, Y.; Zheng, Y. Computer simulation study of nanoparticle interaction with a lipid membrane under mechanical stress. Phys. Chem. Chem. Phys 2012, 15, 270–278. [Google Scholar]
- Lin, J.Q.; Zheng, Y.G.; Zhang, H.W.; Chen, Z. A simulation study on nanoscale holes generated by gold nanoparticles on negative lipid bilayers. Langmuir 2011, 27, 8323–8332. [Google Scholar]
- Li, Y.; Gu, N. Computer Simulation of the Inclusion of Hydrophobic Nanoparticles into a Lipid Bilayer. J. Nanosci. Nanotech 2010, 10, 7616–7619. [Google Scholar]
- Lin, J.Q.; Zhang, H.W.; Chen, Z.; Zheng, Y.G. Penetration of Lipid Membranes by Gold Nanoparticles: Insights into Cellular Uptake, Cytotoxicity, and Their Relationship. ACS Nano 2010, 4, 5421–5429. [Google Scholar]
- Wong-Ekkabut, J.; Baoukina, S.; Triampo, W.; Tang, I.M.; Tieleman, D.P.; Monticelli, L. Computer simulation study of fullerene translocation through lipid membranes. Nat. Nanotechnol 2008, 3, 363–368. [Google Scholar]
- Smith, W.; Forester, T.R. DL_POLY_2.0: A general-purpose parallel molecular dynamics simulation package. J. Mol. Graphics 1996, 14, 136–141. [Google Scholar]
- Smondyrev, A.M.; Berkowitz, M.L. Structure of dipalmitoylphosphatidylcholine/cholesterol bilayer at low and high cholesterol concentrations: molecular dynamics simulation. Biophys. J 1999, 77, 2075–2089. [Google Scholar]
- Smondyrev, A.M.; Berkowitz, M.L. United atom force field for phospholipid membranes: Constant pressure molecular dynamics simulation of dipalmitoylphosphatidicholine/water system. J. Comput. Chem 1999, 20, 531–545. [Google Scholar]
- Fiedler, S.L.; Violi, A. Simulation of Nanoparticle Permeation through a Lipid Membrane. Biophys. J 2010, 99, 144–152. [Google Scholar]
- Eun, C.; Berkowitz, M.L. Molecular dynamics simulation study of the water-mediated interaction between zwitterionic and charged surfaces. J. Chem. Phys 2012, 136, 024501–024507. [Google Scholar]
- Zimmerli, U.; Koumoutsakos, P. Simulations of electrophoretic RNA transport through transmembrane carbon nanotubes. Biophys. J 2008, 94, 2546–2557. [Google Scholar]
- Liu, B.; Li, X.Y.; Li, B.L.; Xu, B.Q.; Zhao, Y.L. Carbon Nanotube Based Artificial Water Channel Protein: Membrane Perturbation and Water Transportation. Nano Lett 2009, 9, 1386–1394. [Google Scholar]
- Walde, P. Phospholipid Membranes as Regulators of Localized Activity. Chem. Biol 2010, 17, 922–923. [Google Scholar]
- Tsuji, A.; Yoshikawa, K. ON-OFF Switching of Transcriptional Activity of Large DNA through a Conformational Transition in Cooperation with Phospholipid Membrane. J. Am. Chem. Soc 2010, 132, 12464–12471. [Google Scholar]
- Liu, C.; Ming, W.; An, J. Esben Thormann, Andra Dėdinaitė Hyaluronan and phospholipids in boundary lubrication. Soft Matter 2012, 8, 10241–10244. [Google Scholar]
- Mark, W.; Grinstaff, C.A.H.P.; Li, Y.; Luo, D.; Tom, J. MacIntosh Charge Switchable Helper Lipids for Gene Delivery. Mol. Ther. 2006, 13, S206. [Google Scholar]
- Backus, E.H.G.; Kuiper, J.M.; Engberts, J.B.F.N.; Poolman, B.; Bonn, M. Reversible Optical Control of Monolayers on Water through Photoswitchable Lipids. J. Phys. Chem. B 2011, 115, 2294–2302. [Google Scholar]
- Duan, P.F.; Li, Y.G.; Li, L.C.; Deng, J.G.; Liu, M.H. Multiresponsive Chiroptical Switch of an Azobenzene-Containing Lipid: Solvent, Temperature, and Photoregulated Supramolecular Chirality. J. Phys. Chem. B 2011, 115, 3322–3329. [Google Scholar]
- Qiao, W.H.; Zheng, Z.B.; Peng, H. Synthesis of switchable amphipathic molecules triggered by CO2 through carbonyl-amine condensation. Eur. J. Lipid Sci. Tech 2011, 113, 841–847. [Google Scholar]





| Nano-object | Size of the nano-object | Lipid | Lipid structure | Simulation time | Force field/Software | Water model | Ref |
|---|---|---|---|---|---|---|---|
| Gold Nanoparticle | Radius of gyration:11.45 Å, Gold core:11.13 Å | DPPC/DPPG (3:1) | Bilayer | 40 ns | MARTINI | P4 | [289] |
| Fullerene | 1 nm | Pure DOPC, Pure DPPC | Bilayer | 88 μs | MARTINI | P4 | [290] |
| C180, C60 C20 | 1.2, 0.72, 0.4 nm | Pure DPPC, Pure DLPC, Pure DSPC | Bilayer | 800 ns | MARTINI | P4 | [286] |
| C60, C68H29 | 1.07 ×1.1 nm2 | DMPC/Cholesterol (3:1) | Bilayer | 1.1 ps | DL_POLY 2.17 GUI [291], UA-OPLS [292,293] GROMACS v.3.3.1 and v. 4.0.5 | TIP3P | [294] |
| Graphene | 5.9 × 6.2 nm2 | POPC | Bilayer | 516 ns | MARTINI | P4 | [284] |
| Graphene | 2.425 × 2.380 nm2 | CRPC | PC-Plate | 10 ns | GROMOS | SPC/E | [295] |
| CNT | L = 2.35 nm, R = 1.87 nm | DMPC | Bilayer | 1.58ns | AMBER 96 | TIP3P | [296] |
| Hydrophobic NP | 10 nm | DPPC | Bilayer | 20 ns | MARTINI | P4 | [42] |
| Semihydrophilic NP | 10nm | DPPC | Bilayer | 15 ns | MARTINI | P4 | [42] |
| CG NP | 6.8 nm | DPPC | Bilayer | 320 ns | MARTINI | P4 | [41] |
| CNT | L = 20 Å, R = 10 Å | Pure POPC, POPC/Cholesterol (7:3) | Bilayer | 6 ns | CHARMM | TIP3 | [278] |
| DWCNTs | L =33 Å, R =3.4Å | DMPC | Monomer | 3 ns | CHARM27 | TIP3 | [297] |
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mashaghi, S.; Jadidi, T.; Koenderink, G.; Mashaghi, A. Lipid Nanotechnology. Int. J. Mol. Sci. 2013, 14, 4242-4282. https://doi.org/10.3390/ijms14024242
Mashaghi S, Jadidi T, Koenderink G, Mashaghi A. Lipid Nanotechnology. International Journal of Molecular Sciences. 2013; 14(2):4242-4282. https://doi.org/10.3390/ijms14024242
Chicago/Turabian StyleMashaghi, Samaneh, Tayebeh Jadidi, Gijsje Koenderink, and Alireza Mashaghi. 2013. "Lipid Nanotechnology" International Journal of Molecular Sciences 14, no. 2: 4242-4282. https://doi.org/10.3390/ijms14024242
APA StyleMashaghi, S., Jadidi, T., Koenderink, G., & Mashaghi, A. (2013). Lipid Nanotechnology. International Journal of Molecular Sciences, 14(2), 4242-4282. https://doi.org/10.3390/ijms14024242





