Effect of Artocarpus communis Extract on UVB Irradiation-Induced Oxidative Stress and Inflammation in Hairless Mice
Abstract
:1. Introduction
2. Results and Discussion
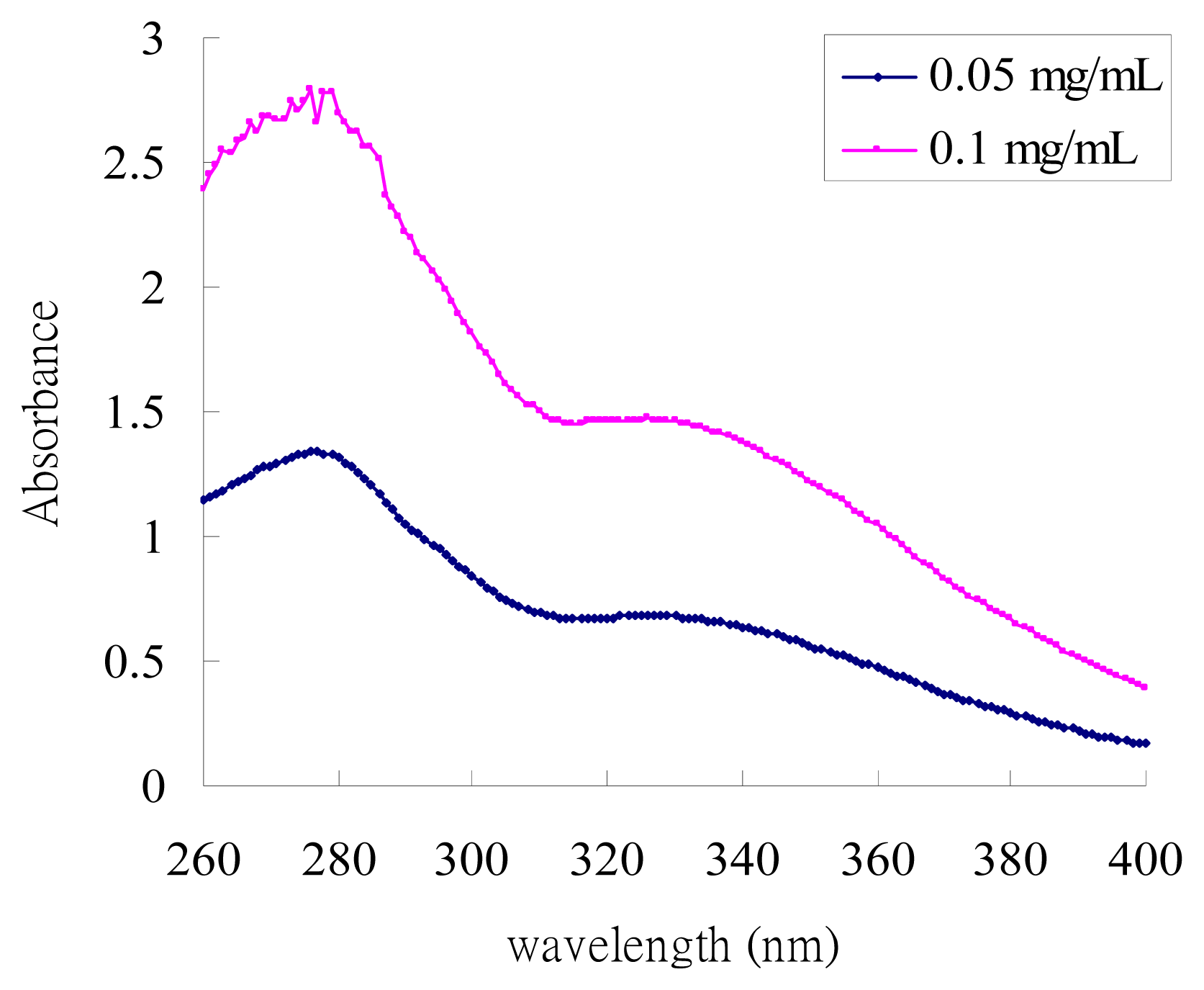
2.1. UV-Absorption Properties of ACM
2.2. Effects of ACM on the Skin Morphology and Histopathological Changes in UVB-Induced Skin Damage
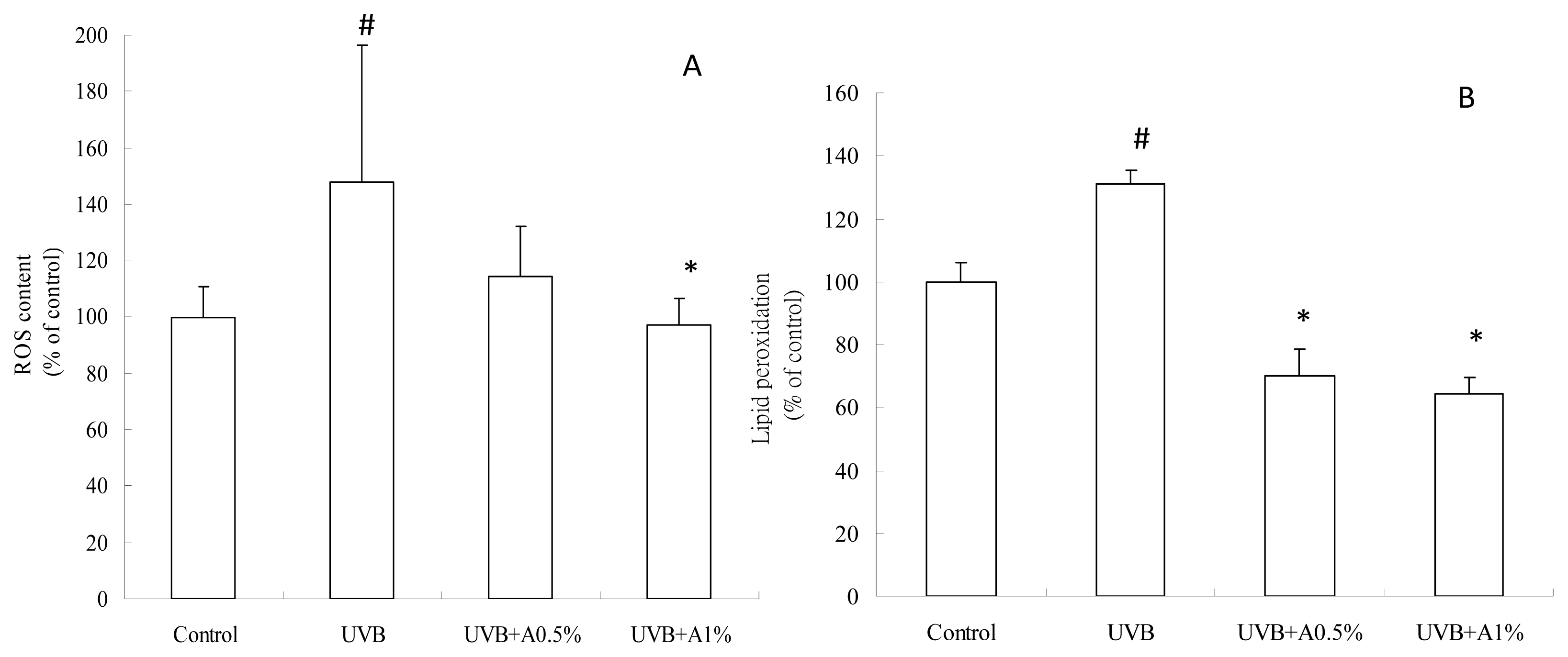
2.3. Antioxidant Activity of ACM on UVB-Induced Oxidative Stress
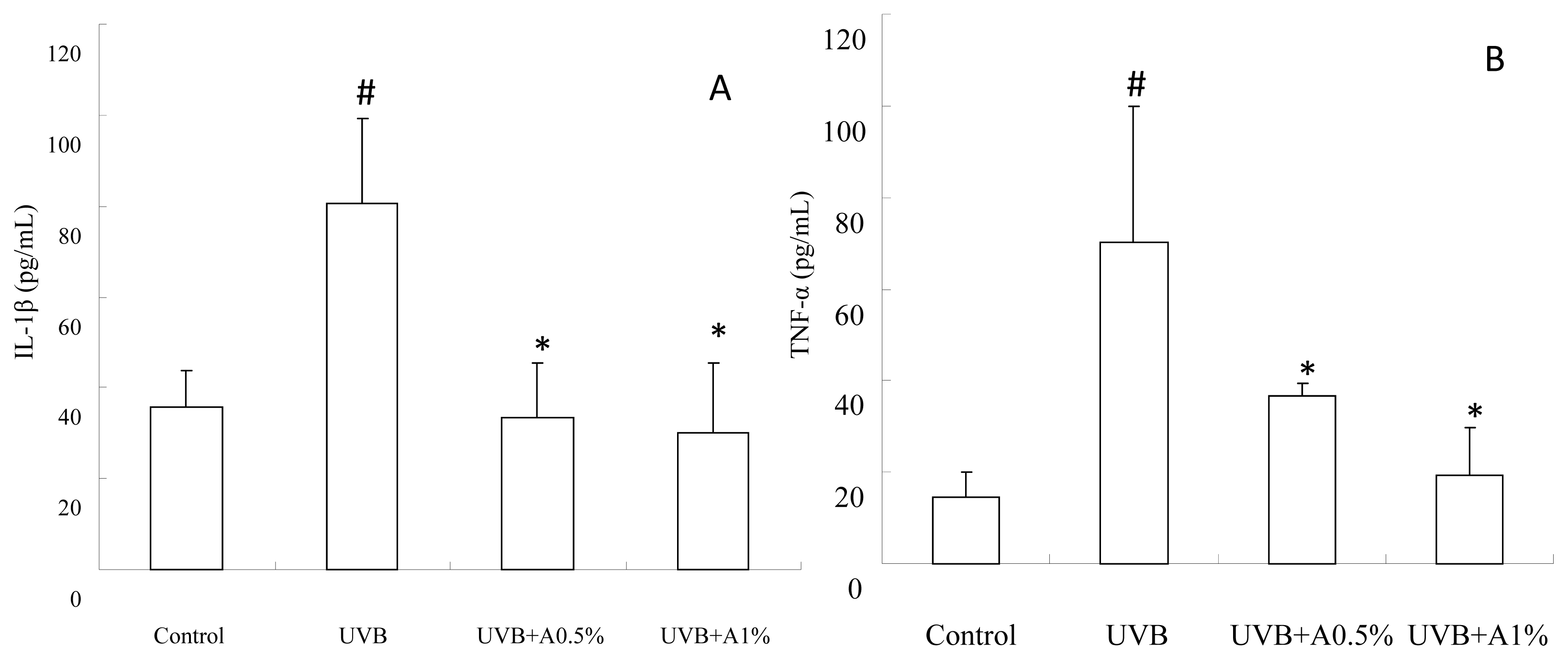
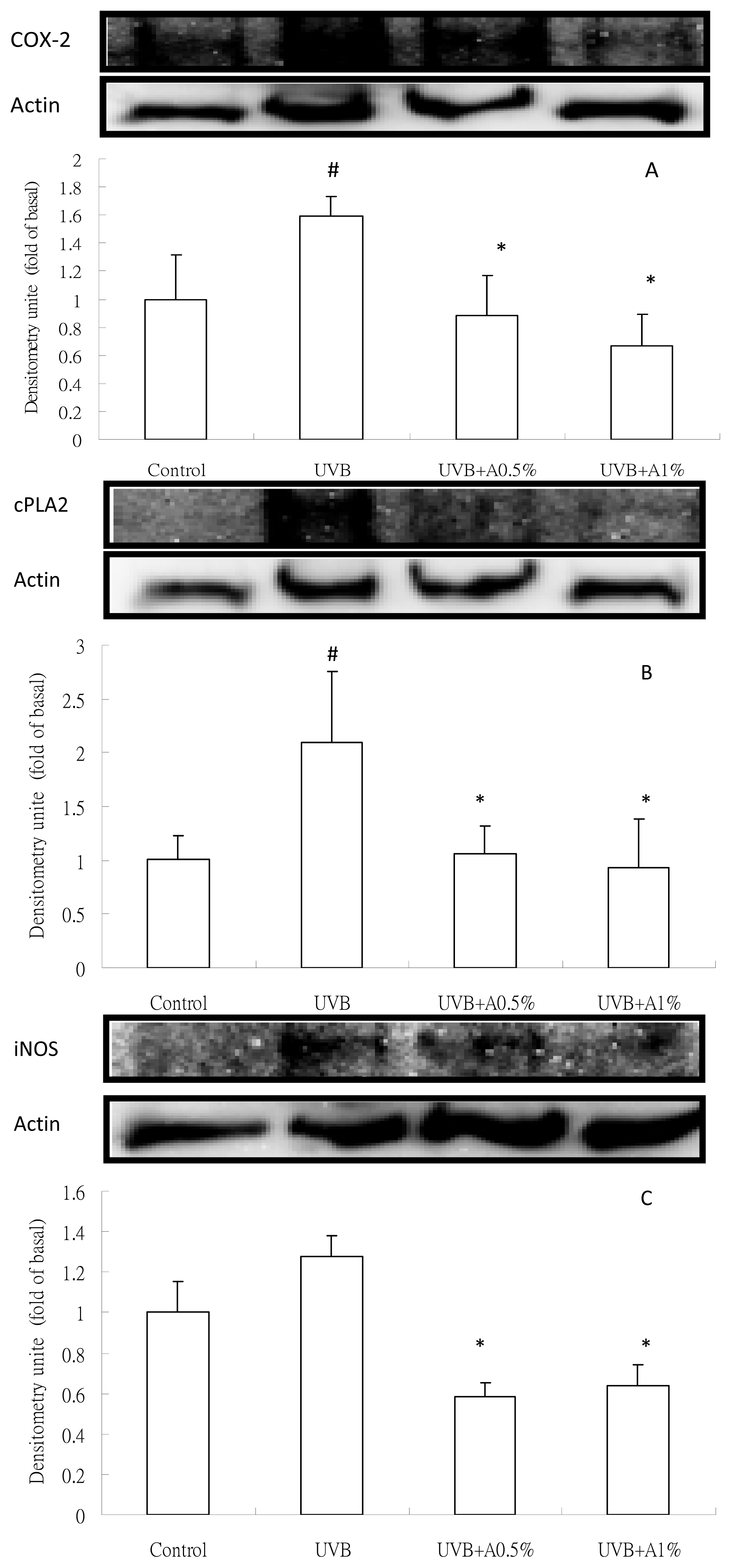
2.4. Anti-Inflammatory Activity of ACM on Cytokine Levels in UVB-Induced Skin Inflammation
3. Experimental Section
3.1. A. communis Methanol Extract (ACM) and Its Preparation of Topical Administration
3.2. UV Absorption Spectrum of ACM
3.3. Administration of ACM on UVB Irradiation-Induced Skin Damage
3.4. Histopathological Observation of the Skin
3.5. Determination of ROS Content
3.6. Determination of Anti-Lipid Peroxidation
3.7. Determination of Inflammatory Cytokines
3.8. Analysis of Inflammatory Protein Expression by Western Blot
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Hruza, L.L.; Pentland, A.P. Mechanisms of UV-induced inflammation. J. Invest. Dermatol 1993, 100, 35S–41S. [Google Scholar]
- Brash, D.E. Sunlight and the onset of skin cancer. Trends Genet 1997, 13, 410–414. [Google Scholar]
- Schwarz, T. Photoimmunosuppression. Photodermatol. Photoimmunol. Photomed 2002, 18, 141–145. [Google Scholar]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci 2008, 30, 87–95. [Google Scholar]
- Zhang, X.; Rosenstein, B.S.; Wang, Y.; Lebwohl, M.; Wei, H. Identification of possible reactive oxygen species involved in ultraviolet radiation-induced oxidative DNA damage. Free Radic. Biol. Med 1997, 23, 980–985. [Google Scholar]
- Kerr, A.; Ferguson, J. Photoallergic contact dermatitis. Photodermatol. Photoimmunol. Photomed 2010, 26, 56–65. [Google Scholar]
- Antoniou, C.; Kosmadaki, M.G.; Stratigos, A.J.; Katsambas, A.D. Photoaging: Prevention and topical treatments. Am. J. Clin. Dermatol 2010, 11, 95–102. [Google Scholar]
- Afaq, F.; Katiyar, S.K. Polyphenols: Skin photoprotection and inhibition of photocarcinogenesis. Mini Rev. Med. Chem 2011, 11, 1200–1215. [Google Scholar]
- Burke, J.R.; Davern, L.B.; Stanley, P.L.; Gregor, K.R.; Banville, J.; Remillard, R.; Russell, J.W.; Brassil, P.J.; Witmer, M.R.; Johnson, G.; et al. BMS-229724 is a tight-binding inhibitor of cytosolic phospholipase A2 that acts at the lipid/water interface and possesses anti-inflammatory activity in skin inflammation models. J. Pharmacol. Exp. Ther 2001, 298, 376–385. [Google Scholar]
- Chen, X.; Gresham, A.; Morrison, A.; Pentland, A.P. Oxidative stress mediates synthesis of cytosolic phospholipase A2 after UVB injury. Biochim. Biophys. Acta 1996, 1299, 23–33. [Google Scholar]
- Isoherranen, K.; Punnonen, K.; Jansen, C.; Uotila, P. Ultraviolet irradiation induces cyclooxygenase-2 expression in keratinocytes. Br. J. Dermatol 1999, 140, 1017–1022. [Google Scholar]
- Medeiros, R.; Figueiredo, C.P.; Passos, G.F.; Calixto, J.B. Reduced skin inflammatory response in mice lacking inducible nitric oxide synthase. Biochem. Pharmacol 2009, 78, 390–395. [Google Scholar]
- Jones, S.M.; Mathew, C.M.; Dixey, J.; Lovell, C.R.; McHugh, N.J. VCAM-1 expression on endothelium in lesions from cutaneous lupus erythematosus is increased compared with systemic and localized scleroderma. Br. J. Dermatol 1996, 135, 678–686. [Google Scholar]
- Kang, T.H.; Park, H.M.; Kim, Y.B.; Kim, H.; Kim, N.; Do, J.H.; Kang, C.; Cho, Y.; Kim, S.Y. Effects of red ginseng extract on UVB irradiation-induced skin aging in hairless mice. J. Ethnopharmacol 2009, 123, 446–451. [Google Scholar]
- Sumiyoshi, M.; Kimura, Y. Effects of a turmeric extract (Curcuma longa) on chronic ultraviolet B irradiation-induced skin damage in melanin-possessing hairless mice. Phytomedicine 2009, 16, 1137–1143. [Google Scholar]
- Tsoyi, K.; Park, H.B.; Kim, Y.M.; Chung, J.I.; Shin, S.C.; Lee, W.S.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.J. Anthocyanins from black soybean seed coats inhibit UVB-induced inflammatory cylooxygenase-2 gene expression and PGE2 production through regulation of the nuclear factor-kappaB and phosphatidylinositol 3-kinase/Akt pathway. J. Agric. Food Chem 2008, 56, 8969–8974. [Google Scholar]
- Casagrande, R.; Georgetti, S.R.; Verri, W.A., Jr; Dorta, D.J.; dos Santos, A.C.; Fonseca, M.J. Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. J. Photochem. Photobiol. B 2006, 84, 21–27. [Google Scholar]
- Han, A.R.; Kang, Y.J.; Windono, T.; Lee, S.K.; Seo, E.K. Prenylated flavonoids from the heartwood of Artocarpus communis with inhibitory activity on lipopolysaccharide-induced nitric oxide production. J. Nat. Prod 2006, 69, 719–721. [Google Scholar]
- Shimizu, K.; Fukuda, M.; Kondo, R.; Sakai, K. The 5 alpha-reductase inhibitory components from heartwood of Artocarpus incisus: structure-activity investigations. Planta Med 2000, 66, 16–19. [Google Scholar]
- Shimizu, K.; Kondo, R.; Sakai, K.; Lee, S.H.; Sato, H. The inhibitory components from Artocarpus incisus on melanin biosynthesis. Planta Med 1998, 64, 408–412. [Google Scholar]
- Pitaksuteepong, T.; Somsiri, A.; Waranuch, N. Targeted transfollicular delivery of artocarpin extract from Artocarpus incisus by means of microparticles. Eur. J. Pharm. Biopharm 2007, 67, 639–645. [Google Scholar]
- Fang, S.C.; Hsu, C.L.; Yu, Y.S.; Yen, G.C. Cytotoxic effects of new geranyl chalcone derivatives isolated from the leaves of Artocarpus communis in SW 872 human liposarcoma cells. J. Agric. Food Chem 2008, 56, 8859–8868. [Google Scholar]
- Erb, P.; Ji, J.; Kump, E.; Mielgo, A.; Wernli, M. Apoptosis and pathogenesis of melanoma and nonmelanoma skin cancer. Adv. Exp. Med. Biol 2008, 624, 283–295. [Google Scholar]
- Pupe, A.; Degreef, H.; Garmyn, M. Induction of tumor necrosis factor-alpha by UVB: A role for reactive oxygen intermediates and eicosanoids. Photochem. Photobiol 2003, 78, 68–74. [Google Scholar]
- Vayalil, P.K.; Elmets, C.A.; Katiyar, S.K. Treatment of green tea polyphenols in hydrophilic cream prevents UVB-induced oxidation of lipids and proteins, depletion of antioxidant enzymes and phosphorylation of MAPK proteins in SKH-1 hairless mouse skin. Carcinogenesis 2003, 24, 927–936. [Google Scholar]
- Kimura, Y.; Sumiyoshi, M. Effects of baicalein and wogonin isolated from Scutellaria baicalensis roots on skin damage in acute UVB-irradiated hairless mice. Eur. J. Pharmacol 2011, 661, 124–132. [Google Scholar]
- Moloney, S.J.; Edmonds, S.H.; Giddens, L.D.; Learn, D.B. The hairless mouse model of photoaging: evaluation of the relationship between dermal elastin, collagen, skin thickness and wrinkles. Photochem. Photobiol 1992, 56, 505–511. [Google Scholar]
- Afaq, F.; Adhami, V.M.; Mukhtar, H. Photochemoprevention of ultraviolet B signaling and photocarcinogenesis. Mutat. Res 2005, 571, 153–173. [Google Scholar]
- Molho-Pessach, V.; Lotem, M. Ultraviolet radiation and cutaneous carcinogenesis. Curr. Probl. Dermatol 2007, 35, 14–27. [Google Scholar]
- Hsieh, C.L.; Yen, G.C.; Chen, H.Y. Antioxidant activities of phenolic acids on ultraviolet radiation-induced erythrocyte and low density lipoprotein oxidation. J. Agric. Food Chem 2005, 53, 6151–6155. [Google Scholar]
- Kohler, H.B.; Huchzermeyer, B.; Martin, M.; de Bruin, A.; Meier, B.; Nolte, I. TNF-alpha dependent NF-kappa B activation in cultured canine keratinocytes is partly mediated by reactive oxygen species. Vet. Dermatol 2001, 12, 129–137. [Google Scholar]
- Ullrich, S.E. The role of epidermal cytokines in the generation of cutaneous immune reactions and ultraviolet radiation-induced immune suppression. Photochem. Photobiol 1995, 62, 389–401. [Google Scholar]
- Inomata, S.; Matsunaga, Y.; Amano, S.; Takada, K.; Kobayashi, K.; Tsunenaga, M.; Nishiyama, T.; Kohno, Y.; Fukuda, M. Possible involvement of gelatinases in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J. Invest. Dermatol 2003, 120, 128–134. [Google Scholar]
- Zhuang, L.; Wang, B.; Shinder, G.A.; Shivji, G.M.; Mak, T.W.; Sauder, D.N. TNF receptor p55 plays a pivotal role in murine keratinocyte apoptosis induced by ultraviolet B irradiation. J. Immunol 1999, 162, 1440–1447. [Google Scholar]
- Schwarz, A.; Bhardwaj, R.; Aragane, Y.; Mahnke, K.; Riemann, H.; Metze, D.; Luger, T.A.; Schwarz, T. Ultraviolet-B-induced apoptosis of keratinocytes: evidence for partial involvement of tumor necrosis factor-alpha in the formation of sunburn cells. J. Invest. Dermatol 1995, 104, 922–927. [Google Scholar]
- Orteu, C.H.; Sontheimer, R.D.; Dutz, J.P. The pathophysiology of photosensitivity in lupus erythematosus. Photodermatol. Photoimmunol. Photomed 2001, 17, 95–113. [Google Scholar]
- Sethi, G.; Sodhi, A. In vitro activation of murine peritoneal macrophages by ultraviolet B radiation: Upregulation of CD18, production of NO, proinflammatory cytokines and a signal transduction pathway. Mol. Immunol 2004, 40, 1315–1323. [Google Scholar]
- Kim, S.Y.; Kim, S.J.; Lee, J.Y.; Kim, W.G.; Park, W.S.; Sim, Y.C.; Lee, S.J. Protective effects of dietary soy isoflavones against UV-induced skin-aging in hairless mouse model. J. Am. Coll. Nutr 2004, 23, 157–162. [Google Scholar]
- Ebaid, H.; Salem, A.; Sayed, A.; Metwalli, A. Whey protein enhances normal inflammatory responses during cutaneous wound healing in diabetic rats. Lipids Health Dis 2011, 10, 235–244. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem 1979, 95, 351–358. [Google Scholar]
- Filip, A.; Clichici, S.; Daicoviciu, D.; Catoi, C.; Bolfa, P.; Postescu, I.D.; Gal, A.; Baldea, I.; Gherman, C.; Muresan, A. Chemopreventive effects of Calluna vulgaris and Vitis vinifera extracts on UVB-induced skin damage in SKH-1 hairless mice. J. Physiol. Pharmacol 2011, 62, 385–392. [Google Scholar]

© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, C.-W.; Ko, H.-H.; Chai, C.-Y.; Chen, W.-T.; Lin, C.-C.; Yen, F.-L. Effect of Artocarpus communis Extract on UVB Irradiation-Induced Oxidative Stress and Inflammation in Hairless Mice. Int. J. Mol. Sci. 2013, 14, 3860-3873. https://doi.org/10.3390/ijms14023860
Lee C-W, Ko H-H, Chai C-Y, Chen W-T, Lin C-C, Yen F-L. Effect of Artocarpus communis Extract on UVB Irradiation-Induced Oxidative Stress and Inflammation in Hairless Mice. International Journal of Molecular Sciences. 2013; 14(2):3860-3873. https://doi.org/10.3390/ijms14023860
Chicago/Turabian StyleLee, Chiang-Wen, Horng-Huey Ko, Chee-Yin Chai, Wan-Tzu Chen, Chun-Ching Lin, and Feng-Lin Yen. 2013. "Effect of Artocarpus communis Extract on UVB Irradiation-Induced Oxidative Stress and Inflammation in Hairless Mice" International Journal of Molecular Sciences 14, no. 2: 3860-3873. https://doi.org/10.3390/ijms14023860
APA StyleLee, C.-W., Ko, H.-H., Chai, C.-Y., Chen, W.-T., Lin, C.-C., & Yen, F.-L. (2013). Effect of Artocarpus communis Extract on UVB Irradiation-Induced Oxidative Stress and Inflammation in Hairless Mice. International Journal of Molecular Sciences, 14(2), 3860-3873. https://doi.org/10.3390/ijms14023860




