Synthesis of 2-Acyloxycyclohexylsulfonamides and Evaluation on Their Fungicidal Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Structure Elucidation
2.2. Screening of Fungicidal Activity and SAR
2.2.1. In Vitro Fungicidal Activity of Compounds III-1–III-18 against B. cinerea
2.2.2. Fungicidal Activity of Compounds III-9 and III-18 against Ten Pathogenic Fungi
2.2.3. In Vivo Fungicidal Activity of Compounds III-18, 19 and 20 against B. cinerea
3. Experimental Section
3.1. General Information
3.2. Synthetic Procedures
3.2.1. Synthesis of Compounds I
3.2.2. Synthesis of Acyl Chloride II
- II-1: Benzoyl chloride, colorless liquid, bp 91–92 °C/31 mmHg, yield 85%.
- II-2: 3-Methylbenzoyl chloride, colorless liquid, bp 90–92 °C/10 mmHg, yield 86%.
- II-3: 4-Methylbenzoyl chloride, colorless liquid, bp 117–119 °C/15 mmHg, yield 88%.
- II-4: 4-Methoxybenzoyl chloride, white solid, bp 153–156 °C/15mmHg, yield 80%.
- II-5: 4-Fluorobenzoyl chloride, colorless liquid, bp 87–88 °C/25 mmHg, yield 88%.
- II-6: 2-Chlorobenzoyl chloride, colorless liquid, bp 110–114 °C/8 mmHg, yield 85%.
- II-7: Propionyl chloride, colorless, bp 77–79 °C, yield 84%.
- II-8: Phenoxyacetyl chloride, colorless liquid, bp 103–105 °C/7 mmHg, yield 82%.
- II-9: 2-Ethoxyacetyl chloride, colorless liquid, bp 65–67 °C/33 mmHg, yield 82%.
- II-10: 2-Methoxyacetyl chloride, colorless liquid, bp 112–113 °C, yield 85%.
3.2.3. Synthesis of Compounds III-1–III-18
N-(2-Trifluoromethyl-4-chlorophenyl)-2-benzoyloxy-cyclohexylsulfonamide (III-1)
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3-methyl-benzoyloxy)-cyclohexylsulfonamide (III-2)
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(4-methyl-benzoyloxy)-cyclohexylsulfonamide (III-3)
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(4-methoxy-benzoyloxy)-cyclohexylsulfonamide (III-4)
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(4-fluoro-benzoyloxy)-cyclohexylsulfonamide (III-5)
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(2-chloro-benzoyloxy)-cyclohexylsulfonamide (III-6)
N-(2-Trifluoromethyl-4-chlorophenyl)-2-propionyloxy-cyclohexylsulfonamide (III-7)
N-(2-Trifluoromethyl-4-chlorophenyl)-2-phenoxyacetoxy-cyclohexylsulfonamide (III-8)
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(2-ethoxyacetoxy)-cyclohexylsulfonamide (III-9)
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(2-methoxyacetoxy)-cyclohexylsulfonamide (III-10)
N-(2,4,5-Trichlorophenyl)-2-benzoyloxy-cyclohexylsulfonamide (III-11)
N-(2,4,5-Trichlorophenyl)-2-(3-methylbenzoyloxy)-cyclohexylsulfonamide (III-12)
N-(2,4,5-Trichlorophenyl)-2-(4-methylbenzoyloxy)-cyclohexylsulfonamide (III-13)
N-(2,4,5-Trichlorophenyl)-2-(4-fluorobenzoyloxy)-cyclohexylsulfonamide (III-14)
N-(2,4,5-Trichlorophenyl)-2-(2-chlorobenzoyloxy)-cyclohexylsulfonamide (III-15)
N-(2,4,5-Trichlorophenyl)-2-propionyloxy-cyclohexylsulfonamide (III-16)
N-(2,4,5-Trichlorophenyl)-2-phenoxyacetoxy-cyclohexylsulfonamide (III-17)
N-(2,4,5-Trichlorophenyl)-2-(2-ethoxyacetoxy)-cyclohexylsulfonamide (III-18)
3.3. Bioassay of Fungicidal Activity
3.3.1. Effect of the Title Compounds on the Mycelial Growth
3.3.2. Effect of Compounds III-9 and III-18 against Ten Major Crop Diseases
3.3.3. Effect on the Ability of B. cinerea to Colonize Detached Leaves of Cucumber
3.3.4. In Vivo Fungicidal Activity against B. cinerea by Pot Culture Test Method in Greenhouse
4. Conclusions
Supplementary Information
ijms-14-22544-s001.pdf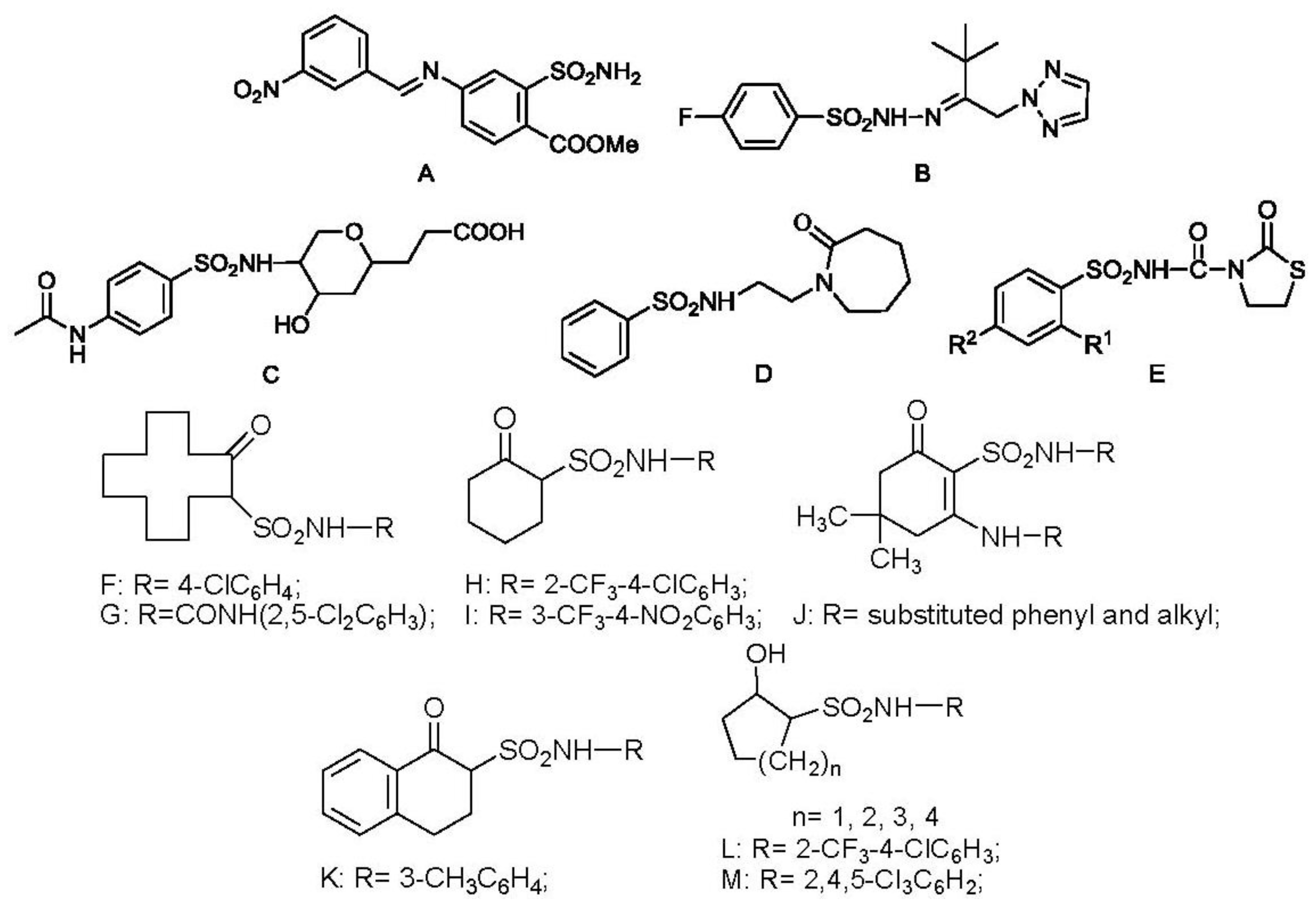



| Compd. | Mycelial growth rate method | Control efficiency on detached leaves of cucumber (500 μg mL−1) (%) (±SEM) | |
|---|---|---|---|
| EC50 (95% confidence limit)/(μg mL−1) | EC80 (95% confidence limit)/(μg mL−1) | ||
| III-1 | 24.36 (10.23–57.99) | >100 | 61.11 (±5.73) de |
| III-2 | 19.94 (10.51–37.80) | >100 | 27.35 (±2.65) kl |
| III-3 | >100 | >100 | 53.33 (±1.20) fg |
| III-4 | 72.28 (7.10–735.67) | >100 | 18.67 (±3.03) kl |
| III-5 | 79.66 (7.90–802.80) | >100 | 43.33 (±8.89) ghi |
| III-6 | 150.33 (50.71–445.65) | >100 | 58.67 (±6.97) defg |
| III-7 | 6.35 (5.33–7.57) | 16.72 (14.03–19.92) | 57.58 (±3.50) fg |
| III-8 | 56.23 (18.13–173.76) | >100 | 48.81 (±9.20) gh |
| III-9 | 7.98 (6.71–9.48) | 20.78 (17.49–24.69) | 80.94 (±2.98) ab |
| III-10 | 7.46 (5.75–9.69) | 31.31 (24.13–40.68) | 70.67 (±8.70) cde |
| III-11 | >100 | - | 43.60 (±3.25) hij |
| III-12 | >100 | - | 39.74 (±7.32) jkl |
| III-13 | 23.86 (16.65–34.19) | 88.48 (61.75–126.78) | 40.47 (±9.55) ij |
| III-14 | 6.20 (5.08–7.58) | 14.37 (11.77–17.55) | 45.75 (±12.55) hi |
| III-15 | 29.05 (9.59–87.99) | >100 | 60.46 (±9.98) efg |
| III-16 | 5.40 (3.66–7.96) | 23.13 (15.69–34.11) | 58.01 (±10.22) gh |
| III-17 | 16.13 (11.02–23.58) | 92.93 (63.55–135.90) | 50.30 (±6.69) ghi |
| III-18 | 4.17 (3.26–5.33) | 17.15 (13.41–21.94) | 96.22 (±13.10) a |
| procymidone | 4.46 (2.28–8.76) | 35.02 (17.94–68.78) | 72.11 (±5.60) bc |
| Fungus | Inhibitory rate (50 μg mL−1) (%) (±SEM) | ||
|---|---|---|---|
| III-9 | III-18 | chlorothalonil | |
| Curvularia lunata (Walker) Boed | 66.78 (±3.07) | 82.93 (±1.58) | 86.39 (±2.05) |
| Bipolaris maydis Shoem | 58.05 (±5.38) | 87.99 (±1.25) | 90.00 (±1.38) |
| Gibberella zeae (Schw.) Petch | 59.01 (±1.48) | 73.23 (±1.80) | 90.96 (±1.48) |
| Colletotrichum gossypii Southw | 75.53 (±1.87) | 74.42 (±1.01) | 51.73 (±1.57) |
| Sclerotinia sclerotiorum (Lib.) de Bary | 78.13 (±0.90) | 73.13 (±1.28) | 95.99 (±0.49) |
| Fusarium oxysporum | 24.02 (±2.13) | 49.77 (±1.40) | 78.07 (±1.35) |
| Macrophoma kuwatsukai Hara | 53.12 (±1.23) | 84.91 (±2.40) | 79.61 (±2.35) |
| Alternaria solani Jones et Grout | 53.76 (±2.30) | 77.03 (±1.45) | 58.94 (±5.17) |
| Corynespora cassiicola | 48.9 (±1.10) | 77.77 (±3.83) | 69.52 (±3.26) |
| Rhizoctonia solani kühn | 19.64 (±3.88) | 44.13 (±2.91) | 68.65 (±2.26) |
 | ||||
|---|---|---|---|---|
| Concentration (μg mL−1) | Compd. |  | Average diameter of the spot (mm) (±SEM) | Inhibitory rate (%) |
| 500 | III-18 | −OCOCH2OC2H5 | 3.0 (±0.6) | 84.12 a |
| 19 | −OH | 4.3 (±0.5) | 77.40 a | |
| 20 | =O | 7.0 (±1.9) | 63.21 b | |
| cyprodinil | 4.1 (±0.5) | 78.47 a | ||
| 125 | III-18 | −OCOCH2OC2H5 | 5.4 (±0.6) | 71.50 a′ |
| 19 | −OH | 6.7 (±0.9) | 64.40 a′b′ | |
| 20 | =O | 8.5 (±1.2) | 55.13 b′ | |
| cyprodinil | 7.0 (±0.8) | 62.80 b′ | ||
| 31.25 | III-18 | −OCOCH2OC2H5 | 9.6 (±1.4) | 49.21 a″ |
| 19 | −OH | 10.5 (±2.0) | 44.22 a″ | |
| 20 | =O | 9.1 (±1.8) | 52.02 a″ | |
| cyprodinil | 8.7 (±0.9) | 54.12 a″ | ||
| 0 | CK | 18.9 (±2.3) | 2.29 | |
Acknowledgments
Conflicts of Interest
References
- Pierre, B.; Martine, V.L.; Arlette, N.; Jean-Michel, L.; Joseph, V.; Stéphane, L.; Gérard, D.; Alain, N. New 2-sulfonamidothiazoles substituted at C-4: Synthesis of polyoxygenated aryl derivatives and in vitro evaluation of antifungal activity. Eur. J. Med. Chem 1999, 34, 773–779. [Google Scholar]
- Ashis, K.; Saha, L.L.; Patrick, M.; Frank, O. Novel antifungals based on 4-substituted imidazole: Solid-phase synthesis of substituted aryl sulfonamides towards optimization of in vitro activity. Bioorg. Med. Chem. Lett 2000, 10, 2735–2739. [Google Scholar]
- Iraj, R.E.; Charalabos, C.; Panagiotis, Z.; Athina, G.; Marina, S.; Jasmina, G.; Ana, C. Sulfonamide-1,2,4-triazole derivatives as antifungal and antibacterial agents: Synthesis, biological evaluation, lipophilicity, and conformational studies. Bioorg. Med. Chem 2008, 16, 1150–1161. [Google Scholar]
- Mahantesha, B.K.; Shivashankar, M.V.; Kulkarni, V.P.; Rasal, H.; Patel, S.S.; Mutha, A.A.M. Synthesis and antimicrobial studies on novel sulfonamides containing 4-azidomethyl coumarin. Eur. J. Med. Chem 2010, 45, 1151–1157. [Google Scholar]
- Zahid, H.; Chohan, M.H.; Youssoufi, Aliasghar J.; Taibi, B.H. Identification of antibacterial and antifungal pharmacophore sites for potent bacteria and fungi inhibition: Indolenyl sulfonamide derivatives. Eur. J. Med. Chem 2010, 45, 1189–1199. [Google Scholar]
- Zoumpoulakis, P.; Camoutsis, C.; Pairas, G.; Soković, M.; Glamočlija, J.; Potamitis, C.; Pitsas, A. Synthesis of novel sulfonamide-1,2,4-triazoles, 1,3,4-thiadiazoles and 1,3,4-oxadiazoles, as potential antibacterial and antifungal agents. Biological evaluation and conformational analysis studies. Eur. J. Med. Chem 2012, 20, 1569–1583. [Google Scholar]
- Keche, A.P.; Hatnapure, G.D.; Tale, R.H.; Rodge, A.H.; Birajdar, S.S.; Kamble, V.M. A novel pyrimidine derivatives with aryl urea, thiourea and sulfonamide moieties: Synthesis, anti-inflammatory and antimicrobial evaluation. Bioorg. Med. Chem. Lett 2012, 22, 3445–3448. [Google Scholar]
- Keche, A.P.; Hatnapure, G.D.; Tale, R.H.; Rodge, A.H.; Kamble, V.M. Synthesis, anti-inflammatory and antimicrobial evaluation of novel 1-acetyl-3,5-diaryl-4,5-dihydro (1H) pyrazole derivatives bearing urea, thiourea and sulfonamide moieties. Bioorg. Med. Chem. Lett 2012, 22, 6611–6615. [Google Scholar]
- Fujita, T. Nebijin (flusulfamide, MTF-651), a new soil fungicide. Agrochem. Jpn 1994, 65, 17–19. [Google Scholar]
- Tanaka, S.; Kochi, S.I.; Kunita, H.; Ito, S.I.; Kameya-Iwaki, M. Biological mode of action of the fungicide, Flusulfamide, against Plasmodiophora brassicae (clubroot). Eur. J. Plant. Pathol 1999, 105, 577–584. [Google Scholar]
- Tabuchi, T.; Yamamoto, T.; Nakayama, M. Preparation of Aryl- or Heterocyclyl-Sulfonamide Derivatives as Agricultural and Horticultural Microbicides. WO Patent WO 2,000,065,913, 9 November 2000. [Google Scholar]
- Shigeru, M.; Satoshi, A.; Tomona, Y.; Yasuko, T.; Takeshi, O.; Norifusa, M. Fungicidal activity of the novel fungicide cyazofamid against Phytophthora infestans and other plant pathogenic fungi in vitro. Pestic. Biochem. Physiol. 2001, 71, 92–99. [Google Scholar]
- Yang, J.C.; Wu, Q.; Liu, R.L.; Ma, S.C.; Liu, C.L. Recent advances on the development of agricultural fungicides. Agrochemicals 2008, 47, 402–405. [Google Scholar]
- Wang, M.Y.; Li, Z.M. Synthesis and Bioactivities of Novel 2-Methoxycarbonyl-5-arylazomethine Benzenesulfonamide. Chin. J. Synth. Chem 2012, 20, 65–68. [Google Scholar]
- Zeng, D.Q.; Zhang, Y.M.; Chen, J. Studies on the synthesis and fungicidal activity of N-phenylsulfonyl-α-triazolyl-pinacolone-hydrazone. J. Guangxi Agric. Biol. Sci 2004, 23, 313–315. [Google Scholar]
- Zhong, Z.M.; Chen, R.; Xing, R.G.; Chen, X.L.; Liu, S.; Guo, Z.Y.; Ji, X.; Wang, L.; Li, P.C. Synthesis and antifungal properties of sulfanilamide derivatives of chitosan. Carbohydr. Res 2007, 342, 2390–2395. [Google Scholar]
- Liang, X.M.; Fang, X.Q.; Dong, Y.H.; Yuan, H.Z.; Qi, S.H.; Wang, D.Q.; Wu, J.P. Synthesis and fungicidal activity of N-(arylsulfonylaminoethyl)-1,6-caprolactam. Chin. J. Pestic. Sci 2008, 10, 156–160. [Google Scholar]
- Chen, Q.W.; Shen, D.L. Synthesis and biological activity of novel 2-thiazolidiones sulfonylurea derivatives. J. Zhejiang Univ. Technol 2008, 36, 562–564. [Google Scholar]
- Wang, X.P.; Wang, D.Q. Synthesis and antifungal activity of N-substituted-2-oxocyclododecylsulphonamides. Chem. J. Chin. Univ 1997, 18, 889–893. [Google Scholar]
- Li, X.H.; Yang, X.L.; Ling, Y.; Fan, Z.J.; Wang, D.Q.; Li, Z.M. The Synthesis and fungicidal Activity of Novel α-Oxocycloalkylsulfonylureas. J. Agric. Food Chem 2005, 53, 2202–2206. [Google Scholar]
- Li, X.H.; Yang, X.L.; Liang, X.M.; Kai, Z.P.; Yuan, H.Z.; Wang, D.Q. Synthesis and biological activities of 2-oxocycloalkylsulfonamides. Bioorg. Med. Chem 2008, 16, 4538–4544. [Google Scholar]
- Yang, H.Y.; Yan, X.J.; Yuan, H.Z.; Liang, X.M.; Zhang, J.J.; Wang, D.Q. Synthesis and fungicidal activity of 2-oxocyclohexylsulfonamides containing fluorine. Chin. J. Pestic. Sci 2010, 12, 449–452. [Google Scholar]
- Li, X.H.; Ji, M.S.; Qi, Z.Q.; Li, X.W.; Shen, Y.X.; Gu, Z.M.; Zhang, Y.; Wei, S.H.; Wang, Y.Z.; Wang, D.Q. Synthesis of 2-amino-6-oxocyclohexenylsulfonamides and their activity againstBotrytis cinerea. Pest Manag. Sci 2011, 67, 986–992. [Google Scholar]
- Liang, X.M.; Wang, D.Q.; Yang, H.Y.; Wu, J.P.; Zhang, J.J.; Yan, X.J. Preparation method of 1-oxotetralyl-2-sulfonamide and its usage as a fungicide. Patent CN 101,503,381, 12 August 2009. [Google Scholar]
- Li, X.H.; Wu, D.C.; Qi, Z.Q.; Li, X.W.; Gu, Z.M.; Wei, S.H.; Zhang, Y.; Wang, Y.Z.; Ji, M.S. Synthesis, fungicidal activity, and structure-activity relationship of 2-oxo and 2-hydroxycycloalkylsulfonamides. J. Agric. Food Chem 2010, 58, 11384–11389. [Google Scholar]
- Li, X.H.; Wu, D.C.; Qi, Z.Q.; Gu, Z.M.; Li, X.W.; Ji, M.S. Bactericidal activity of 2-oxo and 2-hydroxycycloalkylsulfonamide to 14 kinds of pathogenic fungi. Chin. J. Pestic. Sci 2011, 13, 423–426. [Google Scholar]
- Li, X.H.; Pan, Q.; Cui, Z.N.; Ji, M.S.; Qi, Z.Q. Synthesis and Fungicidal Activity of N-(2,4,5-trichlorophenyl)-2-Oxo- and 2-Hydroxycycloalkylsulfonamides. Lett. Drug Des. Discov 2013, 10, 353–359. [Google Scholar]
- Sano, T.; Ohashi, K.; Oriyama, T. Remarkably fast acylation of alcohols with benzoyl chloride promoted by TMEDA. Synthesis 1999, 7, 1141–1144. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, X.; Cui, Z.; Chen, X.; Wu, D.; Qi, Z.; Ji, M. Synthesis of 2-Acyloxycyclohexylsulfonamides and Evaluation on Their Fungicidal Activity. Int. J. Mol. Sci. 2013, 14, 22544-22557. https://doi.org/10.3390/ijms141122544
Li X, Cui Z, Chen X, Wu D, Qi Z, Ji M. Synthesis of 2-Acyloxycyclohexylsulfonamides and Evaluation on Their Fungicidal Activity. International Journal of Molecular Sciences. 2013; 14(11):22544-22557. https://doi.org/10.3390/ijms141122544
Chicago/Turabian StyleLi, Xinghai, Zining Cui, Xiaoyuan Chen, Decai Wu, Zhiqiu Qi, and Mingshan Ji. 2013. "Synthesis of 2-Acyloxycyclohexylsulfonamides and Evaluation on Their Fungicidal Activity" International Journal of Molecular Sciences 14, no. 11: 22544-22557. https://doi.org/10.3390/ijms141122544
APA StyleLi, X., Cui, Z., Chen, X., Wu, D., Qi, Z., & Ji, M. (2013). Synthesis of 2-Acyloxycyclohexylsulfonamides and Evaluation on Their Fungicidal Activity. International Journal of Molecular Sciences, 14(11), 22544-22557. https://doi.org/10.3390/ijms141122544





