Identification and Dynamic Regulation of microRNAs Involved in Salt Stress Responses in Functional Soybean Nodules by High-Throughput Sequencing
Abstract
:1. Introduction
2. Results and Discussion
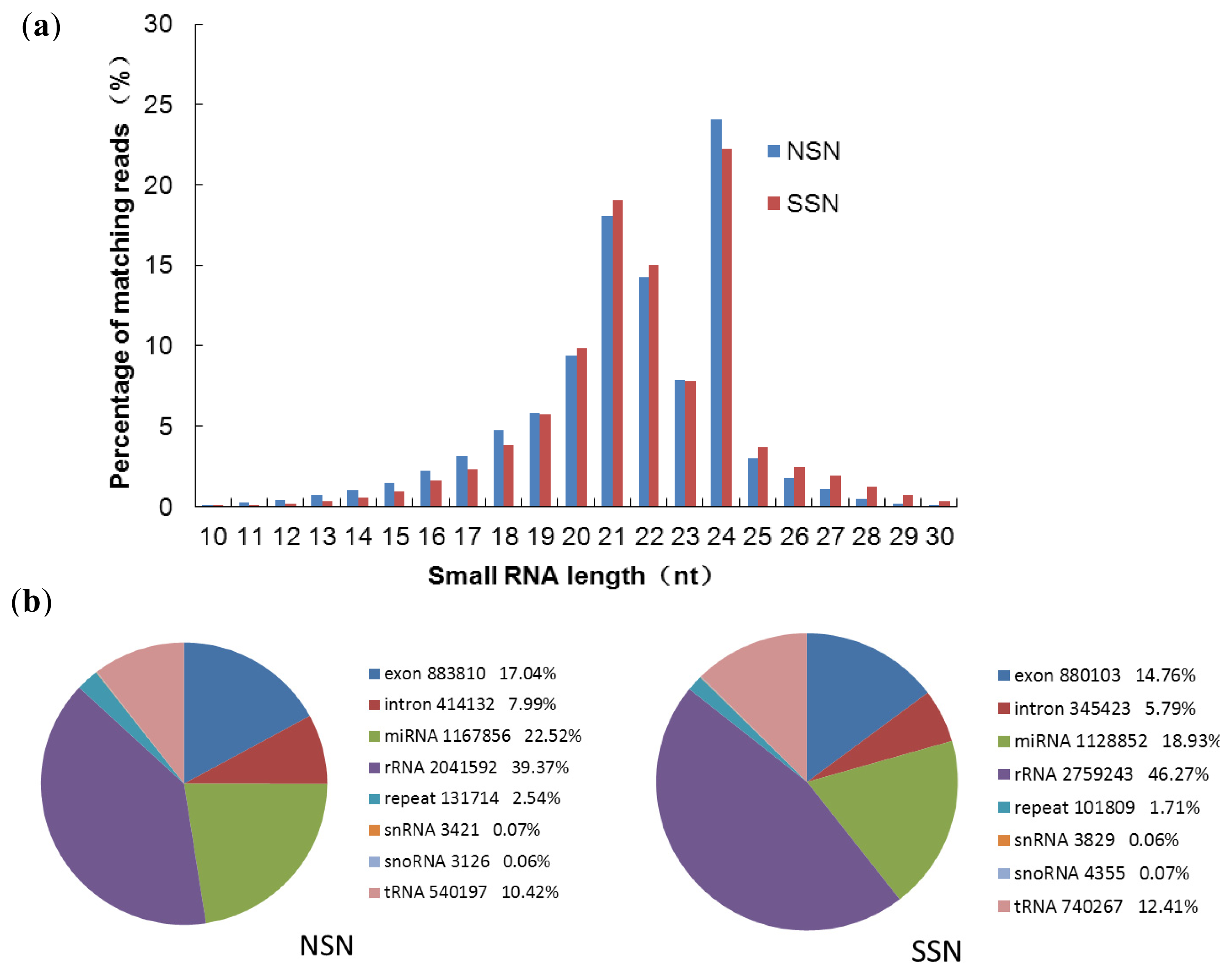
2.1. Deep Sequencing of Small RNAs of Stressed and Non-Stressed Nodules
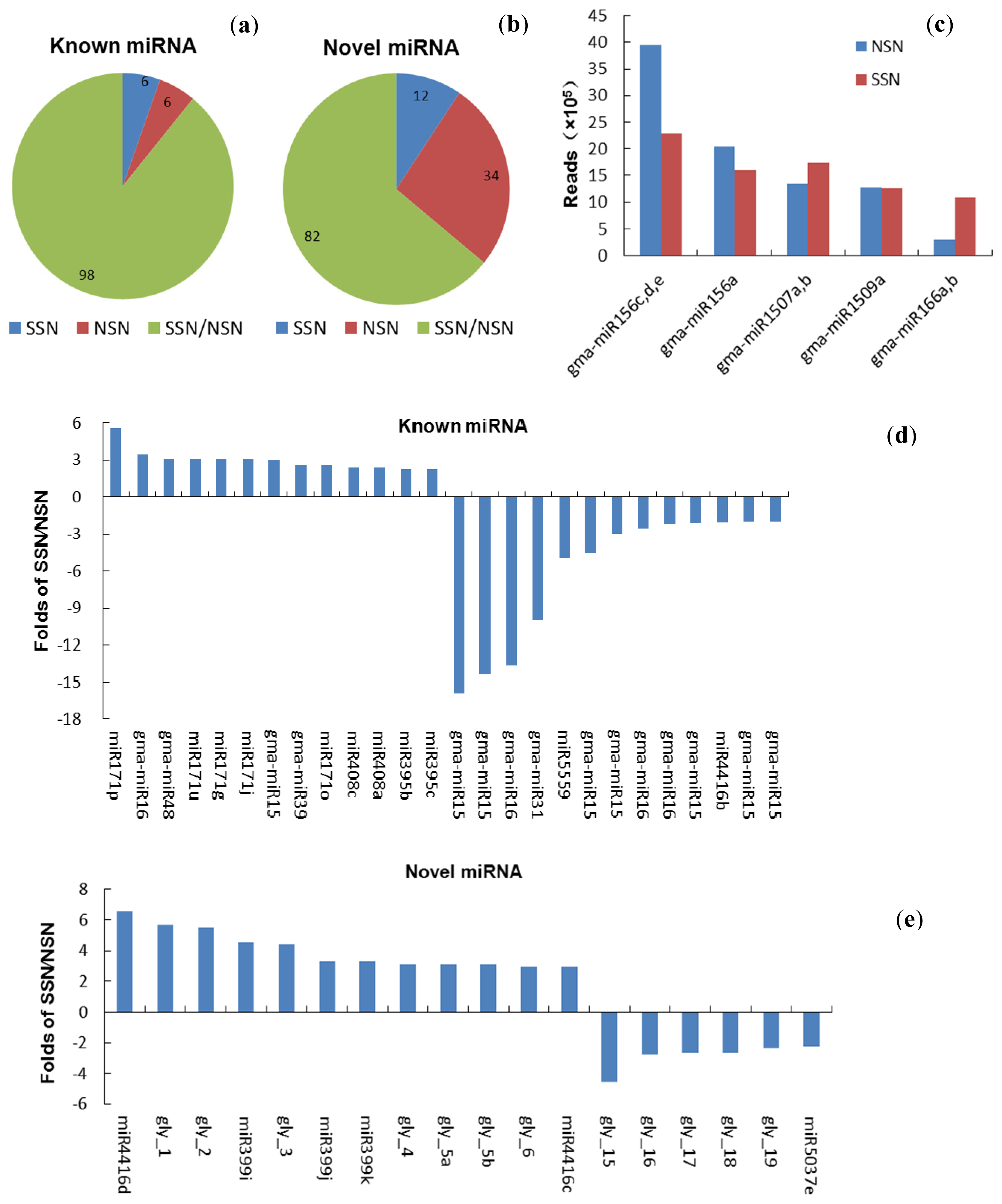
2.2. Identification and Conservation of Soybean Nodule miRNAs
2.3. Expression of Known miRNAs in Nodules under Salt Stress
2.4. Expression of Novel miRNAs in the Salt Stressed Nodules
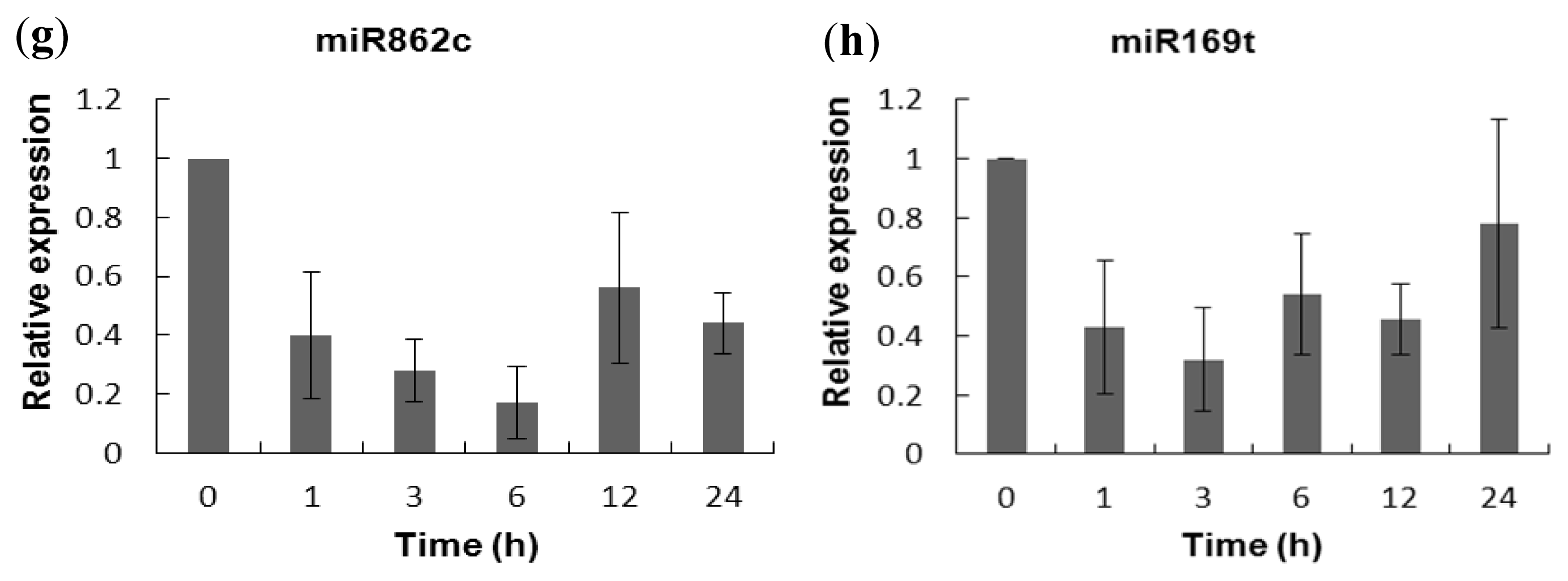
2.5. Experimental Validation of the Salt-Responsive Expression of miRNAs
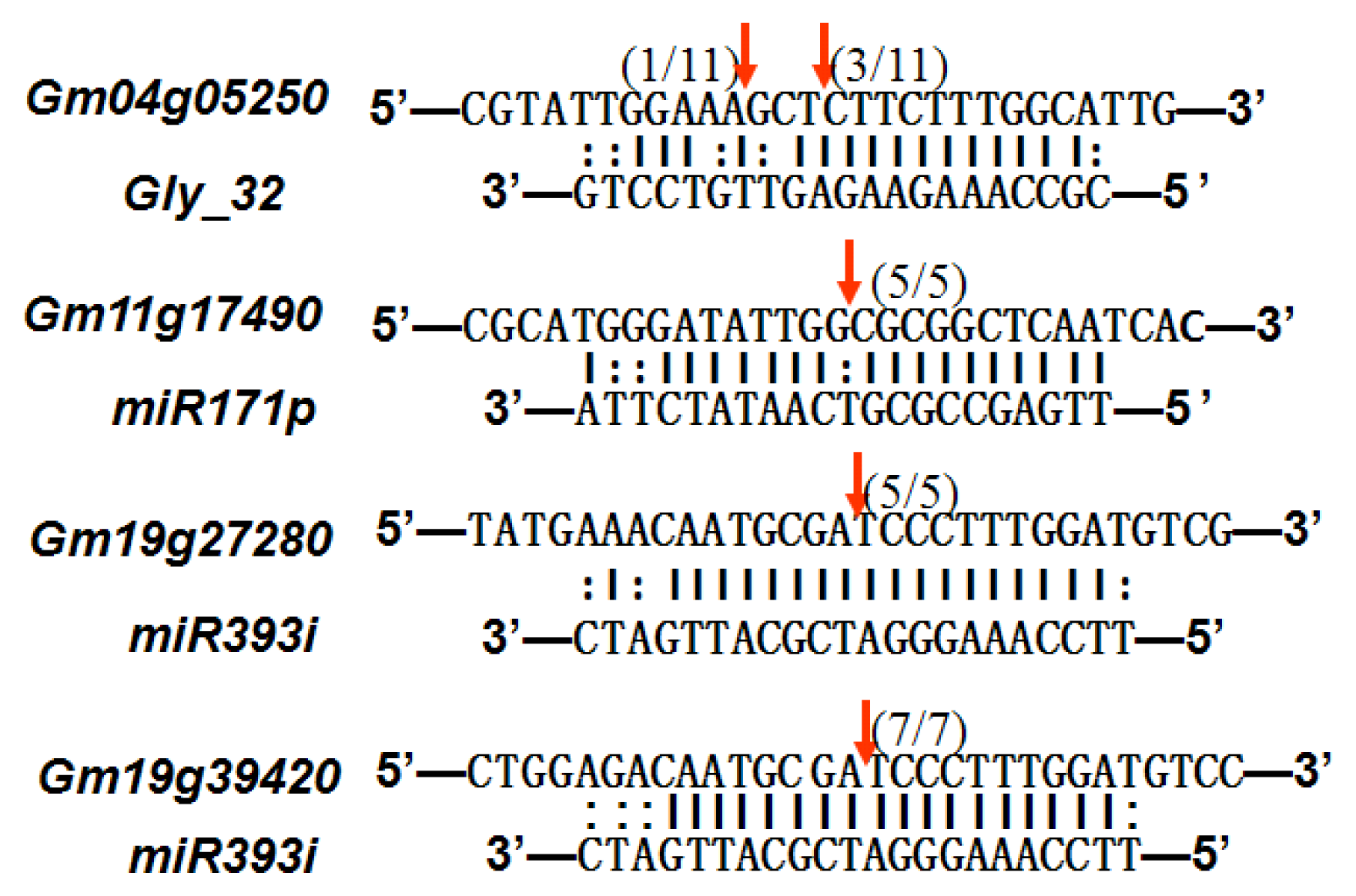
2.6. Gma-miRNAs Target Prediction
3. Experimental Section
3.1. Plant Growth Conditions, Inoculation with B. japonicum and Salt Treatment
3.2. Small RNA Library Construction and Solexa Sequencing
3.3. Data Analysis
3.4. Quantitative Real-Time PCR
3.5. Target Prediction and Experimental Validation
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Spaink, H.P. Root nodulation and infection factors produced by rhizobial bacteria. Ann. Rev. Microbiol 2000, 54, 257–288. [Google Scholar]
- Gage, D.J. Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol. Mol. Biol. Rev 2004, 68, 280–300. [Google Scholar]
- Zahran, H.H. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev 1999, 63, 968–989. [Google Scholar]
- Vance, C.P. Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant Physiol 2001, 127, 390–397. [Google Scholar]
- Graham, P.H.; Vance, C.P. Legumes: Importance and constraints to greater use. Plant Physiol 2003, 131, 872–877. [Google Scholar]
- Unkovich, M.J.; Pate, J.S. An appraisal of recent field measurements of symbiotic N2 fixation by annual legumes. Field Crops Res 2000, 65, 211–228. [Google Scholar]
- Verdoy, D.; Lucas, M.; Manrique, E.; Covarrubias, A.; de Felipe, M.; Pueyo, J. Differential organ-specific response to salt stress and water deficit in nodulated bean (Phaseolus vulgaris). Plant Cell Environ 2004, 27, 757–767. [Google Scholar]
- Natural Resources and Environment. Available online: hppt:// www.fao.org/ag/agl/agll/spush/ accessed on 10 November 2012.
- Wu, W.; Zhang, Q.; Zhu, Y.; Lam, H.M.; Cai, Z.; Guo, D. Comparative metabolic profiling reveals secondary metabolites correlated with soybean salt tolerance. J. Agric. Food Chem 2008, 56, 11132–11138. [Google Scholar]
- Ikeda, J. The effect of short term withdrawal of NaCl stress on nodulation of white clover. Plant Soil 1994, 158, 23–27. [Google Scholar]
- Coba de la Pena, T.; Verdoy, D.; Redondo, F.J.; Pueyo, J.J. Salt Tolerance in the Rhizobium-Legume Symbiosis: An Overview. In Recent Research Developments in Plant Molecular Biology; Pandalai, S.G., Ed.; CAB Direct: Oxford, UK, 2003; pp. 187–205. [Google Scholar]
- El-Hamdaoui, A.; Redondo-Nieto, M.; Rivilla, R.; Bonilla, I.; Bolanos, L. Effects of boron and calcium nutrition on the establishment of the Rhizobium leguminosarum–pea (Pisum sativum) symbiosis and nodule development under salt stress. Plant Cell Environ 2003, 26, 1003–1011. [Google Scholar]
- Pedersen, A.; Feldner, H.; Rosendahl, L. Effect of proline on nitrogenase activity in symbiosomes from root nodules of soybean (Glycine max L.) subjected to drought stress. J. Exp. Bot 1996, 47, 1533–1539. [Google Scholar]
- Matamoros, M.A.; Baird, L.M.; Escuredo, P.R.; Dalton, D.A.; Minchin, F.R.; Iturbe-Ormaetxe, I.; Rubio, M.C.; Moran, J.F.; Gordon, A.J.; Becana, M. Stress-induced legume root nodule senescence. Physiological, biochemical, and structural alterations. Plant Physiol 1999, 121, 97–112. [Google Scholar]
- Coba de la Pe a, T.; Redondo, F.J.; Manrique, E.; Lucas, M.M.; Pueyo, J.J. Nitrogen fixation persists under conditions of salt stress in transgenic Medicago truncatula plants expressing a cyanobacterial flavodoxin. Plant Biotechnol. J 2010, 8, 954–965. [Google Scholar]
- Serraj, R.; Vasquez-Diaz, H.; Drevon, J. Effects of salt stress on nitrogen fixation, oxygen diffusion, and ion distribution in soybean, common bean, and alfalfa. J. Plant Nutr 1998, 21, 475–488. [Google Scholar]
- Gargantini, P.R.; Gonzalez-Rizzo, S.; Chinchilla, D.; Raices, M.; Giammaria, V.; Ulloa, R.M.; Frugier, F.; Crespi, M.D. A CDPK isoform participates in the regulation of nodule number in Medicago truncatula. Plant J 2006, 48, 843–856. [Google Scholar]
- Carlos, M.; Mainassara, Z.A.; Faheema, K.; Nadia, F.; Ralf, H.; Diana, S.; Laurie, A.; Jean-Jacques, D.; Peter, W.; Günter, K. The salt-responsive transcriptome of chickpea roots and nodules via deepSuperSAGE. BMC Plant Biol 2011, 11, 1–26. [Google Scholar]
- Brodersen, P.; Sakvarelidze-Achard, L.; Bruun-Rasmussen, M.; Dunoyer, P.; Yamamoto, Y.Y.; Sieburth, L.; Voinnet, O. Widespread translational inhibition by plant miRNAs and siRNAs. Science 2008, 320, 1185–1190. [Google Scholar]
- Chen, X. A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 2004, 303, 2022–2025. [Google Scholar]
- Sunkar, R.; Zhu, J.K. Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 2004, 16, 2001–2019. [Google Scholar]
- Jones-Rhoades, M.W.; Bartel, D.P.; Bartel, B. MicroRNAs and their regulatory roles in plants. Ann. Rev. Plant Biol 2006, 57, 19–53. [Google Scholar]
- Shukla, L.I.; Chinnusamy, V.; Sunkar, R. The role of microRNAs and other endogenous small RNAs in plant stress responses. Biochim. Biophys. Acta 2008, 1779, 743–748. [Google Scholar]
- Gao, P.; Bai, X.; Yang, L.; Lv, D.; Pan, X.; Li, Y.; Cai, H.; Ji, W.; Chen, Q.; Zhu, Y. Osa-MIR393: A salinity- and alkaline stress-related microRNA gene. Mol. Biol. Rep 2011, 38, 237–242. [Google Scholar]
- Ding, D.; Zhang, L.; Wang, H.; Liu, Z.; Zhang, Z.; Zheng, Y. Differential expression of miRNAs in response to salt stress in maize roots. Ann. Bot 2009, 103, 29–38. [Google Scholar]
- Lu, S.; Sun, Y.H.; Chiang, V.L. Stress-responsive microRNAs in Populus. Plant J 2008, 55, 131–151. [Google Scholar]
- Liu, H.H.; Tian, X.; Li, Y.J.; Wu, C.A.; Zheng, C.C. Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. RNA 2008, 14, 836–843. [Google Scholar]
- Li, H.; Dong, Y.; Yin, H.; Wang, N.; Yang, J.; Liu, X.; Wang, Y.; Wu, J.; Li, X. Characterization of the stress associated microRNAs in Glycine max by deep sequencing. BMC Plant Biol 2011, 11, 1–12. [Google Scholar]
- Gao, P.; Bai, X.; Yang, L.; Lv, D.; Li, Y.; Cai, H.; Ji, W.; Guo, D.; Zhu, Y. Over-expression of osa-MIR396c decreases salt and alkali stress tolerance. Planta 2010, 231, 991–1001. [Google Scholar]
- Sunkar, R.; Zhou, X.; Zheng, Y.; Zhang, W.; Zhu, J.K. Identification of novel and candidate miRNAs in rice by high throughput sequencing. BMC Plant Biol 2008, 8, 1–17. [Google Scholar]
- Combier, J.P.; Frugier, F.; de Billy, F.; Boualem, A.; El-Yahyaoui, F.; Moreau, S.; Vernié, T.; Ott, T.; Gamas, P.; Crespi, M. MtHAP2-1 is a key transcriptional regulator of symbiotic nodule development regulated by microRNA169 in Medicago truncatula. Genes Dev 2006, 20, 3084–3088. [Google Scholar]
- Subramanian, S.; Fu, Y.; Sunkar, R.; Barbazuk, W.B.; Zhu, J.K.; Yu, O. Novel and nodulation-regulated microRNAs in soybean roots. BMC Genomics 2008, 9, 160–173. [Google Scholar]
- Li, H.; Deng, Y.; Wu, T.; Subramanian, S.; Yu, O. Misexpression of miR482, miR1512, and miR1515 increases soybean nodulation. Plant Physiol 2010, 153, 1759–1770. [Google Scholar]
- Lelandais-Briere, C.; Naya, L.; Sallet, E.; Calenge, F.; Frugier, F.; Hartmann, C.; Gouzy, J.; Crespi, M. Genome-wide Medicago truncatula small RNA analysis revealed novel microRNAs and isoforms differentially regulated in roots and nodules. Plant Cell 2009, 21, 2780–2796. [Google Scholar]
- Wang, Y.; Li, P.; Cao, X.; Wang, X.; Zhang, A.; Li, X. Identification and expression analysis of miRNAs from nitrogen-fixing soybean nodules. Biochem. Biophys. Res. Commun 2009, 378, 799–803. [Google Scholar]
- Turner, M.; Yu, O.; Subramanian, S. Genome organization and characteristics of soybean microRNAs. BMC Genomics 2012, 13, 169. [Google Scholar]
- Lv, S.; Nie, X.; Wang, L.; Du, X.; Biradar, S.S.; Jia, X.; Weining, S. Identification and characterization of microRNAs from Barley (Hordeum vulgare L.) by high-throughput sequencing. Int. J. Mol. Sci 2012, 13, 2973–2984. [Google Scholar]
- Song, Q.X.; Liu, Y.F.; Hu, X.Y.; Zhang, W.K.; Ma, B.; Chen, S.Y.; Zhang, J.S. Identification of miRNAs and their target genes in developing soybean seeds by deep sequencing. BMC Plant Biol 2011, 11, 1–16. [Google Scholar]
- Martins, L.; Xavier, G.; Rangel, F.; Ribeiro, J.; Neves, M.; Morgado, L.; Rumjanek, N. Contribution of biological nitrogen fixation to cowpea: A strategy for improving grain yield in the semi-arid region of Brazil. Biol. Fertil. Soils 2003, 38, 333–339. [Google Scholar]
- Mylona, P.; Pawlowski, K.; Bisseling, T. Symbiotic nitrogen fixation. Plant Cell 1995, 7, 869–885. [Google Scholar]
- Joshi, T.; Yan, Z.; Libault, M.; Jeong, D.H.; Park, S.; Green, P.; Sherrier, D.J.; Farmer, A.; May, G.; Meyers, B. Prediction of novel miRNAs and associated target genes in Glycine max. BMC Bioinform 2010, 11, 1–9. [Google Scholar]
- miRBase: The microRNA Database Version 19.0. Available online: http://www.mirbase.org/ accessed on 1 September 2012.
- The mfold Web Server. Available online: http://mfold.rna.albany.edu/?q=mfold accessed on 10 September 2012.
- Wong, C.E.; Zhao, Y.T.; Wang, X.J.; Croft, L.; Wang, Z.H.; Haerizadeh, F.; Mattick, J.S.; Singh, M.B.; Carroll, B.J.; Bhalla, P.L. MicroRNAs in the shoot apical meristem of soybean. J. Exp. Bot 2011, 62, 2495–2506. [Google Scholar]
- Zhang, B.; Pan, X.; Stellwag, E.J. Identification of soybean microRNAs and their targets. Planta 2008, 229, 161–182. [Google Scholar]
- Alonso-Peral, M.M.; Li, J.; Li, Y.; Allen, R.S.; Schnippenkoetter, W.; Ohms, S.; White, R.G.; Millar, A.A. The microRNA159-regulated GAMYB-like genes inhibit growth and promote programmed cell death in Arabidopsis. Plant Physiol 2010, 154, 757–771. [Google Scholar]
- Arenas-Huertero, C.; Pérez, B.; Rabanal, F.; Blanco-Melo, D.; de la Rosa, C.; Estrada-Navarrete, G.; Sanchez, F.; Covarrubias, A.A.; Reyes, J.L. Conserved and novel miRNAs in the legume Phaseolus vulgaris in response to stress. Plant Mol. Biol 2009, 70, 385–401. [Google Scholar]
- Kidner, C.A.; Martienssen, R.A. The developmental role of microRNA in plants. Curr. Opin. Plant Biol 2005, 8, 38–44. [Google Scholar]
- Brechenmacher, L.; Kim, M.Y.; Benitez, M.; Li, M.; Joshi, T.; Calla, B.; Lee, M.P.; Libault, M.; Vodkin, L.O.; Xu, D. Transcription profiling of soybean nodulation by Bradyrhizobium japonicum. Mol. Plant Microbe Interact 2008, 21, 631–645. [Google Scholar]
- McVay, K.; Radcliffe, D.; Hargrove, W. Winter legume effects on soil properties and nitrogen fertilizer requirements. Soil Sci. Soc. Am. J 1989, 53, 1856–1862. [Google Scholar]
- Jia, X.; Wang, W.X.; Ren, L.; Chen, Q.J.; Mendu, V.; Willcut, B.; Dinkins, R.; Tang, X.; Tang, G. Differential and dynamic regulation of miR398 in response to ABA and salt stress in Populustremula and Arabidopsisthaliana. Plant Mol. Biol 2009, 71, 51–59. [Google Scholar]
- Singh, K.B.; Foley, R.C.; Oñate-Sánchez, L. Transcription factors in plant defense and stress responses. Curr. Opin. Plant Biol 2002, 5, 430–436. [Google Scholar]
- Niu, X.; Bressan, R.A.; Hasegawa, P.M.; Pardo, J.M. Ion homeostasis in NaCl stress environments. Plant Physiol 1995, 109, 735–742. [Google Scholar]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol 2003, 6, 441–445. [Google Scholar]
- Li, W.X.; Oono, Y.; Zhu, J.; He, X.J.; Wu, J.M.; Iida, K.; Lu, X.Y.; Cui, X.; Jin, H.; Zhu, J.K. The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. Plant Cell 2008, 20, 2238–2251. [Google Scholar]
- Abe, H.; Yamaguchi-Shinozaki, K.; Urao, T.; Iwasaki, T.; Hosokawa, D.; Shinozaki, K. Role of Arabidopsis MYC and MYB homologs in drought-and abscisic acid-regulated gene expression. The Plant Cell Online 1997, 9, 1859–1868. [Google Scholar]
- Pardo, J.M.; Reddy, M.P.; Yang, S.; Maggio, A.; Huh, G.H.; Matsumoto, T.; Coca, M.A.; Paino-D’Urzo, M.; Koiwa, H.; Yun, D.J. Stress signaling through Ca2+/calmodulin-dependent protein phosphatase calcineurin mediates salt adaptation in plants. Proc. Nat. Acad. Sci 1998, 95, 9681–9686. [Google Scholar]
- Fode, B.; Siemsen, T.; Thurow, C.; Weigel, R.; Gatz, C. The Arabidopsis GRAS protein SCL14 interacts with class II TGA transcription factors and is essential for the activation of stress-inducible promoters. Plant Cell 2008, 20, 3122–3135. [Google Scholar]
- Sakamoto, H.; Maruyama, K.; Sakuma, Y.; Meshi, T.; Iwabuchi, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis Cys2/His2-type zinc-finger proteins function as transcription repressors under drought, cold, and high-salinity stress conditions. Plant Physiol 2004, 136, 2734–2746. [Google Scholar]
- Gluzman, I.; Francis, S.; Oksman, A.; Smith, C.; Duffin, K.; Goldberg, D. Order and specificity of the Plasmodium falciparum hemoglobin degradation pathway. J. Clin. Investig 1994, 93, 1602–1608. [Google Scholar]
- Miller, R.; McRae, D.; Al-Jobore, A.; Berndt, W. Respiration supported nitrogenase activity of isolated Rhizobium meliloti bacteroids. J. Cell. Biochem 1988, 38, 35–49. [Google Scholar]
- Phytozome. Available online: http://www.phytozome.net/soybean accessed on 5 July 2012.
- SOAP: Short Oligonucleotide Analysis Package. Available online: http://soap.genomics.org.cn/ accessed on 5 July 2012.
- Sanger. Available online: http://www.sanger.ac.uk/Software/Rfam accessed on 15 July 2012.
- NCBI Genbank Database. Available online: http://www.ncbi.nlm.nih.gov/blast/Blast.cgi accessed on 15 July 2012.
- MicroRNA Discovery by Deep Sequencing. Available online: http://sourceforge.net/projects/mireap/ accessed on 27 July 2012.
- Meyers, B.C.; Axtell, M.J.; Bartel, B.; Bartel, D.P.; Baulcombe, D.; Bowman, J.L.; Cao, X.; Carrington, J.C.; Chen, X.; Green, P.J. Criteria for annotation of plant microRNAs. Plant Cell 2008, 20, 3186–3190. [Google Scholar]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 2003, 31, 3406–3415. [Google Scholar]
- psRNATarget: A Plant Small RNA Target Analysis Server. Available online: http://plantgrn.noble.org/psRNATarget/ accessed on 12 August 2012.
- Schwab, R.; Palatnik, J.F.; Riester, M.; Schommer, C.; Schmid, M.; Weigel, D. Specific effects of microRNAs on the plant transcriptome. Dev. Cell 2005, 8, 517–527. [Google Scholar]


| Name | Target gene | Function | Alignment between target and miRNA | Expectation (E) |
|---|---|---|---|---|
| gly_1 | Glyma17g37430 | B-Box Zinc Finger |  | 3.0 |
| Glyma04g03110 | Thioredoxin-Related |  | 3.0 | |
| Glyma15g06000 | Glucosyl/Glucuronosyl Transferases | 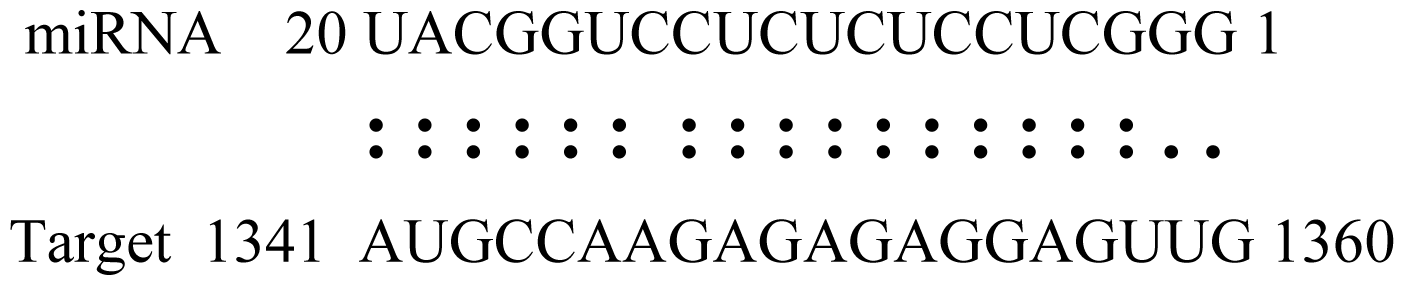 | 3.0 | |
| gly_3 | Glyma09g37130 | Lipoate-Protein Ligase | 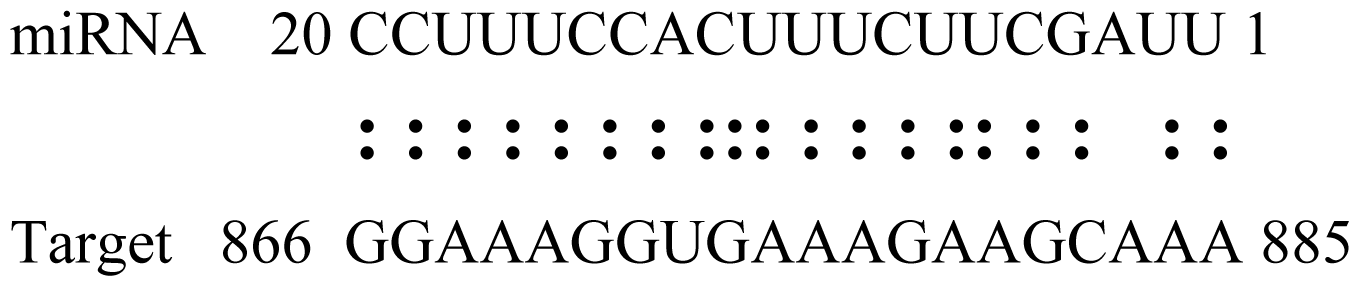 | 1.5 |
| Glyma16g27790 | PPR Repeat Pentatricopetide Repeat-Containing Protein | 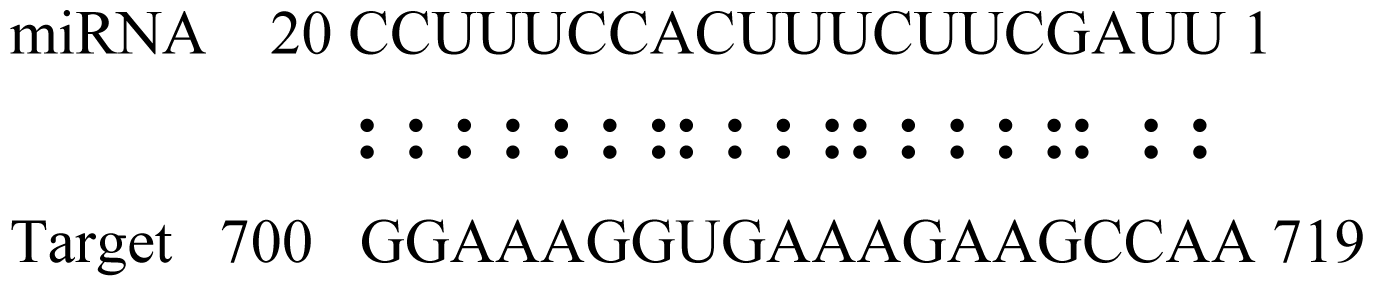 | 1.5 | |
| gly_5 | Glyma10g34820 | Drug Transporter-Related |  | 3.0 |
| gly_6 | Glyma02g05090 | Lupus La Ribonucleoprotein | 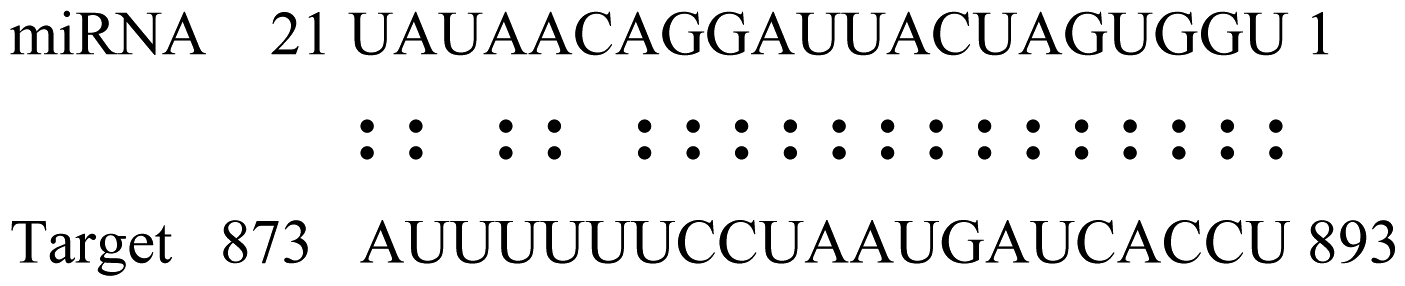 | 3.0 |
| gly_7 | Glyma19g45260 | K+ Potassium Transporter | 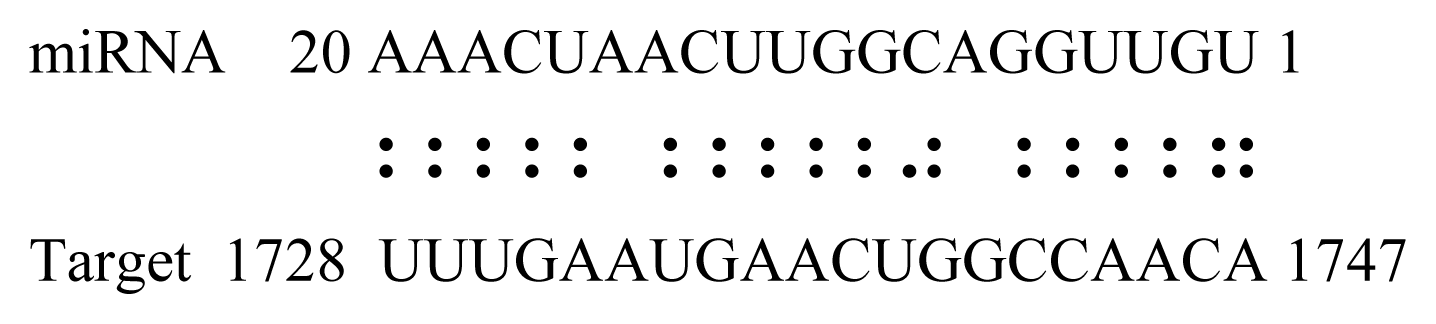 | 3.0 |
| gly_10 | Glyma04g43550 | POT Family | 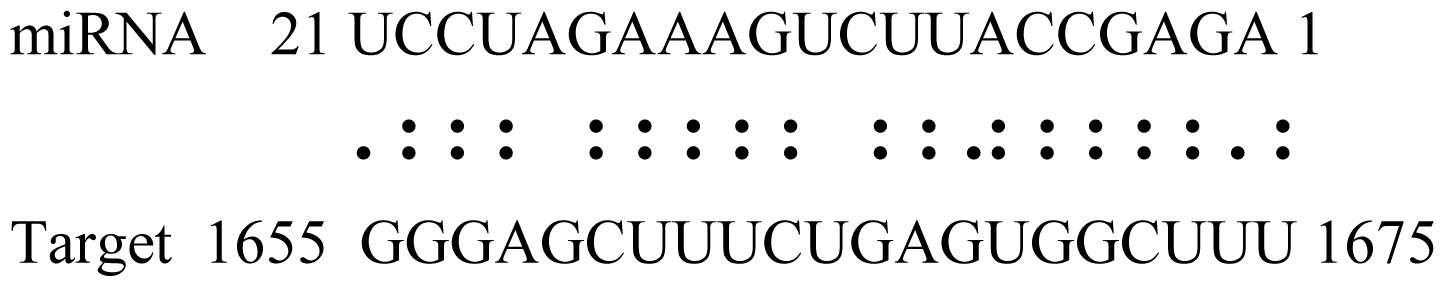 | 3.0 |
| Glyma07g40110 | Protein Tyrosine Kinase |  | 3.0 | |
| Glyma09g37070 | RNA-Binding Protein Related |  | 3.0 | |
| gly_11 | Glyma05g02970 | Ribosomal Protein S21 | 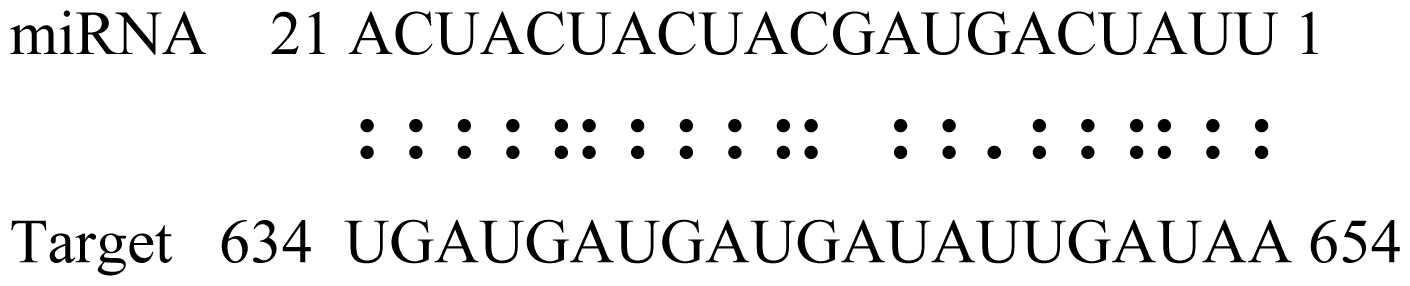 | 2.5 |
| Glyma08g11770 | Ubiquitin-Like Protein Sumo/Smt3-Related |  | 2.5 | |
| Glyma13g17650 | CREG1 Protein |  | 2.5 | |
| gly_12 | Glyma09g30540 | Aldose-1-Epimerase | 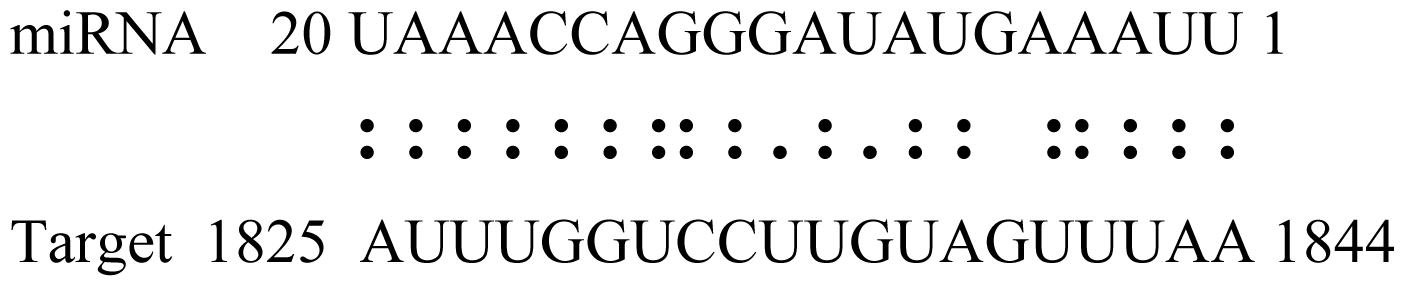 | 2.5 |
| gly_32 | Glyma13g24580 | Mitochondrial Carrier Protein Related | 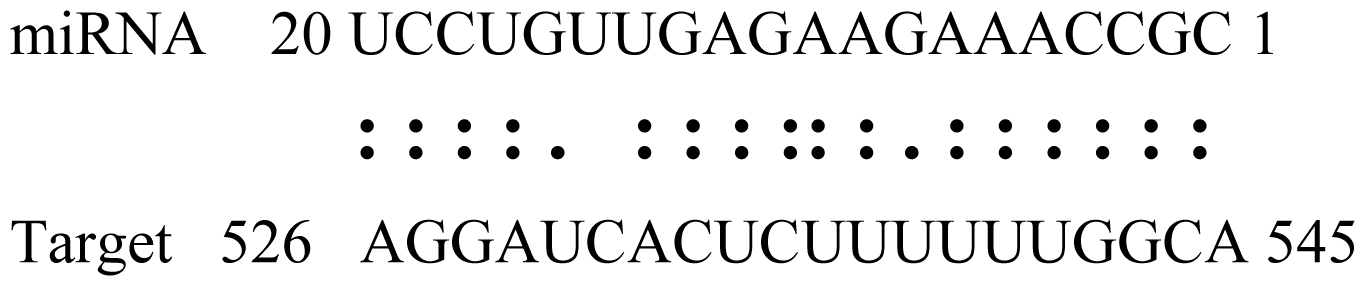 | 3.5 |
| Glyma04g05250 | Hemoglobinase Family Member | 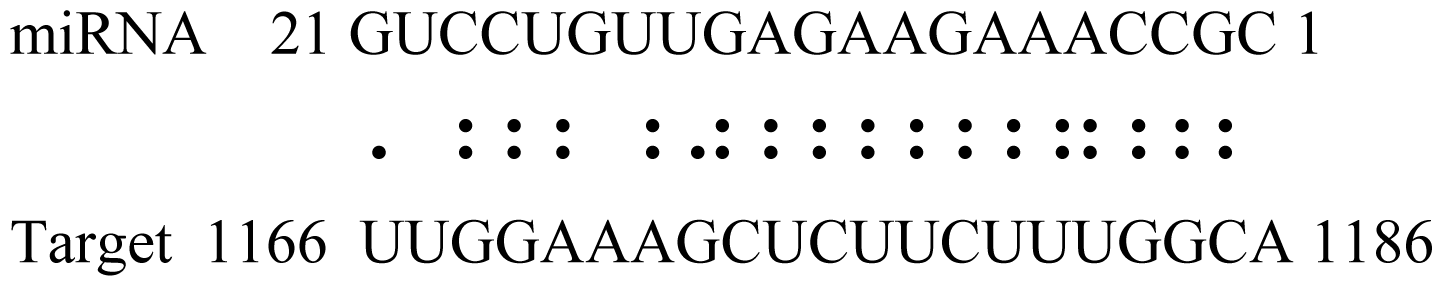 | 3.5 | |
| gly_37 | Glyma08g05740 | Eukaryotic Translation Initation Factor 3, Subunit 8 (Eif3s8)-Related |  | 2.5 |
| miR171p | Glyma11g17490 | GRAS | 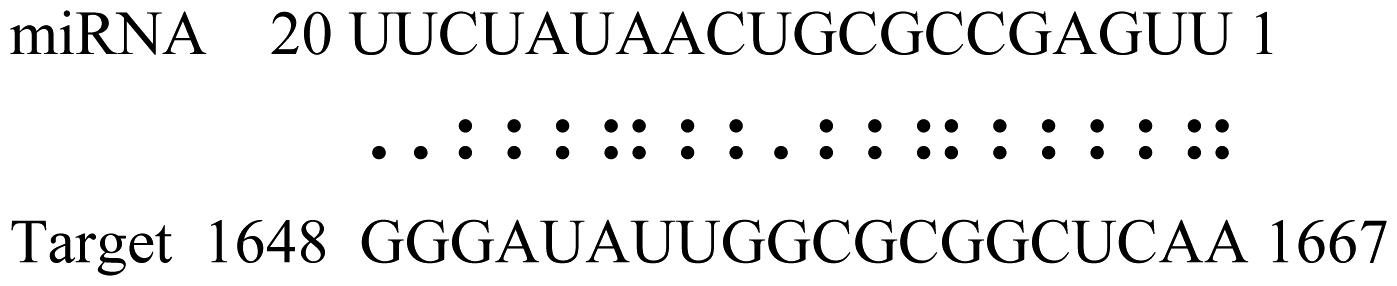 | 2.0 |
| Glyma20g03210 | Glycoside Hydrolases |  | 2.0 | |
| miR393i | Glyma19g27280 | AFB2 Like Protein |  | 1.0 |
| Glyma19g39420 | TIR1 Like Protein | 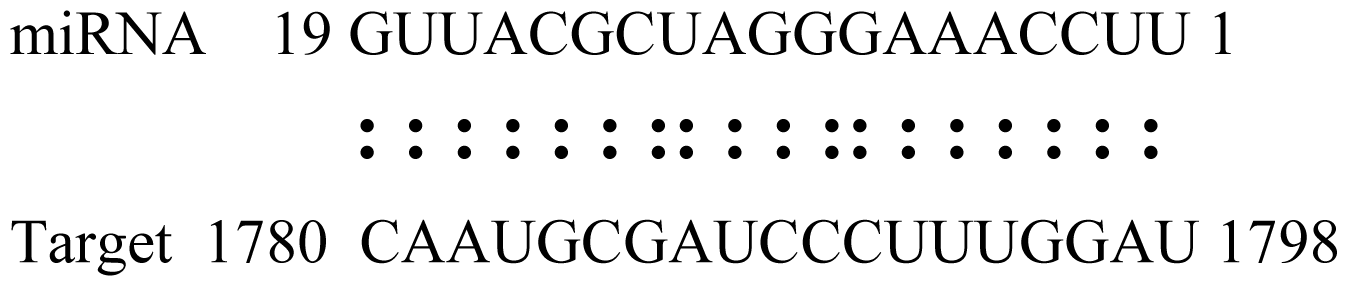 | 1.0 |
Supplementary Files
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dong, Z.; Shi, L.; Wang, Y.; Chen, L.; Cai, Z.; Wang, Y.; Jin, J.; Li, X. Identification and Dynamic Regulation of microRNAs Involved in Salt Stress Responses in Functional Soybean Nodules by High-Throughput Sequencing. Int. J. Mol. Sci. 2013, 14, 2717-2738. https://doi.org/10.3390/ijms14022717
Dong Z, Shi L, Wang Y, Chen L, Cai Z, Wang Y, Jin J, Li X. Identification and Dynamic Regulation of microRNAs Involved in Salt Stress Responses in Functional Soybean Nodules by High-Throughput Sequencing. International Journal of Molecular Sciences. 2013; 14(2):2717-2738. https://doi.org/10.3390/ijms14022717
Chicago/Turabian StyleDong, Zhanghui, Lei Shi, Yanwei Wang, Liang Chen, Zhaoming Cai, Youning Wang, Jingbo Jin, and Xia Li. 2013. "Identification and Dynamic Regulation of microRNAs Involved in Salt Stress Responses in Functional Soybean Nodules by High-Throughput Sequencing" International Journal of Molecular Sciences 14, no. 2: 2717-2738. https://doi.org/10.3390/ijms14022717
APA StyleDong, Z., Shi, L., Wang, Y., Chen, L., Cai, Z., Wang, Y., Jin, J., & Li, X. (2013). Identification and Dynamic Regulation of microRNAs Involved in Salt Stress Responses in Functional Soybean Nodules by High-Throughput Sequencing. International Journal of Molecular Sciences, 14(2), 2717-2738. https://doi.org/10.3390/ijms14022717




