The Preparation of Capsaicin-Chitosan Microspheres (CCMS) Enteric Coated Tablets
Abstract
:1. Introduction
2. Results and Discussion
2.1. Formula Optimisation of CCMS Core Tablets
2.2. Preparation of CCMS Enteric Coating
2.2.1. Determination of Capsaicin Equilibrium Solubility
2.2.2. Methodology Experiment
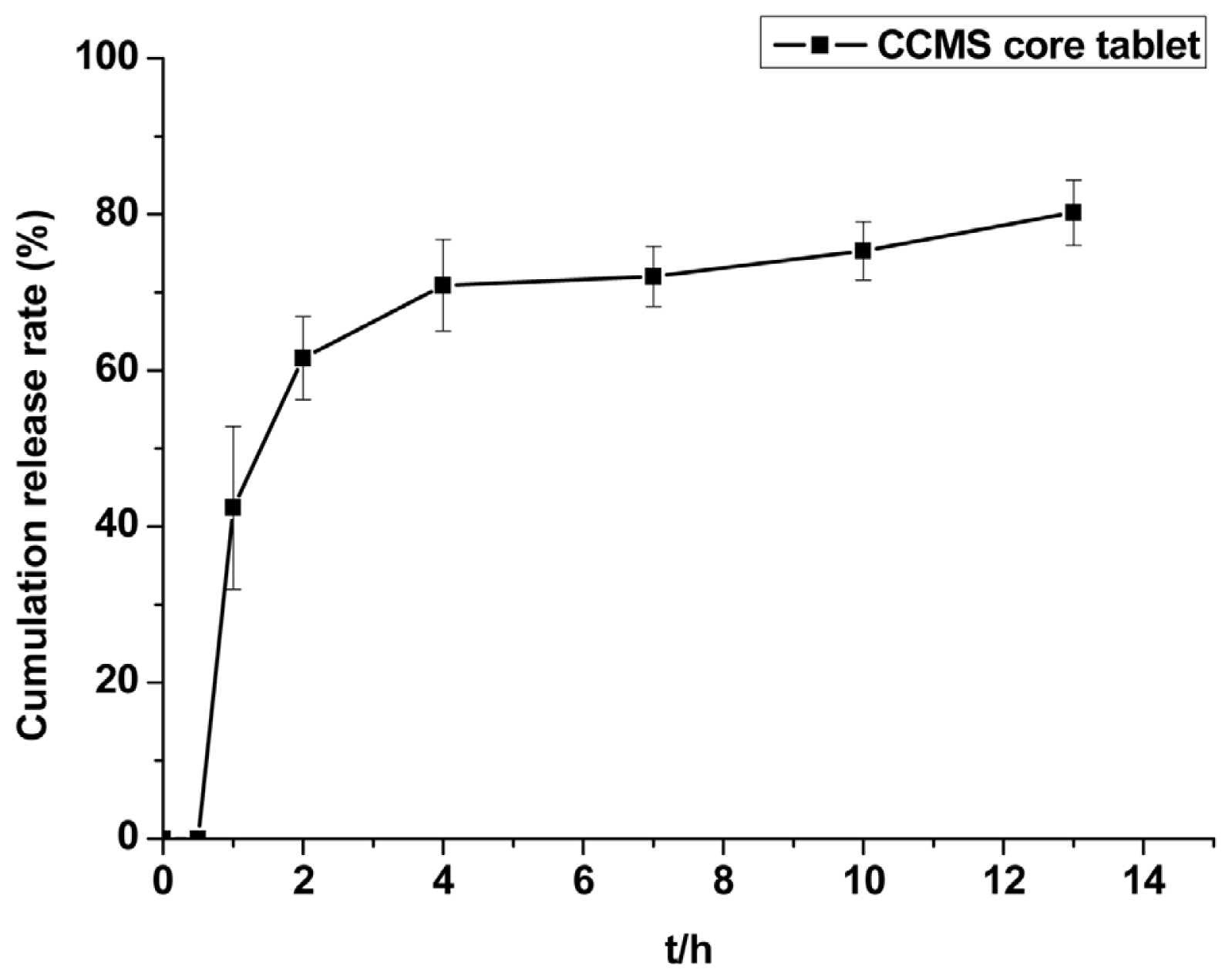
2.2.3. CAP Dissolution Property from CCMS Core Tablet
2.2.4. Evaluation of Talc Content
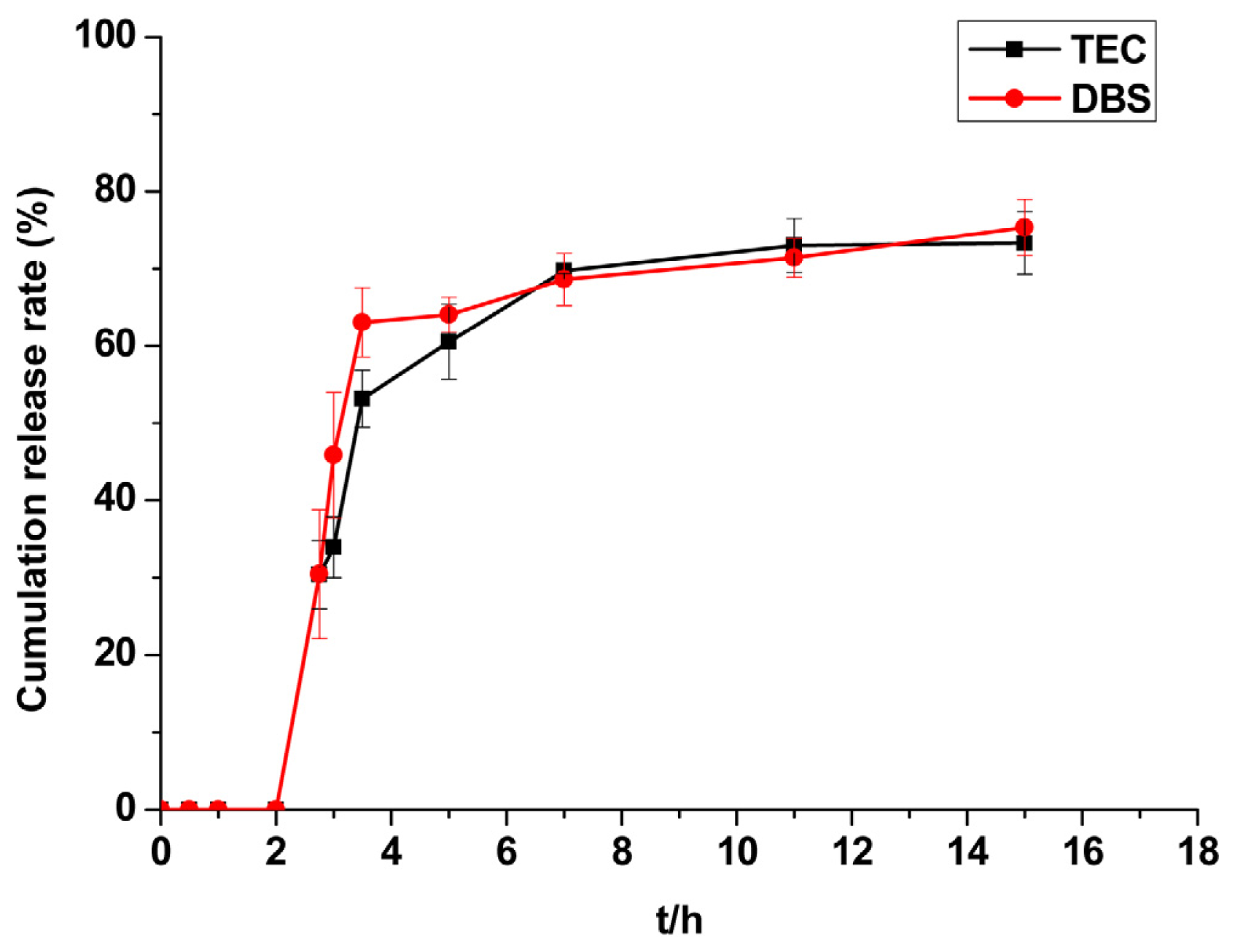
2.2.5. Evaluation of Plasticisers
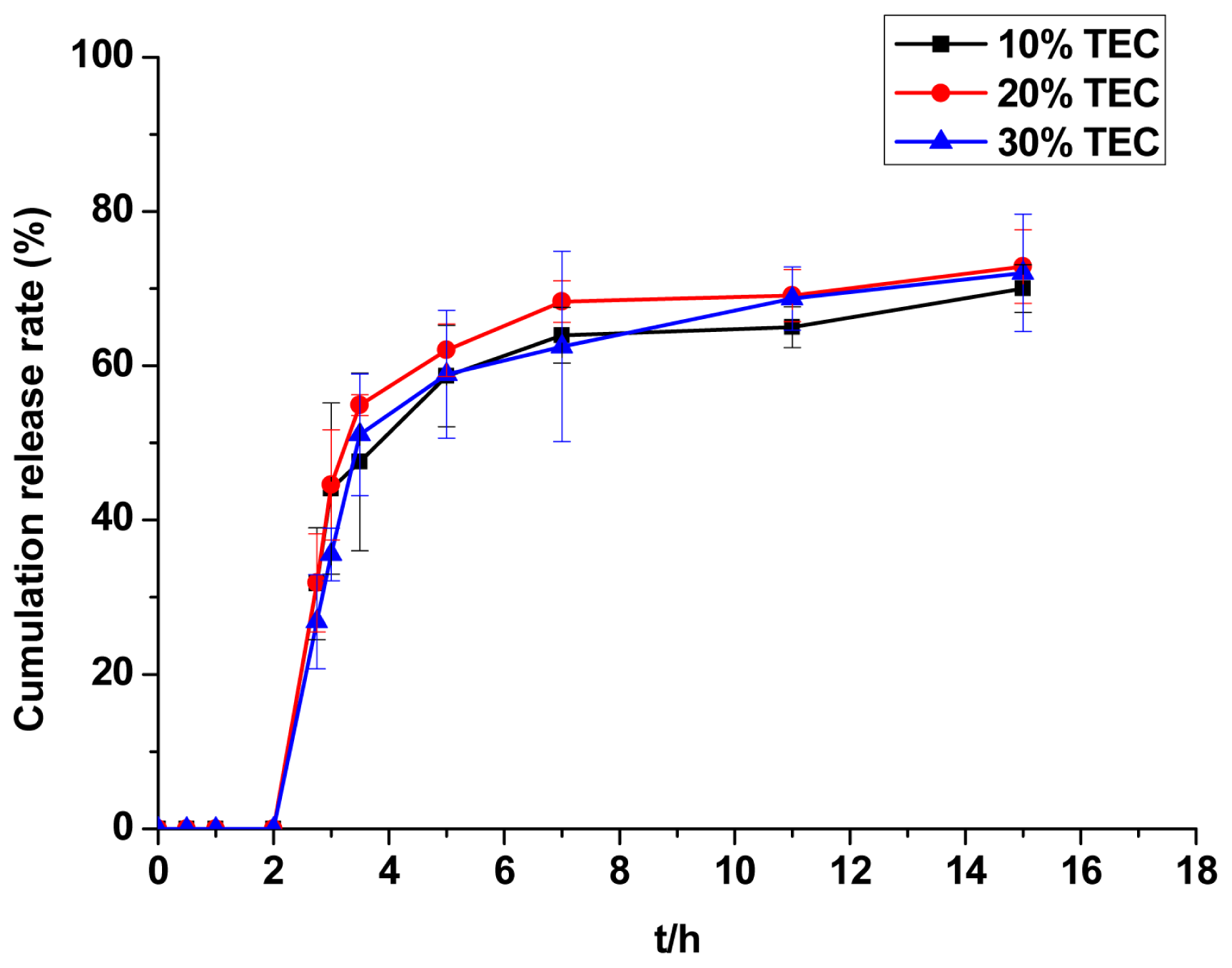
2.2.6. Evaluation of TEC Dosage
2.2.7. Evaluation of Coating Weight
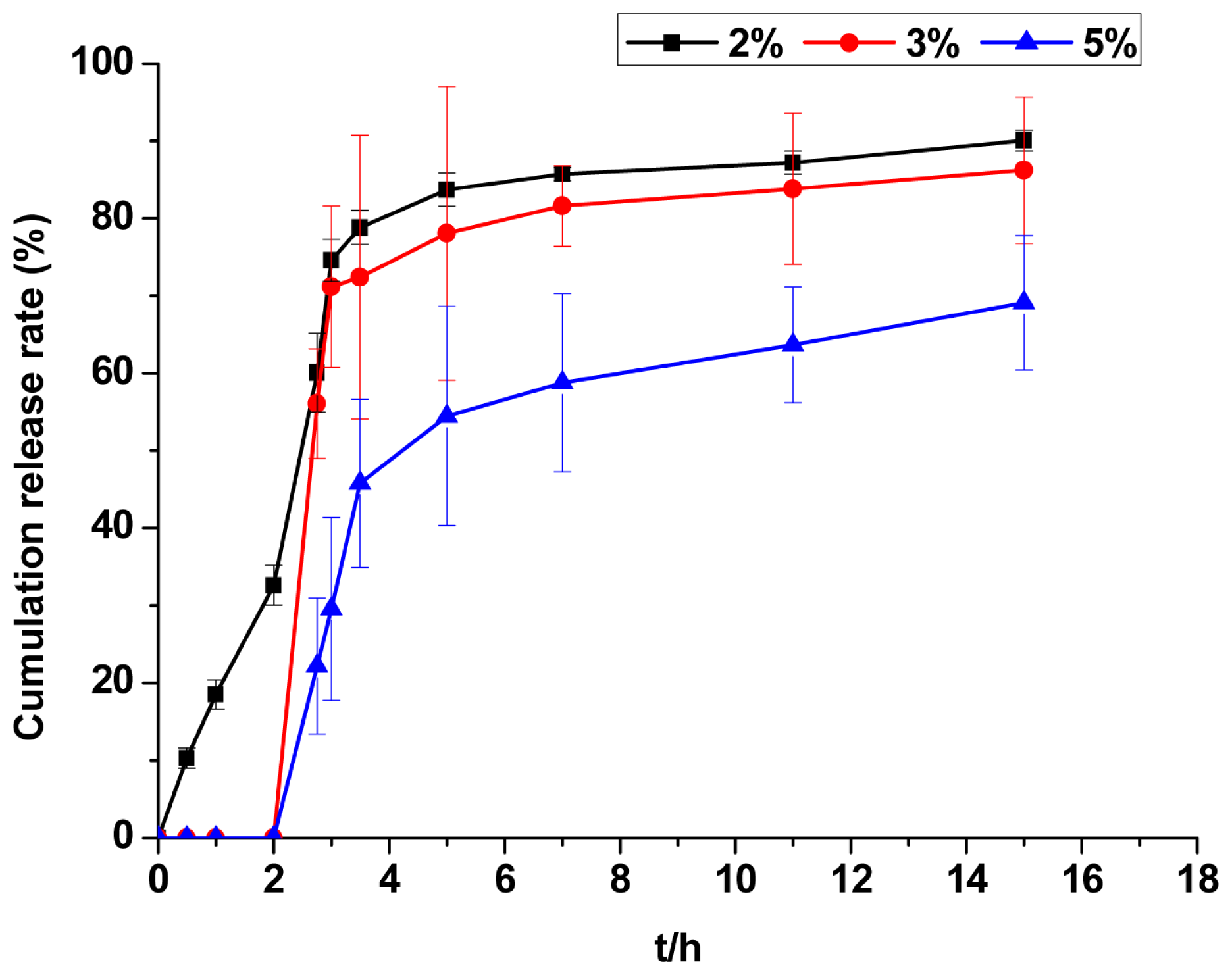
2.2.8. Evaluation of Release Kinetics
2.3. Results of Content Determination
3. Experimental Section
3.1. Chemicals
3.2. Preparation and Characterization of CCMS
3.3. Preparation of CCMS Core Tablets
3.3.1. Method for Preparation of CCMS Core Tablets
3.3.2. Formula Optimisation of CCMS Core Tablets
3.4. Preparation of CCMS Enteric Coated Tablets
3.4.1. Formulation Optimisation of Enteric Coating Solution
3.4.1.1. Content of Talc
3.4.1.2. Plasticisers
3.4.1.3. Content of TEC
3.4.1.4. Coating Weight
3.4.2. Dissolution Research in Vitro
3.4.2.1. Determination of Capsaicin Equilibrium Solubility
3.4.2.2. Dissolution Test of CCMS Core Tablet in Vitro
3.4.2.3. Dissolution Research of CCMS Enteric Coated Tablet in Vitro
3.4.3. Release Kinetics
3.4.4. HPLC Method
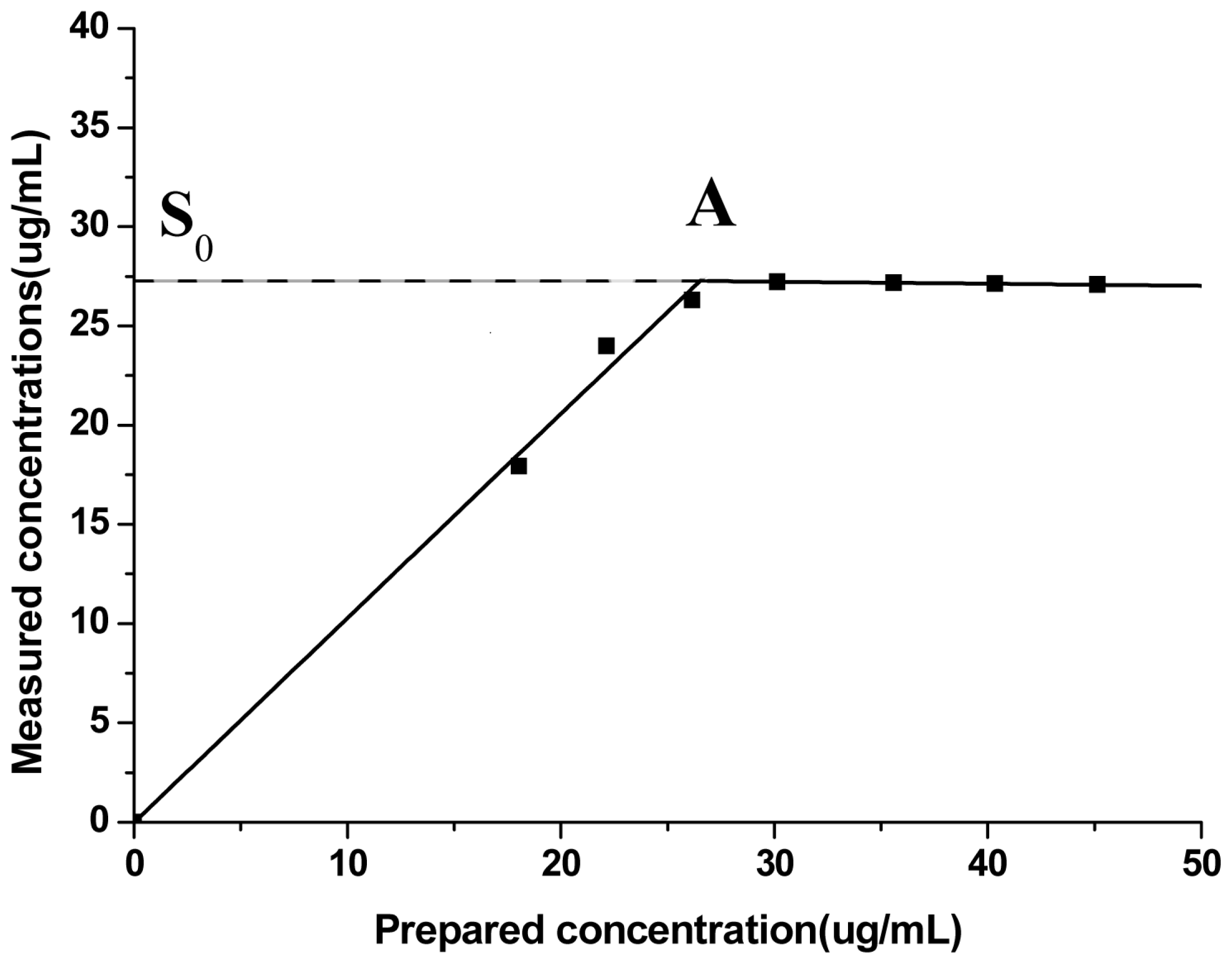
3.4.4.1. Selection of Maximum Absorption Wavelength
3.4.4.2. Chromatography Conditions
3.4.4.3. Specificity Investigation
3.4.4.4. Linear Relationship Investigation
3.4.4.5. Precision Experiment
3.4.4.6. Stability Experiment
3.4.4.7. Recovery Experiment
3.5. Content Determination of CCMS Enteric Coated Tablets
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Jequier, E. Pathways to obesity. Int. J. Obes. Relat. Metab. Disord 2002, 26, 12–17. [Google Scholar]
- Cecchini, M.; Sassi, F.; Lauer, J.A.; Lee, Y.Y.; Guajardo-Barron, V.; Chisholm, D. Tackling of unhealthy diets, physical inactivity, and obesity: Health effects and cost-effectiveness. Lancet 2010, 376, 1775–1784. [Google Scholar]
- Cope, M.B.; Allison, D.B. Obesity: person and population. Obesity 2006, 14, 156S–159S. [Google Scholar]
- Orsi, C.M.; Hale, D.E.; Lynch, J.L. Pediatric obesity epidemiology. Curr. Opin. Endocrinol. Diabetes Obes 2011, 18, 14–22. [Google Scholar]
- Birch, L.L.; Ventura, A.K. Preventing childhood obesity: What works? Int. J. Obes 2009, 33, S74–S81. [Google Scholar]
- Han, J.C.; Lawlor, D.A.; Kimm, S.Y. Childhood obesity. Lancet 2010, 375, 1737–1748. [Google Scholar]
- Luo, J.; Hu, F.B. Time trends of obesity in pre-school children China from 1989 to 1997. Int. J. Obes. Relat. Metab. Disord 2002, 26, 553–558. [Google Scholar]
- Xi, B.; Liang, Y.; He, Y.; Reilly, K.H.; Hu, Y.; Wang, Q.; Yan, Y.; Mi, J. Secular trend in the prevalence of general and abdominal obesity among Chinese adults. Obesity reviews 2012, 13, 287–296. [Google Scholar]
- Haslam, D.W.; James, W.P. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar]
- Ahima, R.S. Digging deeper into obesity. J. Clin. Invest 2011, 121, 2076–2069. [Google Scholar]
- Reilly, J.J.; Methven, E.; McDowell, Z.C.; Hacking, B.; Alexander, D.; Stewart, L.; Kelnar, C.J. Health consequences of obesity. J. Obstet. Gynecol. Neonatal. Nurs 2007, 36, 511–517. [Google Scholar]
- Sharma, A.M. Obesity and cardiovascular risk. Growth Horm. IGF Res 2003, 13, 10–7. [Google Scholar]
- Stoll, B.A. Obesity and breast cancer. Int. J. Obes. Relat. Metab. Disord 1996, 20, 389–392. [Google Scholar]
- Gumbs, A.A. Obesity, pancreatitis, and pancreatic cancer. Obes. Surg 2008, 18, 1183–1187. [Google Scholar]
- Wolin, K.Y.; Carson, K.; Colditz, G.A. Obesity and cancer. Oncologist 2010, 15, 556–565. [Google Scholar]
- Linne, Y.; Rossner, S. Pharmacotherapy of obesity. Clin. Dermatol 2004, 22, 319–324. [Google Scholar]
- Yin, H.; Du, Y.; Zhang, J. Low molecular weight and oligomeric chitosans and their bioactivities. Curr. Top. Med. Chem 2009, 9, 1546–1559. [Google Scholar]
- Yi, H.; Wu, L.Q.; Bentley, W.E.; Ghodssi, R.; Rubloff, G.W.; Culver, J.N.; Payne, G.F. Biofabrication with chitosan. Biomacromolecules 2005, 6, 2881–2894. [Google Scholar]
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V.V. Why is chitosan mucoadhesive? Biomacromolecules 2008, 9, 1837–1842. [Google Scholar]
- Hamman, J.H. Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Mar. Drugs 2010, 8, 1305–1322. [Google Scholar]
- Reinbach, H.C.; Smeets, A.; Mattinussen, T.; Moller, P.; Westerterp-Plantenga, M.S. Effects of capsaicin, green tea and CH-19 sweet pepper on appetite and energy intake in humans in negative and positive energy balance. Clin. Nutr 2009, 28, 260–265. [Google Scholar]
- Irigaray, P.; Newby, J.A.; Lacomme, S.; Belpomme, D. Overweight/obesity and cancer genesis: More than a biological link. Biomed. Pharmacother 2007, 61, 665–678. [Google Scholar]
- Mayumi, Y.; Sylvie, S.P.; Vicky, D.; Isabelle, D.; Masashige, S.; Angelo, T. Effects of red pepper on appetite and energy intake. Br. J. Nutr 1999, 82, 115–123. [Google Scholar]
- Su, Z.Q.; Tao, Y.; Gao, B.; Tan, S.R. Chitosan Microsphere Carrier Capsaicin Preparation Method and Microsphere and Use Chitosan-Loaded Capsaicin Microspheres, Preparation Method Thereof, and Application Thereof CN patent CN201110247679A; 26-Aug-2011, 26 August 2011.
- Tan, S.R.; Wu, S.H.; Gao, B.; Xiao, J.R.; Guo, Z.Y.; Long, S.Y.; Bai, Y.; Su, Z.Q. Experimental investigation on the lipid-lowering activity of three novel antilipidemic materials in vitro. Adv. Mater. Res 2011, 1568, 399–401. [Google Scholar]
- Rowe, R.C.; Sheskey, P.J.; Willer, P.J. Handbook of Pharmaceutical Excipients; RPS: London, UK, 2009; pp. 651–653. [Google Scholar]
- Rowe, R.C.; Sheskey, P.J.; Willer, P.J. Handbook of Pharmaceutical Excipients; RPS: London, UK, 2009; pp. 404–406. [Google Scholar]
- Philip, L.R.; Nikolaos, A.P. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Contr. Release 1987, 5, 37–42. [Google Scholar]
- Reddy, K.R.; Mutalik, S.; Reddy, S. Once-daily sustained release matrix tablets of nicorandil: Formulation and in vitro evaluation. AAPS PharmSciTech 2003, 4, 480–488. [Google Scholar]
- Lu, B. New Techniques and New Doasge. Forms of Drugs; People’s Medical Publishing House: Beijing, China, 2005; pp. 386–388. [Google Scholar]
- Lu, B. New Techniques and New Doasge. Forms of Drugs; People’s Medical Publishing House: Beijing, China, 2005; pp. 257–264. [Google Scholar]

| Formulae | Hardness (N) | Disintegration time (min) | Appearance |
|---|---|---|---|
| 1 | 55.2 ± 2.4 | 15.5 ± 1.5 | + |
| 2 | 57.2 ± 2.6 | 15.0 ± 0.9 | − |
| 3 | 58.4 ± 3.9 | 13.7 ± 0.5 | + |
| 4 | 56.4 ± 3.2 | 15.8 ± 1.3 | + |
| 5 | 55.0 ± 2.4 | 14.3 ± 1.2 | + |
| 6 | 54.6 ± 2.3 | 13.2 ± 0.8 | + |
| 7 | 56.2 ± 2.2 | 14.7 ± 1.0 | + |
| 8 | 53.8 ± 2.3 | 11.7 ± 0.5 | + |
| 9 | 54.8 ± 1.9 | 12.3 ± 0.8 | + |
| Known quantity (mg) | Adding quantity (mg) | Measured quantity (mg) | Recovery ratio (%) | Average recovery ratio (%) | RSD (%) |
|---|---|---|---|---|---|
| 0.1923 | 0.1757 | 0.3673 | 99.60 | ||
| 0.1928 | 0.1757 | 0.3685 | 100.00 | ||
| 0.1955 | 0.1757 | 0.3725 | 100.74 | 99.42 | 1.15 |
| 0.1938 | 0.1757 | 0.3678 | 99.03 | ||
| 0.1883 | 0.1757 | 0.3600 | 97.72 |
| Drug release model | Kinetic equation | Mean relative percentage deviation | R2 |
|---|---|---|---|
| First-order | ln (1 − 0.01Q) = −1.6295t | 21.39% | 0.99 |
| Higuchi | Q = 8.3806t1/2 + 59.2705 | 6.00% | 0.67 |
| Ritger-peppas | lnQ = 0.1185 lnt + 4.1885 | 5.32% | 0.74 |
| Batch | Content of CAP (mg/each tablet) | Mean Content (mg/each tablet) |
|---|---|---|
| 1207014 | 5.45 ± 0.01 | |
| 1207015 | 5.44 ± 0.02 | 5.44 |
| 1207016 | 5.43 ± 0.01 |
| Excipients | Formulae | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| Drug | CCMS (mg) | 400 | 400 | 400 | 400 | 400 | 400 | 400 | 400 | 400 |
| Binders | Carboxymethylcellulose Sodium (Right amount, w/v) | 5% | 3% | 7% | 3% | 3% | 3% | 3% | 3% | |
| HPMC (Right amount, w/v) | - | 5% | - | - | - | - | - | - | - | |
| Lubricants | Magnesium Stearate (mg) | 4 | 4 | 4 | 4 | - | 4 | 4 | 4 | 4 |
| SLS (mg) | - | - | - | - | 4 | - | - | - | - | |
| Disintegrants | Sodium Starch Glycolate (mg) | - | - | - | - | - | 24 | - | 16 | 32 |
| Croscarmellose Sodium (mg) | - | - | - | - | - | - | 24 | - | - | |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, J.; Huang, G.-D.; Tan, S.-R.; Guo, J.; Su, Z.-Q. The Preparation of Capsaicin-Chitosan Microspheres (CCMS) Enteric Coated Tablets. Int. J. Mol. Sci. 2013, 14, 24305-24319. https://doi.org/10.3390/ijms141224305
Chen J, Huang G-D, Tan S-R, Guo J, Su Z-Q. The Preparation of Capsaicin-Chitosan Microspheres (CCMS) Enteric Coated Tablets. International Journal of Molecular Sciences. 2013; 14(12):24305-24319. https://doi.org/10.3390/ijms141224305
Chicago/Turabian StyleChen, Jian, Gui-Dong Huang, Si-Rong Tan, Jiao Guo, and Zheng-Quan Su. 2013. "The Preparation of Capsaicin-Chitosan Microspheres (CCMS) Enteric Coated Tablets" International Journal of Molecular Sciences 14, no. 12: 24305-24319. https://doi.org/10.3390/ijms141224305




