Molecular Cloning and Characterization of a P-Glycoprotein from the Diamondback Moth, Plutella xylostella (Lepidoptera: Plutellidae)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Molecular Cloning of PxPgp1
2.2. Phylogenetic Relationship of PxPgp1 with Other Insect Pgps
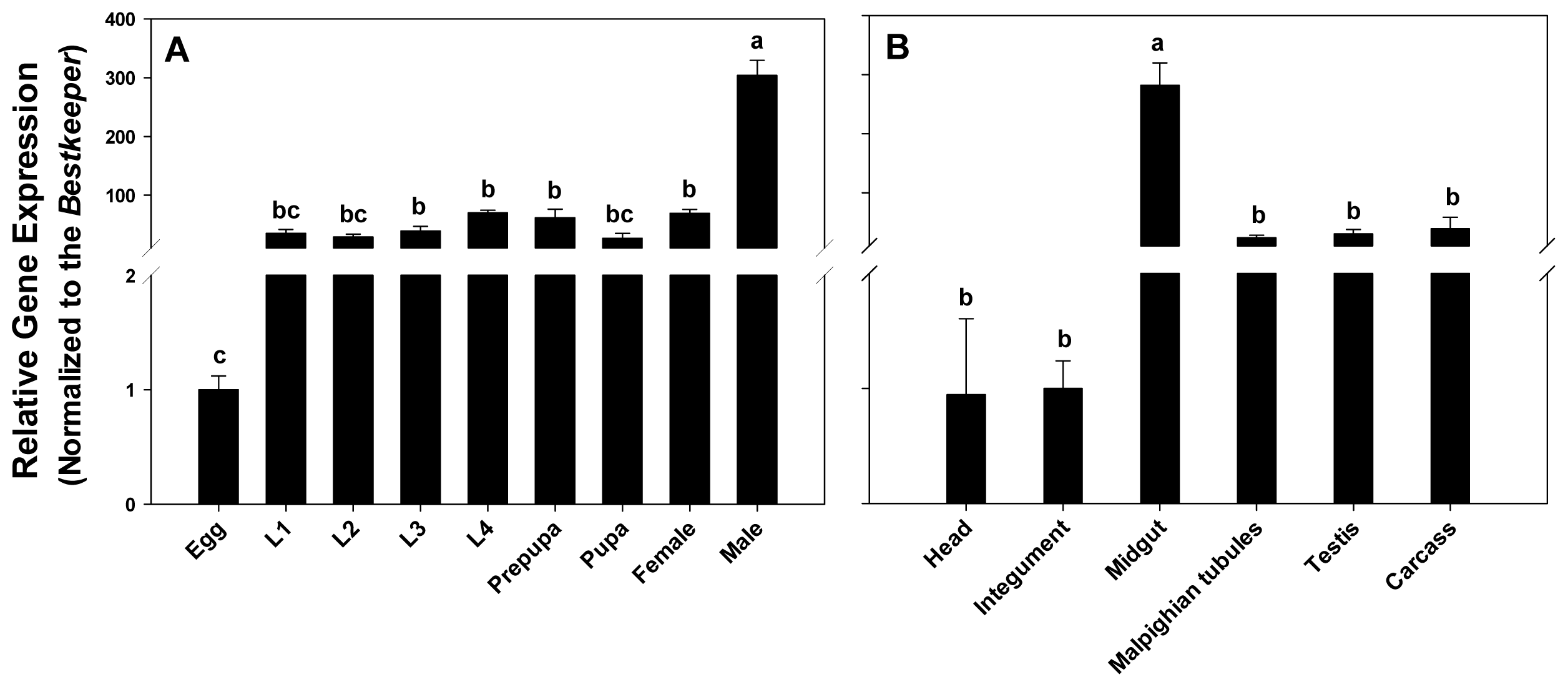
2.3. Expression Profiling of PxPgp1 in Different Developmental Stages and Tissues
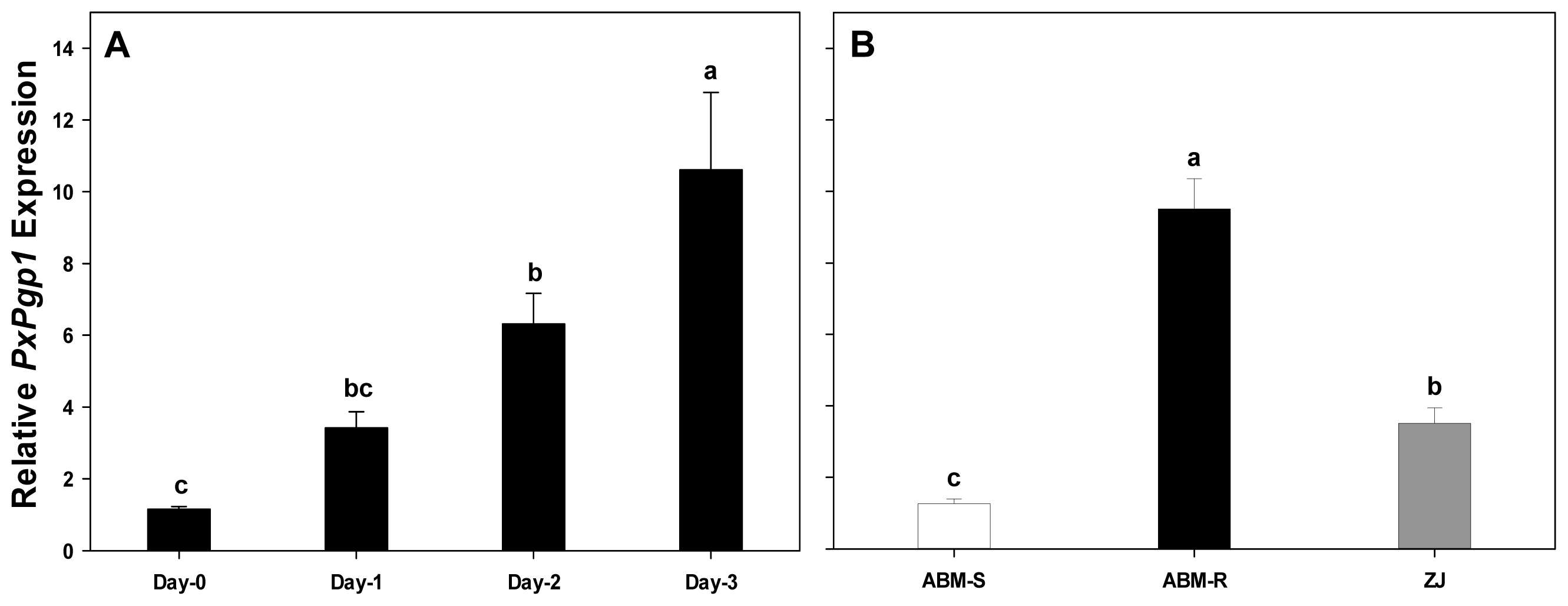
2.4. Transcriptional Response of PxPgp1 to Abamectin Exposure
2.4.1. Acute Response of PxPgp1 to Abamectin Treatment
2.4.2. Constitutive Expression of PxPgp1 in Abamectin-Resistant and -Susceptible Plutella xylostella
3. Experimental Section
3.1. Plutella Xylostella Strains
3.2. Chemicals and Bioassay
3.3. Molecular Cloning of Pgp
3.4. Bioinformatics Analysis
3.5. Quantitative Real-Time RT-PCR
3.6. Statistical Analysis
4. Conclusions
Supplementary Information
ijms-14-22891-s001.pdf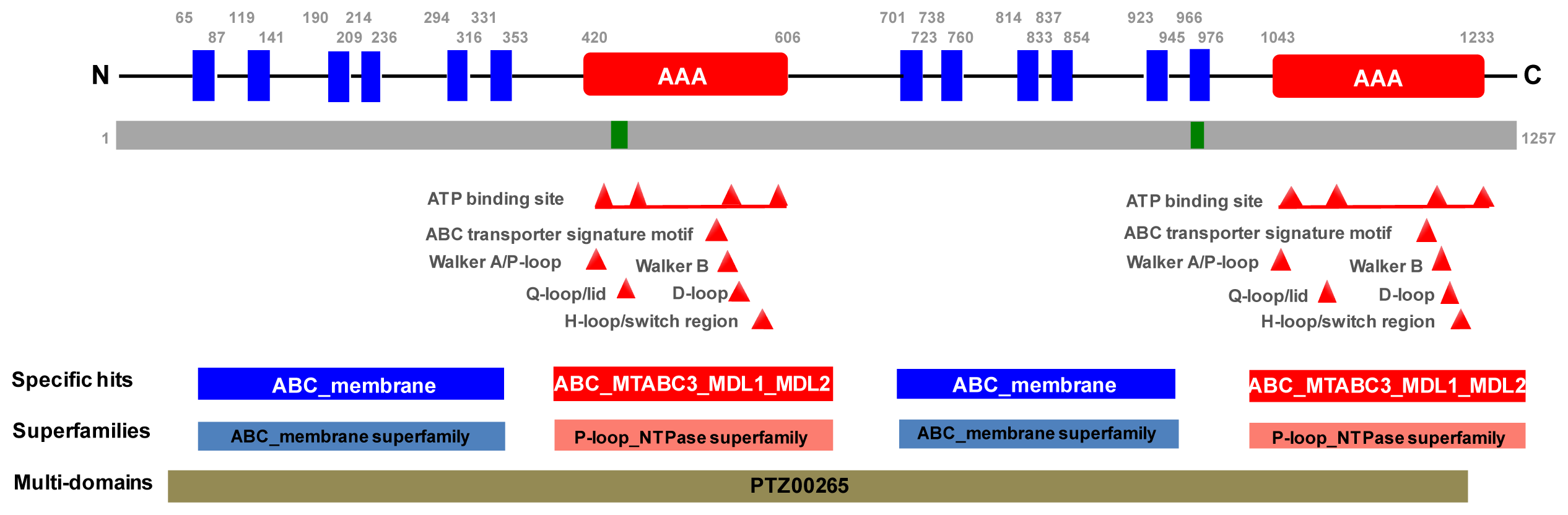

| Strain | LC50 (95% FL) (mg/L−1) | Slope (±SE) | RR a |
|---|---|---|---|
| ABM-S | 0.0025 (0.0015–0.042) | 1.651 (±0.218) | 1 |
| ABM-R | 5.442 (3.487–8.493) | 1.882 (±0.252) | 217.68 |
| ZJ | 0.557 (0.242–0.915) | 1.682 (±0.221) | 23.08 |
| Primer | Position | Sequence (5′-3′) | Length (bp) |
|---|---|---|---|
| 1 | 1060 1566 | F: TAYGCTCTKGCMTTCTGG R: GGYTCCTGACYCACSASRCC | 506 |
| 2 | 3289 3722 | F: AGYGGCTGYGGVAAGAGYAC R: CTTGVACMACCTTTTCACTTTC | 433 |
| 3 | 1429 3309 | F: CGGCAAGTCGACCATCATAC R: GTACTCTTCCCGCAGCCGC | 1880 |
| GSP1 | 3645 | GCTGAGACGACCGAAGATGCTCCTAC | 520 |
| GSP2 | 1273 | TAGCAGGGGGTTGATGGACGGCAC | 1273 |
| Gene | Accession number | Primer sequence (5′-3′) |
|---|---|---|
| ACTB | AB282645 | F: TGGGTATGGAATCTTGCGG R: GGACATGACGGTGTTGGCG |
| EF1 | EF417849 | F: GCCTCCCTACAGCGAATC R: CCTTGAACCAGGGCATCT |
| GAPDH | AJ489521 | F: GCCACCACTGCCACTC R: CGGGACGGGAACACG |
| RPL32 | AB180441 | F: CCAATTTACCGCCCTACC R: TACCCTGTTGTCAATACCTCT |
| RPS13 | AY174891 | F: TCAGGCTTATTCTCGTCG R: GCTGTGCTGGATTCGTAC |
| RPS23 | AB180672 | F: ATGGGCTGACAAGGATTAC R: TGCGGATGGCAGAGTT |
| PxPgp1 | F: GGAATAGTGGCACAAGATGG R: TCTGGAGTCCTGGAGTTATCG |
Acknowledgments
Conflicts of Interest
References
- Talekar, N.S.; Shelton, A.M. Biology, ecology, and management of the diamondback moth. Annu. Rev. Entomol 1993, 38, 275–301. [Google Scholar]
- Zalucki, M.P.; Shabbir, A.; Silva, R.; Adamson, D.; Liu, S.S.; Furlong, M.J. Estimating the economic cost of one of the world’s major insect pests, Plutella xylostella: Just how long is a piece of string? J. Econ. Entomol 2012, 105, 1115–1129. [Google Scholar]
- Furlong, M.J.; Wright, D.J.; Dosdall, L.M. Diamondback moth ecology and management: Problems, progress and prospects. Annu. Rev. Entomol 2013, 58, 517–541. [Google Scholar]
- Iqbal, M.; Verkerk, R.H.J.; Furlong, M.J.; Ong, P.C.; Rahman, S.A.; Wright, D.J. Evidence for resistance to Bacillus thuringiensis (Bt) subsp. kurstaki HD-1, Bt subsp. aizawai and abamectin in field populations of Plutella xylostella from Malaysia. Pestic. Sci 1996, 48, 89–97. [Google Scholar]
- Pu, X.; Yang, Y.H.; Wu, S.W.; Wu, Y.D. Characterisation of abamectin resistance in a field-evolved multiresistant population of Plutella xylostella. Pest Manag. Sci 2010, 66, 371–378. [Google Scholar]
- Liang, P.; Gao, X.W.; Zheng, B. Genetic basis of resistance and studies on cross-resistance in a population of diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Pest Manag. Sci 2003, 59, 1232–1236. [Google Scholar]
- Qian, L.; Cao, G.C.; Song, J.X.; Yin, Q.; Han, Z. Biochemical mechanisms conferring cross-resistance between tebufenozide and abamectin in Plutella xylostella. Pestic. Biochem. Physiol 2008, 91, 175–179. [Google Scholar]
- Sarfraz, M.; Keddie, B.A. Conserving the efficacy of insecticides against Plutella xylostella (L.) (Lep., Plutellidae). J. Appl. Enotomol 2005, 129, 149–157. [Google Scholar]
- Huang, H.S.; Hu, N.T.; Yao, Y.E.; Wu, C.Y.; Chiang, S.W.; Sun, C.N. Molecular cloning and heterologous expression of a glutathione S-transferase involved in insecticide resistance from the diamondback moth Plutella xylostella. Insect Biochem. Mol. Biol 1998, 28, 651–658. [Google Scholar]
- Wu, Q.J.; Zhang, W.J.; Zhang, Y.J.; Xu, B.Y.; Zhu, G.R. Cuticular penetration and desensitivity of GABAA receptor in abamectin resistant Plutella xylostella. L. Acta Entomol. Sin 2002, 45, 336–340. (in Chinese). [Google Scholar]
- Perry, T.; Batterham, P.; Daborn, P.J. The biology of insecticidal activity and resistance. Insect Biochem. Mol. Biol 2011, 41, 411–422. [Google Scholar]
- Ludmerer, S.W.; Warren, V.A.; Williams, B.S.; Zheng, Y.C.; Hunt, D.C.; Ayer, M.B.; Wallace, M.A.; Chaudhary, A.G.; Egan, M.A.; Meinke, P.T.; et al. Ivermectin and nodulisporic acid receptors in Drosophila melanogaster contain both γ-aminobutyric acid-gated rdl and glutamate-gated GluClα chloride channel subunits. Biochemistry 2002, 41, 6548–6560. [Google Scholar]
- Ffrench-Constant, R.H.; Daborn, P.J.; Goff, G.L. The genetics and genomics of insecticide resistance. Trends Genet 2004, 20, 163–170. [Google Scholar]
- Wolstenholme, A.J.; Rogers, A.T. Glutamate-gated chloride channels and the mode of action of the abamectin/milbemycin anthelmintics. Parasitology 2005, 131, S85–S95. [Google Scholar]
- Zhou, X.M.; Wu, Q.J.; Zhang, Y.J.; Bai, L.Y.; Huang, X.Y. Cloning and characterization of a GABA receptor from Plutella xylostella (Lepidoptera: Plutellidae). J. Econ. Entomol 2008, 101, 1888–1896. [Google Scholar]
- Casida, J.E.; Durkin, K.A. Neuroactive insecticides: Targets, selectivity, resistance and secondary effects. Annu. Rev. Entomol 2013, 58, 99–117. [Google Scholar]
- Liu, S.M.; Zhou, S.; Tian, L.; Guo, E.E.; Luan, Y.X.; Zhang, J.Z.; Li, S. Genome-wide identification and characterization of ATP-binding cassette transporters in the silkworm Bombyx mori. BMC Genomics 2011, 12, 1471–2164. [Google Scholar]
- Xie, X.D.; Cheng, T.C.; Wang, G.H.; Duan, J.; Niu, W.H.; Xia, Q.Y. Genome-wide analysis of the ATP-binding cassette (ABC) transporter gene family in the silkworm Bombyx mori. Mol. Biol. Rep 2012, 39, 7281–7291. [Google Scholar]
- Labbé, R.; Caveney, S.; Donly, C. Genetic analysis of the xenobiotic resistance- associated ABC gene subfamilies of the Lepidoptera. Insect Mol. Biol 2011, 20, 243–256. [Google Scholar]
- Xie, W.; Lei, Y.Y.; Fu, W.; Yang, Z.X.; Zhu, X.; Guo, Z.J.; Wu, Q.J.; Wang, S.L.; Xu, B.Y.; Zhou, X.G.; et al. Tissue-specific transcriptome profiling of Plutella Xylostella third instar larval midgut. Int. J. Biol. Sci 2012, 8, 1142–1155. [Google Scholar]
- Baxter, S.W.; Badenes-Pérez, F.R.; Morrison, A.; Vogel, H.; Crickmore, N.; Kain, W.; Wang, P.; Heckel, D.G.; Jiggins, C.D. Parallel evolution of Bacillus thuringiensis toxin resistance in Lepidoptera. Genetics 2011, 189, 675–679. [Google Scholar]
- Dassa, E.; Bouige, P. The ABC of ABCs: A phylogenetic and functional classification of ABC systems in living organisms. Res. Microbiol 2001, 152, 211–229. [Google Scholar]
- Dean, M.; Hamon, Y.; Chimini, G. The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res 2001, 42, 1007–1017. [Google Scholar]
- Holland, I.B.; Cole, S.P.C.; Kuchler, K.; Higgins, C.F. ABC Proteins: From Bacteria to Man; Academic Press: London, UK, 2003. [Google Scholar]
- Dean, M.; Annilo, T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu. Rev. Genomics Hum. Genet 2005, 6, 123–142. [Google Scholar]
- Sturm, A.; Cunningham, P.; Dean, M. The ABC transporter gene family of Daphnia pulex. BMC Genomics 2009, 10, 170–188. [Google Scholar]
- Sheps, J.A.; Ralph, S.; Zhao, Z.Y.; Baillie, D.L.; Ling, V. The ABC transporter gene family of Caenorhabditis elegans has implications for the evolutionary dynamics of the ABC transporter gene family multidrug resistance in eukaryotes. Genome Biol 2004, 5, R15:1–R15:17. [Google Scholar]
- Dermauw, W.; Osborne, E.J.; Clark, R.M.; Grbić, M.; Tirry, L.; Leeuwen, T.V. A burst of ABC genes in the genome of the polyphagous spider mite Tetranychus urticae. BMC Genomics 2013, 14, 317. [Google Scholar]
- Lespine, A.; Alvinerie, M.; Vercruysse, J.; Prichard, R.K.; Geldhof, P. ABC transporter modulation: A strategy to enhance the activity of macrocyclic lactone anthelmintics. Trends Parasitol 2008, 24, 293–298. [Google Scholar]
- Juliano, R.L.; Ling, V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. BBA-Biomembranes 1976, 455, 152–162. [Google Scholar]
- Sharom, F.J. The P-glycoprotein multidrug transporter. Essays Biochem 2011, 50, 161–178. [Google Scholar]
- Gottesman, M.M.; Ling, V. The molecular basis of multidrug resistance in cancer: The early years of P-glycoprotein research. FEBS Lett 2006, 580, 998–1009. [Google Scholar]
- Aller, S.G.; Yu, J.; Ward, A.; Weng, Y.; Chittaboina, S.; Zhuo, R.; Harrell, P.M.; Trinh, Y.T.; Zhang, Q.H.; Urbatsch, I.L.; et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 2009, 323, 1718–1722. [Google Scholar]
- Didier, A.; Loor, F. The abamectin derivative ivermectin is a potent P-glycoprotein inhibitor. Anti-Cancer Drugs 1996, 7, 745–751. [Google Scholar]
- Lespine, A.; Dupuy, J.; Orlowski, S.; Nagy, T.; Glavinas, H.; Krajcsi, P.; Alvinerie, M. Interaction of ivermectin with multidrug resistance proteins (MRP1, 2 and 3). Chem. Biol. Interact 2006, 159, 169–179. [Google Scholar]
- Lespine, A.; Martin, S.; Dupuy, J.; Roulet, A.; Pineay, T.; Orlowski, S.; Alvinerie, M. Interaction of macrocyclic lactones with P-glycoprotein: Structure–affinity relationship. Eur. J. Pharm. Sci 2007, 30, 84–94. [Google Scholar]
- Lespine, A.; Dupuy, J.; Alvinerie, M.; Comera, C.; Nagy, T.; Krajcsi, P.; Orlowski, S. Interaction of macrocyclic lactones with the multidrug transporters: The bases of the pharmacokinetics of lipid-like drugs. Curr. Drug Metab 2009, 10, 272–288. [Google Scholar]
- Aurade, R.; Jayalakshmi, S.K.; Sreeramulu, K. Stimulatory effect of insecticides on partially purified P-glycoprotein ATPase from the resistant pest Helicoverpa armigera. Biochem. Cell Biol 2006, 84, 1045–1050. [Google Scholar]
- Aurade, R.; Jayalakshmi, S.K.; Sreeramulu, K. P-glycoprotein ATPase from the resistant pest, Helicoverpa armigera: Purification, characterization and effect of various insecticides on its transport function. BBA-Biomembranes 2010, 1798, 1135–1143. [Google Scholar]
- Aurade, R.; Jayalakshmi, S.K.; Sreeramulu, K. Modulation of P-glycoprotein ATPase of Helicoverpa armigera by cholesterol: Effects on ATPase activity and interaction of insecticides. Arch. Insect Biochem 2012, 79, 47–60. [Google Scholar]
- Heumann, J.; Carmichael, S.; Bron, J.E.; Tildesley, A.; Sturm, A. Molecular cloning and characterisation of a novel P-glycoprotein in the salmon louse Lepeophtheirus salmonis. Comp. Biochem. Phys. C 2012, 155, 198–205. [Google Scholar]
- Lanning, C.L.; Fine, R.L.; Corcoran, J.J.; Ayad, H.M.; Rose, R.L.; Abou-Donia, M.B. Tobacco budworm P-glycoprotein: Biochemical characterization and its involvement in pesticide resistance. BBA-Gen. Subj 1996, 1291, 155–162. [Google Scholar]
- Srinivas, R.; Udikeri, S.S.; Jayalakshmi, S.K.; Sreeramulu, K. Identification of factors reponsible for insecticide resistance in Helicoverpa armigera. Comp. Biochem. Phys. C 2004, 137, 261–269. [Google Scholar]
- Huang, Y.J.; Prichard, R.K. Identification and stage-specific expression of two putative P-glycoprotein coding genes in Onchocerca volvulus. Mol. Biochem. Parasitol 1999, 102, 273–281. [Google Scholar]
- James, C.E.; Davey, M.W. Increased expression of ABC transport proteins is associated with ivermectin resistance in the model nematode Caenorhabditis elegans. Int. J. Parasitol 2009, 39, 213–220. [Google Scholar]
- Luo, L.; Sun, Y.J.; Wu, Y.J. Abamectin resistance in Drosophila is related to increased expression of P-glycoprotein via the dEGFR and dAkt pathways. Insect Biochem. Mol 2013, 43, 627–634. [Google Scholar]
- Proudfoot, N.J.; Brownlee, G.G. 3′ non-coding region sequences in eukaryotic messenger RNA. Nature 1976, 263, 211–214. [Google Scholar]
- Senior, A.E.; Bhagat, S. P-glycoprotein shows strong catalytic cooperativity between the two nucleotide sites. Biochemistry 1998, 37, 831–836. [Google Scholar]
- Locher, K.P. Structure and mechanism of ATP-binding cassette transporters. Philos. Trans. R. Soc. B 2009, 364, 239–245. [Google Scholar]
- Sauna, Z.E.; Ambudkar, S.V. About a switch: How P-glycoprotein (ABCB1) harnesses the energy of ATP binding and hydrolysis to do mechanical work. Mol. Cancer Ther 2007, 6, 13–23. [Google Scholar]
- Jin, M.S.; Oldham, M.L.; Zhang, Q.J.; Chen, J. Crystal structure of the multidrug transporter P-glycoprotein from Caenorhabditis elegans. Nature 2012, 490, 566–569. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol 2011, 28, 2731–2739. [Google Scholar]
- Simmons, J.; D’Souza, O.; Rheault, M.; Donly, C. Multidrug resistance protein gene expression in Trichoplusia ni caterpillars. Insect Mol. Biol 2013, 22, 62–71. [Google Scholar]
- Gaertner, L.S.; Murray, C.L.; Morris, C.E. Transepithelial transport of nicotine and vinblastine in isolated malpighian tubules of the tobacco hornworm (Manduca sexta) suggests a P-glycoprotein-like mechanism. J. Exp. Biol 1998, 201, 2637–2645. [Google Scholar]
- Tapadia, M.G.; Lakhotia, S.C. Expression of mdr49 and mdr65 multidrug resistance genes in larval tissues of Drosophila melanogaster under normal and stress conditions. Cell Stress Chaperones 2005, 10, 7–11. [Google Scholar]
- Fromm, M.F. Importance of P-glycoprotein at blood-tissue barriers. Trends Pharmacol. Sci 2004, 25, 423–429. [Google Scholar]
- Hennessy, M.; Spiers, J.P. A primer on the mechanics of P-glycoprotein the multidrug transporter. Pharmacol. Res 2007, 55, 1–15. [Google Scholar]
- Xu, M.; Molento, M.; Blackhall, W.; Ribeiro, P.; Beech, R.; Prichard, R. Ivermectin resistance in nematodes may be caused by alteration of P-glycoprotein homolog. Mol. Biochem. Parasitol 1998, 91, 327–335. [Google Scholar]
- Stege, A.; Priebsch, A.; Nieth, C.; Lage, H. Stable and complete overcoming of MDR1/P-glycoprotein-mediated multidrug resistance in human gastric carcinoma cells by RNA interference. Cancer Gene Ther 2004, 11, 699–706. [Google Scholar]
- Pichler, A.; Zelcer, N.; Prior, J.L.; Kuil, A.J.; Piwnica-Worms, D. In vivo RNA interference-mediated ablation of MDR1 P-glycoprotein. Clin. Cancer Res 2005, 11, 4487–4494. [Google Scholar]
- Shi, Z.; Liang, Y.J.; Chen, Z.S.; Wang, X.W.; Wang, X.H.; Ding, Y.; Chen, L.M.; Yang, X.P.; Fu, L.W. Reversal of MDR1/P-glycoprotein-mediated multidrug resistance by vector-based RNA interference in vitro and in vivo. Cancer Biol. Ther. 2006, 5, 39–47. [Google Scholar]
- Tabashnik, B.E.; Cushing, N.L. Leaf residue vs. topical bioassays for assessing insecticide resistance in the diamondback moth, Plutella xylostella L. FAO Plant Prot. Bull 1987, 35, 11–14. [Google Scholar]
- Bjellqvist, B.; Hughes, G.J.; Pasquali, C.; Paquet, N.; Ravier, F.; Sanchez, J.-C.; Frutiger, S.; Hochstrasser, D.F. The focusing positions of polypeptides in immobilized pH gradients can be predicted from their amino acid sequences. Electrophoresis 1993, 14, 1023–1031. [Google Scholar]
- Bjellqvist, B.; Basse, B.; Olsen, E.; Celis, J.E. Reference points for comparisons of two-dimensional maps of proteins from different human cell types defined in a pH scale where isoelectric points correlate with polypeptide compositions. Electrophoresis 1994, 15, 529–539. [Google Scholar]
- Petersen, T.N.; Brunak, S.; Heijne, G.V.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar]
- Hofmann, K.; Stoffel, W. Tmbase—A database of membrane spanning proteins segments. Biol. Chem. Hoppe-Seyler 1993, 374, 166. [Google Scholar]
- Marchler-Bauer, A.; Lu, S.; Anderson, J.B.; Chitsaz, F.; Derbyshire, M.K.; DeWeese-Scott, C.; Fong, J.H.; Geer, L.Y.; Geer, R.C.; Gonzales, N.R.; et al. CDD: A Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res 2011, 39, 225–229. [Google Scholar]
- Arnold, K.; Bordoli, L.; Kopp, J.; Schwede, T. The SWISS-MODEL Workspace: A web-based environment for protein structure homology modeling. Bioinformatics 2006, 22, 195–201. [Google Scholar]
- Kiefer, F.; Arnold, K.; Künzli, M.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res 2009, 37, D387–D392. [Google Scholar]
- The PyMOL Molecular Graphics System, Version 1.5.0.4; Schrödinger, LLC: New York, NY, USA, 2011.
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol 1987, 4, 406–425. [Google Scholar]
- You, M.; Yue, Z.; He, W.; Yang, X.; Yang, G.; Xie, M.; Zhan, D.; Baxter, S.W.; Vasseur, L.; Gurr, G.M.; et al. A heterozygous moth genome provides insights into herbivory and detoxification. Nat. Genet 2013, 45, 220–225. [Google Scholar] [Green Version]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 1994, 22, 4673–4680. [Google Scholar]
- Fu, W.; Xie, W.; Zhang, Z.; Wang, S.L.; Wu, Q.J.; Liu, Y.; Zhou, X.M.; Zhou, X.G.; Zhang, Y.J. Exploring valid reference genes for quantitative real-time PCR analysis in Plutella xylostella (Lepidoptera: Plutellidae). Int. J. Biol. Sci 2013, 9, 792–802. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001, 29, e45. [Google Scholar]
- Finney, D.J. Probit Analysis, 3d ed; Cambridge University Press: London, UK, 1971. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tian, L.; Yang, J.; Hou, W.; Xu, B.; Xie, W.; Wang, S.; Zhang, Y.; Zhou, X.; Wu, Q. Molecular Cloning and Characterization of a P-Glycoprotein from the Diamondback Moth, Plutella xylostella (Lepidoptera: Plutellidae). Int. J. Mol. Sci. 2013, 14, 22891-22905. https://doi.org/10.3390/ijms141122891
Tian L, Yang J, Hou W, Xu B, Xie W, Wang S, Zhang Y, Zhou X, Wu Q. Molecular Cloning and Characterization of a P-Glycoprotein from the Diamondback Moth, Plutella xylostella (Lepidoptera: Plutellidae). International Journal of Molecular Sciences. 2013; 14(11):22891-22905. https://doi.org/10.3390/ijms141122891
Chicago/Turabian StyleTian, Lixia, Jiaqiang Yang, Wenjie Hou, Baoyun Xu, Wen Xie, Shaoli Wang, Youjun Zhang, Xuguo Zhou, and Qingjun Wu. 2013. "Molecular Cloning and Characterization of a P-Glycoprotein from the Diamondback Moth, Plutella xylostella (Lepidoptera: Plutellidae)" International Journal of Molecular Sciences 14, no. 11: 22891-22905. https://doi.org/10.3390/ijms141122891








