Biodegradation of Di-n-Butyl Phthalate by a Newly Isolated Halotolerant Sphingobium sp.
Abstract
:1. Introduction
2. Results and Discussion
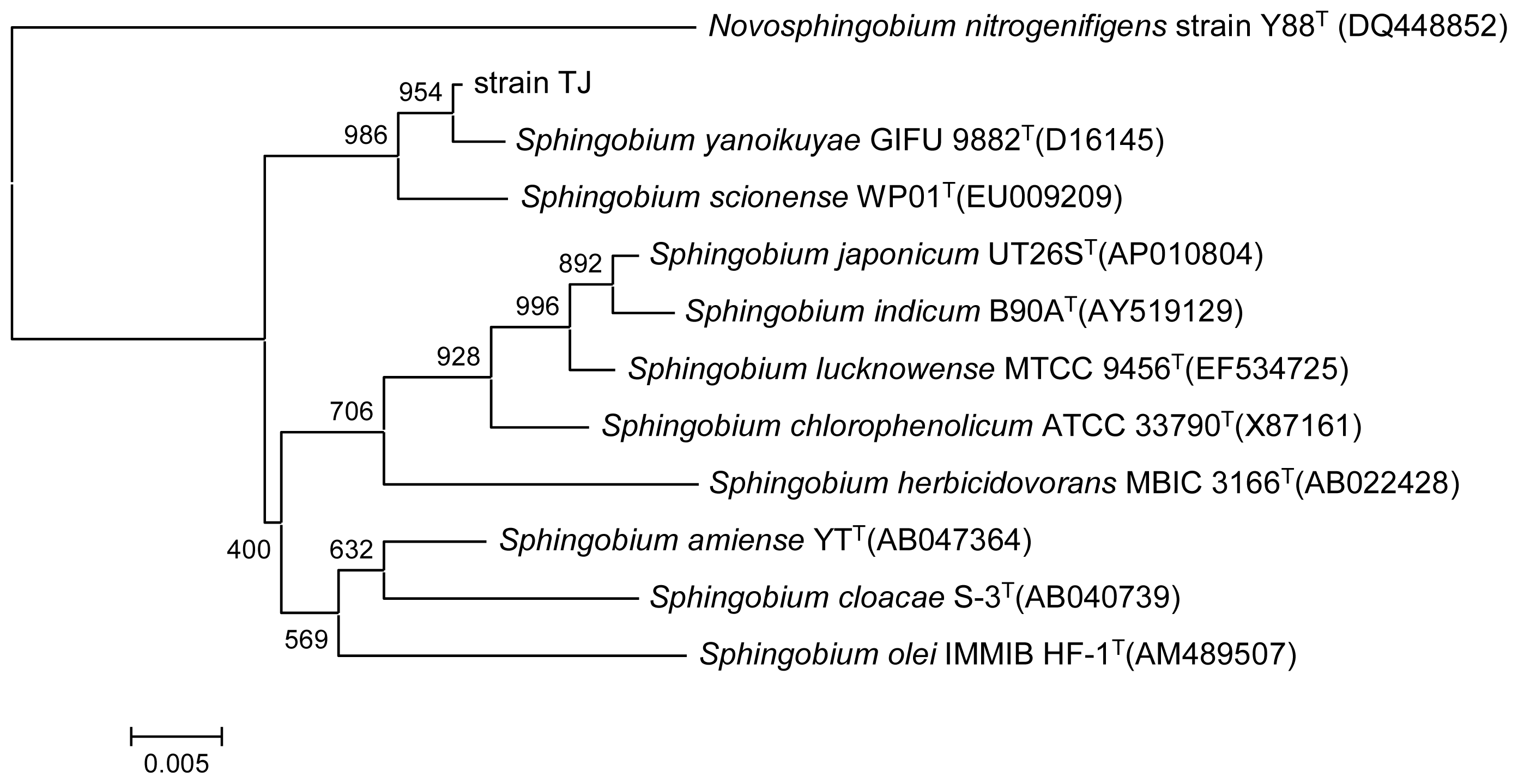
2.1. Identification and Characterization of the PAEs Degrading Strain
2.2. Substrate Utilization Tests
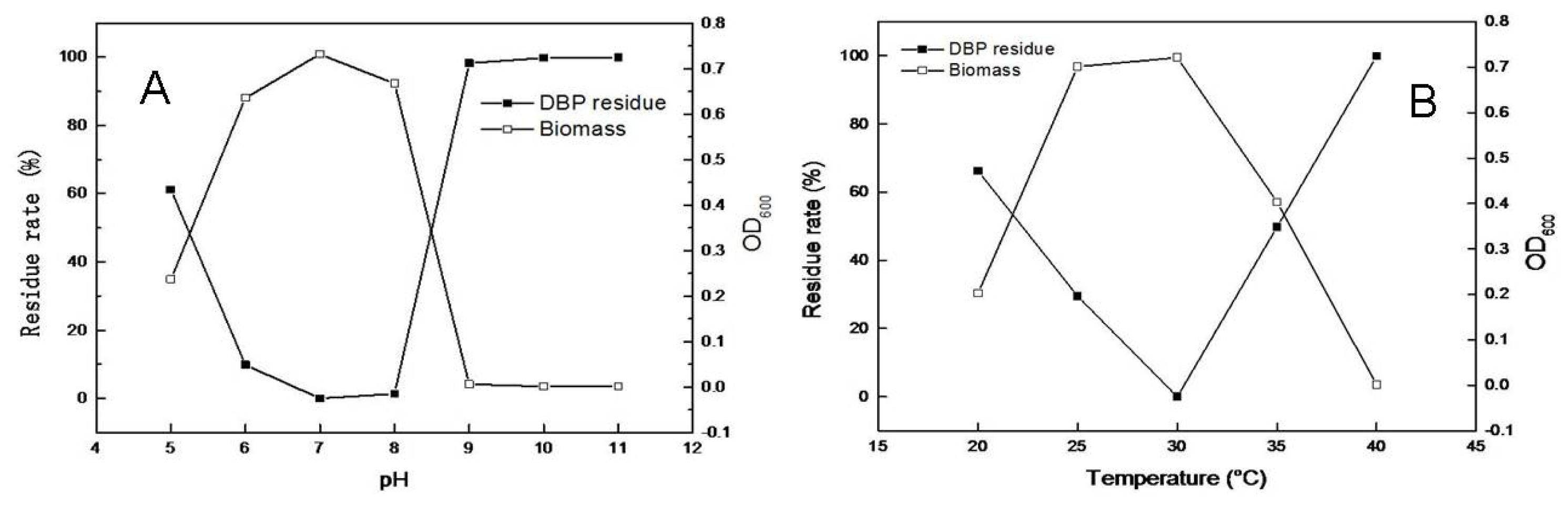
2.3. Effects of pH and Temperature on Microbial Growth and DBP Degradation
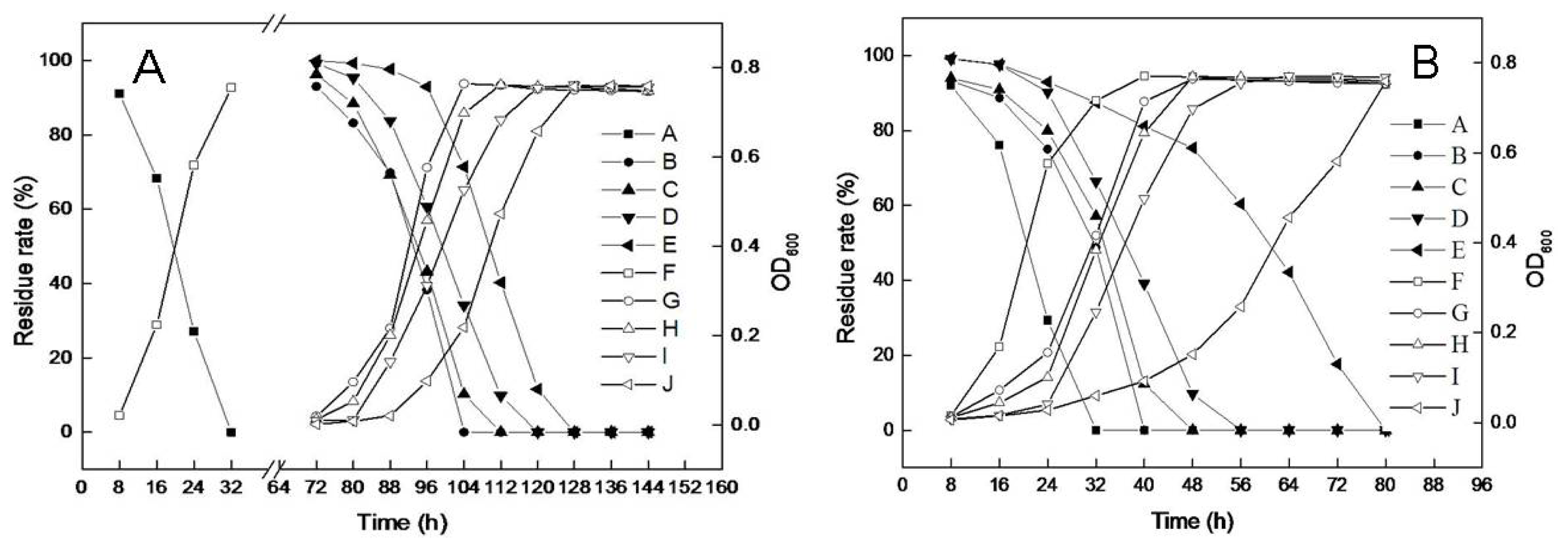
2.4. Effects of NaCl Induction on Microbial Growth and DBP Degradation
3.1. Chemicals
3.2. Enrichment Culture and Isolation of Bacteria
3.3. Identification of a Pure Culture
3.4. Substrate Utilization Tests
3.5. Biodegradation of DBP
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Yuan, S.Y.; Liu, C.; Liao, C.S.; Chang, B.V. Occurrence and microbial degradation of phthalate esters in Taiwan river sediments. Chemosphere 2002, 49, 1295–1299. [Google Scholar]
- Nishioka, T.; Iwata, M.; Imaoka, T.; Mutoh, M.; Egashira, Y.; Nishiyama, T.; Shin, T.; Fujii, T. A mono-2-ethylhexyl phthalate hydrolase from a Gordonia sp. that is able to dissimilate di-2-ethylhexyl phthalate. Appl. Environ. Microbiol 2006, 72, 2394–2399. [Google Scholar]
- Lim, D.S.; Shin, B.S.; Yoo, S.D.; Kim, H.S.; Kwack, S.J.; Ahn, M.Y.; Lee, B.M. Toxicokinetics of phthalic acid: The common final metabolite of phthalic acid esters in rats. J. Toxicol. Environ. Health A 2007, 70, 1344–1349. [Google Scholar]
- Lottrup, G.; Andersson, A.; Leffers, H.; Mortensen, G.; Toppari, J.; Skakkebaek, N.E.; Main, K.M. Possible impact of phthalates on infant reproductive health. Int. J. Androl 2006, 29, 172–180. [Google Scholar]
- Wang, J.L.; Liu, P.; Qian, Y. Microbial degradation of di-n-butyl phthalate. Chemosphere 1995, 31, 4051–4056. [Google Scholar]
- Wang, J.L.; Shi, H.C.; Qian, Y. Biodegradation of plasticizer di-n-butyl phthalate (DBP) by immobilized microbial cells. Toxicol. Environ. Chem 2000, 74, 195–202. [Google Scholar]
- Wu, X.L.; Wang, Y.Y.; Liang, R.X.; Dai, Q.Y.; Jin, D.C.; Chao, W.L. Biodegradation of an endocrine-disrupting chemical di-n-butyl phthalate by newly isolated Agrobacterium sp. and the biochemical pathway. Process Biochem 2011, 46, 1090–1094. [Google Scholar]
- Lu, Y.; Tang, F.; Wang, Y.; Zhao, J.; Zeng, X.; Luo, Q.; Wang, L. Biodegradation of dimethyl phthalate, diethyl phthalate and di-n-butyl phthalate by Rhodococcus sp. L4 isolated from activated sludge. J. Hazard. Mater 2009, 168, 938–943. [Google Scholar]
- Liao, C.S.; Chen, L.C.; Chen, B.S.; Lin, S.H. Bioremediation of endocrine disruptor di-n-butyl phthalate ester by Deinococcus radiodurans and Pseudomonas stutzeri. Chemosphere 2010, 78, 342–346. [Google Scholar]
- Babu, B.; Wu, J.T. Biodegradation of phthalate esters by Cyanobacteria. J. Phycol 2010, 46, 1106–1113. [Google Scholar]
- Wu, X.L.; Wang, Y.Y.; Liang, R.X.; Dai, Q.Y.; Chao, W.L. Degradation of di-n-butyl phthalate by newly isolated Ochrobactrum sp. Bull. Environ. Contam. Toxicol 2010, 85, 235–237. [Google Scholar]
- Zheng, G.Y.; Selvam, A.; Wong, J.W.C. Rapid degradation of lindane (g-hexachlorocyclohexane) at low temperature by Sphingobium strains. Int. Biodeter. Biodegr 2011, 65, 612–618. [Google Scholar]
- Sun, J.Q.; Huang, X.; Chen, Q.L.; Liang, B.; Qiu, J.G.; Ali, S.W.; Li, S.P. Isolation and characterization of three Sphingobium sp. strains capable of degrading isoproturon and cloning of the catechol 1,2-dioxygenase gene from these strains. World J. Microbiol. Biotechnol 2009, 25, 259–268. [Google Scholar]
- Dai, M.; Copley, S.D. Genome shuffling improves degradation of the anthropogenic pesticide pentachlorophenol by Sphingobium chlorophenolicum ATCC 39723. Appl. Environ. Microbiol 2004, 70, 2391–2397. [Google Scholar]
- Wang, Y.; Yin, B.; Hong, Y.; Yan, Y.; Gu, J.D. Degradation of dimethyl carboxylic phthalate ester by Burkholderia cepacia DA2 isolated from marine sediment of South China Sea. Ecotoxicology 2008, 17, 845–852. [Google Scholar]
- Yoon, J.H.; Oh, T.K. Sphingopyxis flavimaris sp. nov., isolated from sea water of the Yellow Sea in Korea. Int. J. Syst. Evol. Microbiol 2005, 55, 369–373. [Google Scholar]
- Jin, D.C.; Liang, R.X.; Dai, Q.Y.; Zhang, R.Y.; Wu, X.L.; Chao, W.L. Biodegradation of di-n-butyl phthalate by Rhodococcus sp. JDC-11 and molecular detection of 3,4-phthalate dioxygenase gene. J. Microbiol. Biotechnol 2010, 20, 1440–1445. [Google Scholar]
- Yamamoto, S.; Harayama, S. PCR amplification and direct sequencing of gyrb genes with universal primers and their application to the detection and taxonomic analysis of Pseudomonas putida strains. Appl. Environ. Microbiol 1995, 61, 1104–1109. [Google Scholar]
- Jin, D.C.; Bai, Z.H.; Chang, D.D.; Hoefel, D.; Jin, B.; Wang, P.; Wei, D.B.; Zhuang, G.Q. Biodegradation of di-n-butyl phthalate by an isolated Gordonia sp. strain QH-11: Genetic identification and degradation kinetics. J. Hazard. Mater 2012, 221–222, 80–85. [Google Scholar]

| Substrate | Utilization | Substrate | Utilization | Substrate | Utilization |
|---|---|---|---|---|---|
| DMP | + | DOP | − | Phthalic acid | − |
| DEP | + | DIOP | − | Protocatechuic acid | − |
| DBP | + | MBP | + | Benzoic acid | + |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jin, D.; Kong, X.; Cui, B.; Bai, Z.; Zhang, H. Biodegradation of Di-n-Butyl Phthalate by a Newly Isolated Halotolerant Sphingobium sp. Int. J. Mol. Sci. 2013, 14, 24046-24054. https://doi.org/10.3390/ijms141224046
Jin D, Kong X, Cui B, Bai Z, Zhang H. Biodegradation of Di-n-Butyl Phthalate by a Newly Isolated Halotolerant Sphingobium sp. International Journal of Molecular Sciences. 2013; 14(12):24046-24054. https://doi.org/10.3390/ijms141224046
Chicago/Turabian StyleJin, Decai, Xiao Kong, Bingjian Cui, Zhihui Bai, and Hongxun Zhang. 2013. "Biodegradation of Di-n-Butyl Phthalate by a Newly Isolated Halotolerant Sphingobium sp." International Journal of Molecular Sciences 14, no. 12: 24046-24054. https://doi.org/10.3390/ijms141224046






