Effects of Soybean Agglutinin on Mechanical Barrier Function and Tight Junction Protein Expression in Intestinal Epithelial Cells from Piglets
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of SBA on Intestinal Permeability Barrier Function
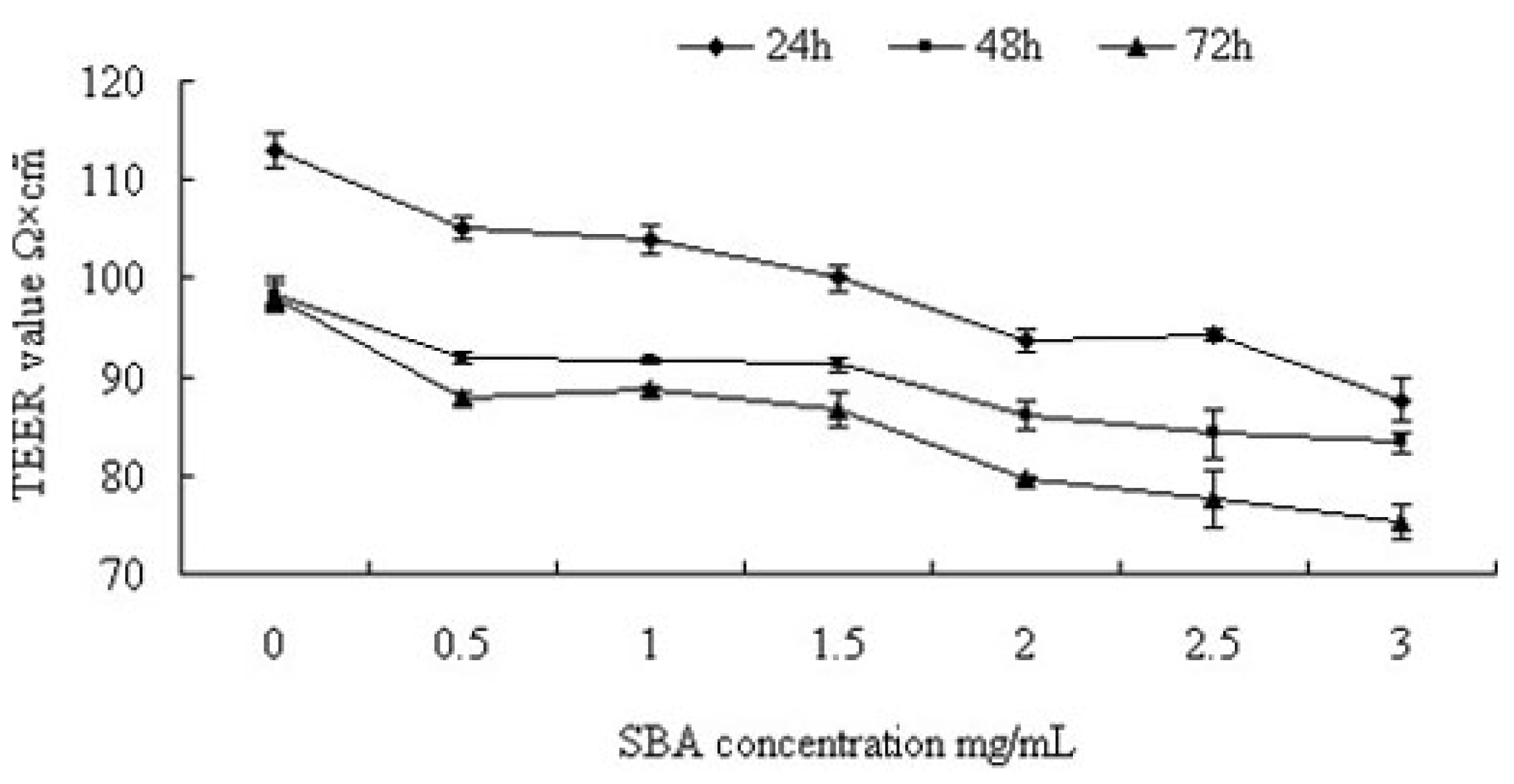
2.1.1. Trans-Epithelial Electrical Resistance (TEER) Value Analysis
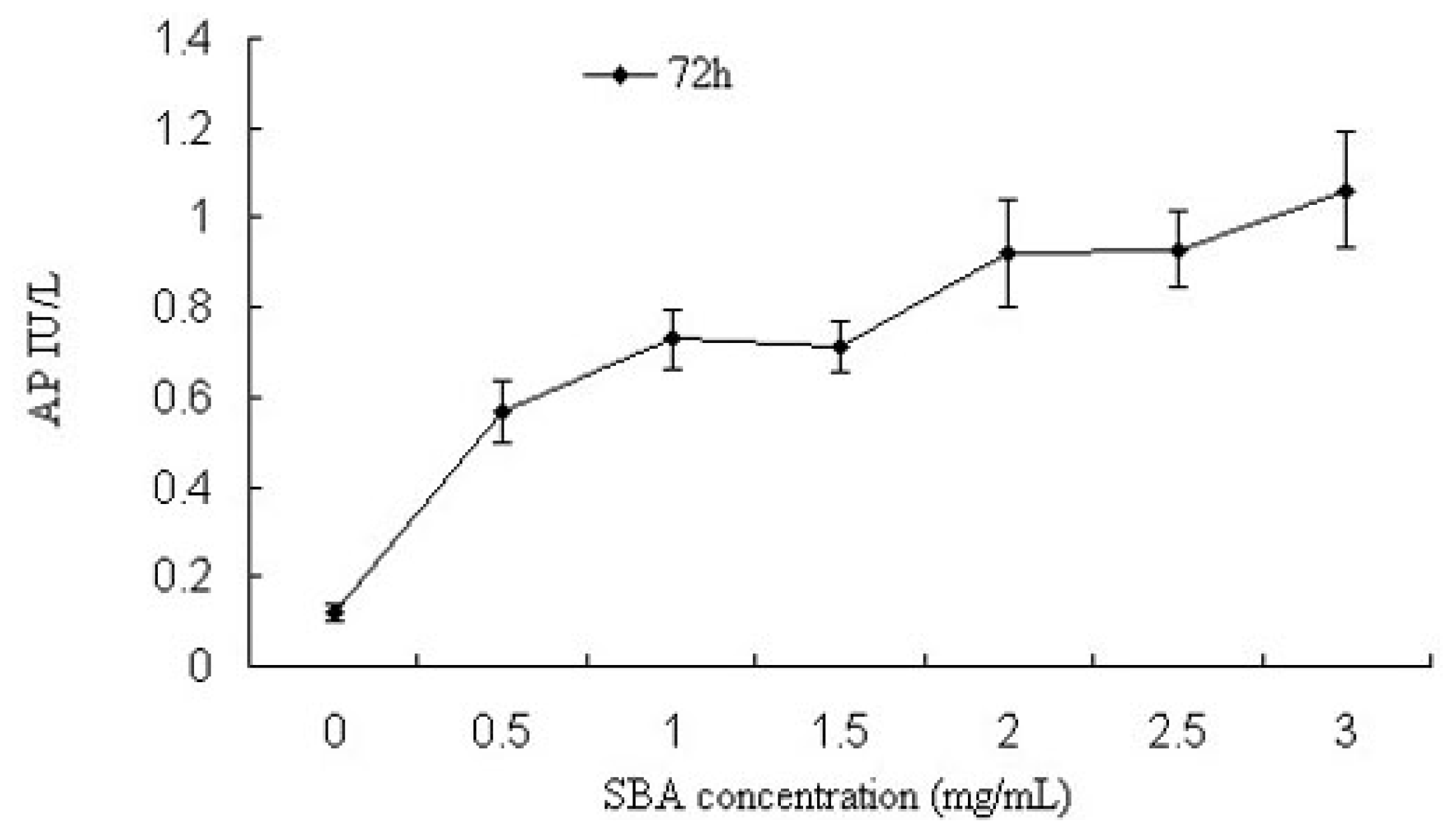
2.1.2. Alkaline Phosphatase (AP) Activity Analysis
2.2. Effects of SBA on Intestinal Epithelial Cell Viability and Cellular Morphology
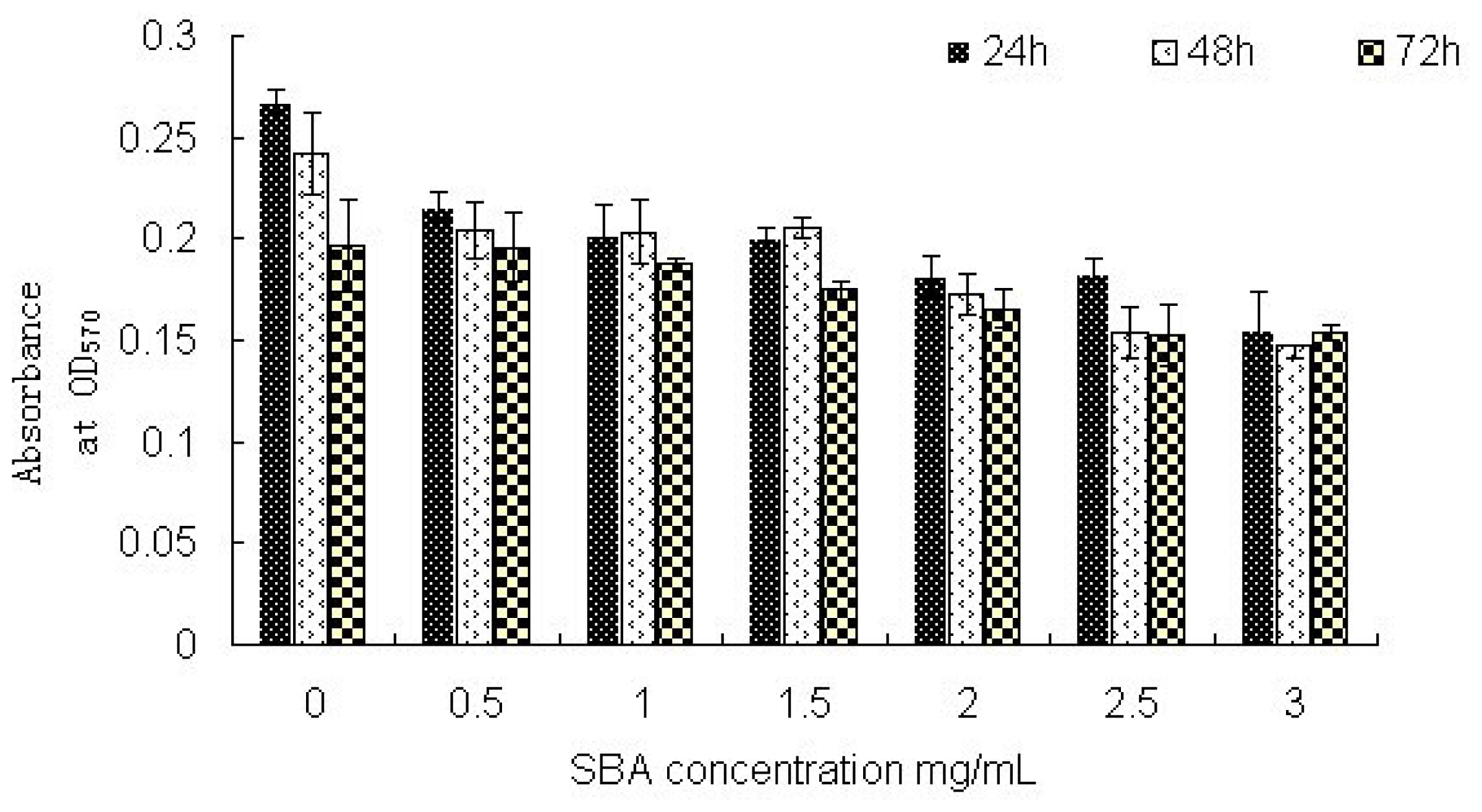
2.2.1. Cell Viability: MTT Assay Analysis
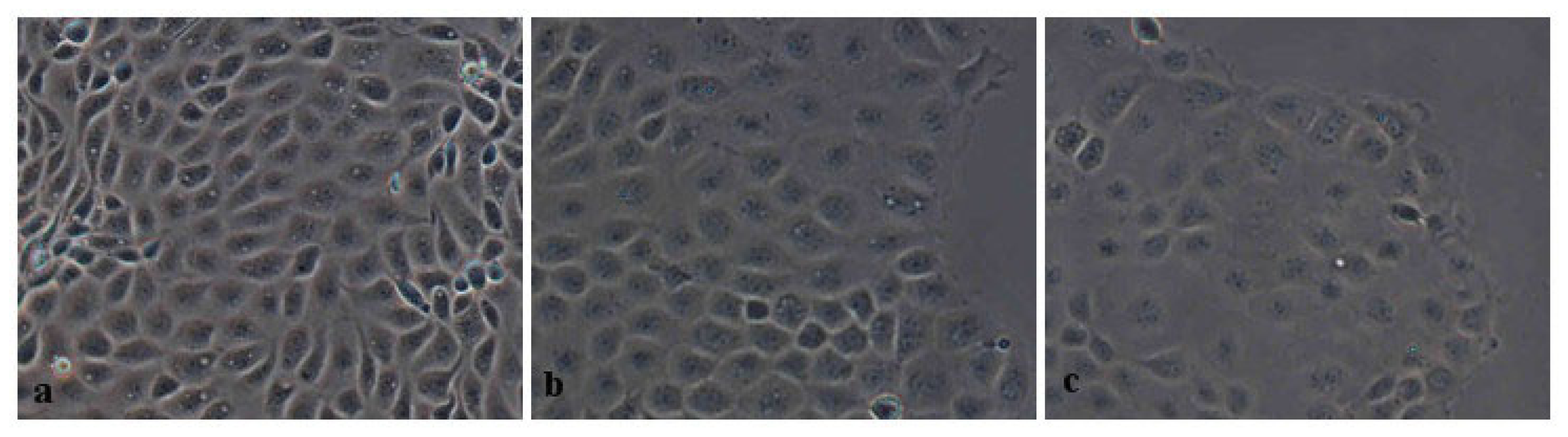
2.2.2. Morphometric Analysis
2.2.3. Pearson Correlation Analysis
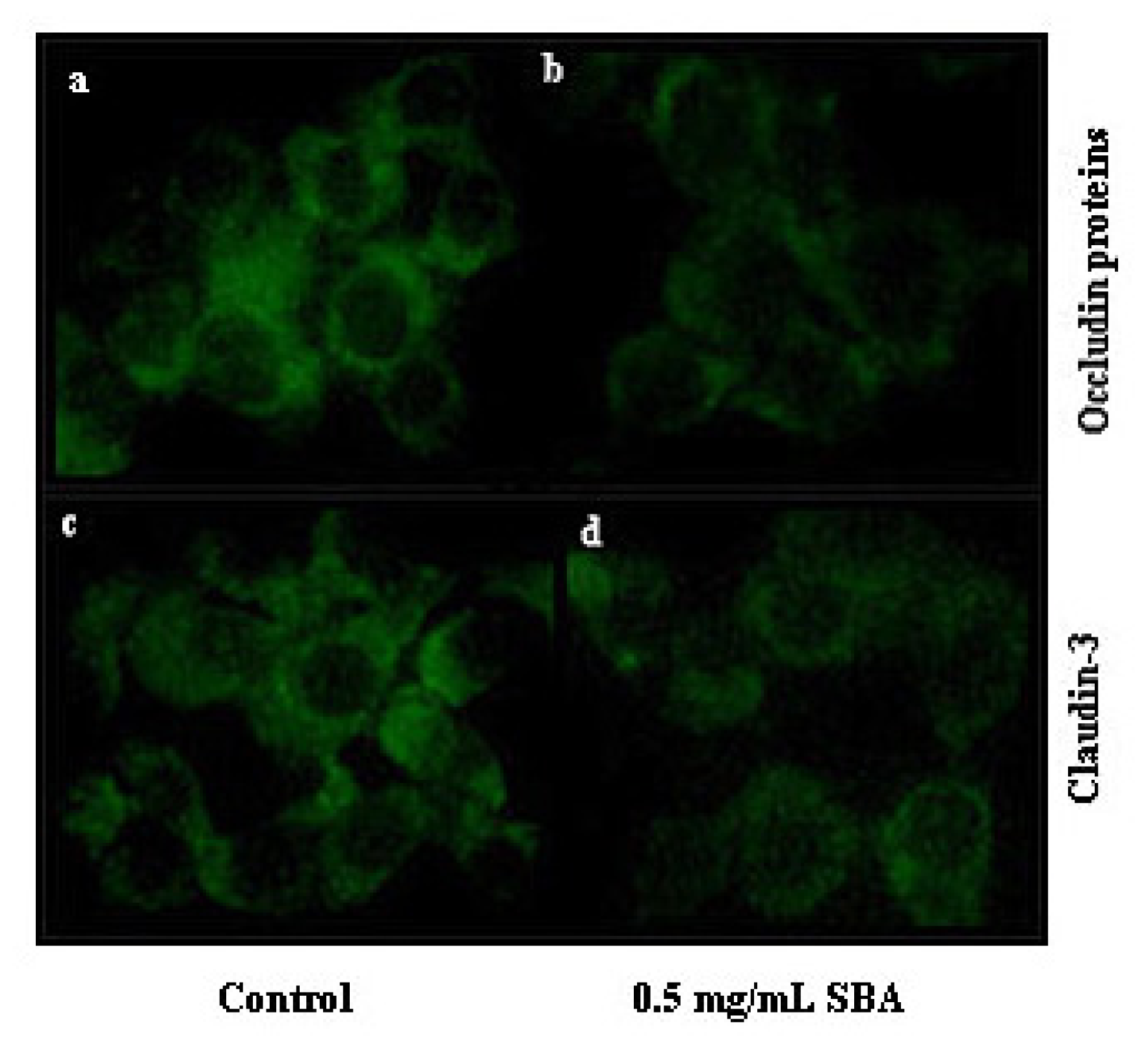
2.3. Effects of SBA on the Distribution and Expression of TJ Proteins
2.3.1. Effects of SBA on the Distribution of TJ Proteins
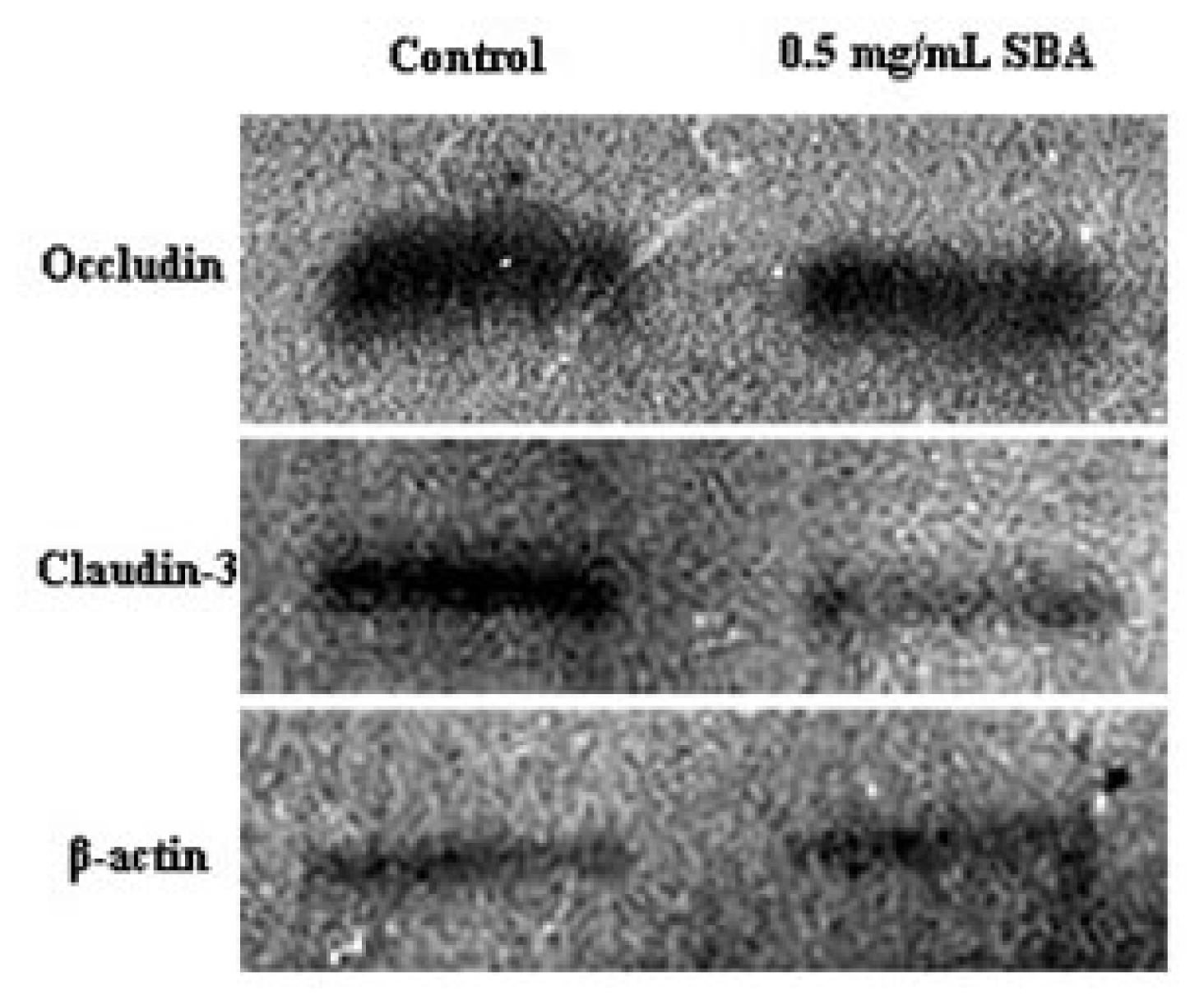
2.3.2. Effects of SBA on the Expression of TJ Proteins
3. Experimental Section
3.1. Cell Culture
3.2. Effects of SBA on Intestinal Integrity and Permeability Barrier Function
3.2.1. Measurement of Transepithelial Electrical Resistance (TEER)
3.2.2. Enzyme Assay: Determination of Alkaline Phosphatase Activity
3.3. Effects of SBA on Intestinal Epithelial Cell Viability
3.3.1. MTT Assay
3.3.2. Cell Morphological Observation
3.4. Effects of SBA on the Distribution and Expression of TJ Proteins
3.4.1. Immunofluorescence
3.4.2. Preparation of Cell Total Protein Extracts
3.4.3. SDS-PAGE and Western Blotting
3.5. Statistical Analysis
4. Conclusions

| Heading | MTT | AP | TEER | |
|---|---|---|---|---|
| MTT | Pearson correlation | 1 | ||
| N | 28 | |||
| AP | Pearson correlation | −0.682 * | 1 | |
| N | 28 | 28 | ||
| TEER | Pearson correlation | 0.688 * | −0.940 * | 1 |
| N | 21 | 21 | 21 | |
Acknowledgments
Conflicts of Interest
References
- Irish, G.G.; Maenz, D.D.; Classen, H.L. A new assay for functional lectins: the brush border lectin agglutinin assay (BBLAA). Anim. Feed Sci. Technol 1999, 76, 321–333. [Google Scholar]
- Dorland, L.; van-Halbcek, H.; Vliegenthart, J.F.G.; Lis, H.; Sharon, N. Primary structure of the carbohydrate chain of soybean agglutinin. J. Biol. Chem 1981, 256, 7708–7711. [Google Scholar]
- King, T.P.; Begbie, R.; Cadenhead, A. Nutritional toxicity of raw kidney beans in pigs. Immunocytochemical and cytopathological studies on the gut and the pancreas. J. Sci. Food Agric 1983, 34, 1404–1412. [Google Scholar]
- Quaroni, A.; Hochman, J. Development of intestinal cell culture models for drug transport and metabolism studies. Adv. Drug Deliv. Rev 1996, 22, 3–52. [Google Scholar]
- Pinton, P.; Nougayrède, J.P.; Del Rio, J.C.; Moreno, C.; Marin, D.E.; Ferrier, L.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol 2009, 237, 41–48. [Google Scholar] [Green Version]
- Shin, K.; Fogg, V.C.; Margolis, B. Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol 2006, 22, 207–235. [Google Scholar]
- Mazzon, E.; Sturniolo, G.C.; Puzzolo, D.; Frisina, N.; Fries, W. Effect of stress on the paracellular barrier in the rat ileum. Gut 2002, 51, 507–513. [Google Scholar]
- Simonovic, I.; Rosenberg, J.; Koutsouris, A.; Hecht, G. Enteropathogenic Escherichia coli dephosphorylates and dissociates occluding from intestinal epithelial tight junctions. Cell Microbiol 2000, 2, 305–315. [Google Scholar]
- Gonzalez-Mariseal, L.; Betanzos, A.; Nava, P.; Jaramillo, B.E. Tight junction proteins. Prog. Biophys. Mol. Biol 2003, 81, 1–44. [Google Scholar]
- Dublineau, I.; Lebrun, F.; Grison, S.; Griffiths, N.M. Functional and structural alterations of epithelial barrier properties of rat ileum following X-irradiation. Can. J. Physiol. Pharmacol 2004, 82, 84–93. [Google Scholar]
- Furuse, M.; Hirase, T.; Itoh, M.; Nagafuchi, A.; Yonemura, S.; Tsukita, S.; Tsukita, S. Occludin: A novel integral membrane protein localizing at tight junctions. J. Cell Biol 1993, 123, 1777–1788. [Google Scholar]
- Furuse, M.; Itoh, M.; Hirase, T.; Nagafuchi, A.; Yonemura, S.; Tsukita, S.; Tsukita, S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J. Cell Biol 1994, 127, 1617–1626. [Google Scholar]
- Gonzalez-Mariscal, L.; Namorado, M.C.; Martin, D.; Luna, J.; Alarcon, L.; Islas, S.; Valencia, L.; Muriel, P.; Ponce, L.; Reyes, J.L. Tight junction proteins ZO-1, ZO-2, and occludin along isolated renal tubules. Kidney Int 2000, 57, 2386–2402. [Google Scholar]
- Itoh, M.; Furuse, M.; Morita, K.; Kubota, K.; Saitou, M.; Tsukita, S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J. Cell Biol 1999, 147, 1351–1363. [Google Scholar]
- Furuse, M. Molecular basis of the core structure of tight junctions. Cold Spring Harb. Perspect. Biol 2010, 2, 1–14. [Google Scholar]
- Zhang, L.L.; Hou, X.Q.; Wang, L.M.; Sun, Z.W.; Liu, L.N.; Zhao, Y.; Qin, G.X. Stability of soybean agglutinin to pepsin and trypsin. Anim. Husband Vet. Med 2009, 12, 1–6. [Google Scholar]
- Pellegrina, C.D.; Rizzi, C.; Mosconi, S.; Zoccatelli, G.; Peruffo, A.; Chignola, R. Plant lectin as carriers for oral drugs: is wheat germ agglutinin a suitable candidate? Toxicol. Appl. Pharm 2005, 207, 170–178. [Google Scholar]
- Pellegrina, C.D.; Perbellini, O.; Scupoli, M.T.; Tomelleri, C.; Zanetti, C.; Zoccatell, G.; Fusi, M.; Peruffo, A.; Rizzi, C.; Chignola, R. Effects of wheat germ agglutinin on human gastrointestinal epithelium: Insights from an experimental model of immune/epithelial cell 304 interaction. Toxicol. Appl. Pharm 2009, 237, 146–153. [Google Scholar]
- Barrett, K.E. Positive and negative regulation of chloride secretion in T84 cells. Am. J. Physiol 1993, 265, C859–C868. [Google Scholar]
- Wang, L.M.; Hu, H.X.; Yang, S.B.; Luan, W.M.; Qin, G.X. Study of immunohistochemistry on specificity combined with soybean agglutinin of small intestinal mucosa in piglet. Nutr. Feedstuffs 2010, 46, 53–55. [Google Scholar]
- Konishi, Y. Modulations of food-derived substances on intestinal permeability in Caco-2 cell monolayers. Biosci. Biotechnol. Biochem 2003, 67, 2297–2299. [Google Scholar]
- Wang, L.M. Comparative study on digestive dynamics and antinutritional effects of soybean agglutinin in different species of animals. Ph.D. Thesis, Jilin Agricultural University, Changchun, May 2007. [Google Scholar]
- Goldberg, R.F.; Austen, W.G., Jr; Zhang, X.; Munene, G.; Mostafa, G.; Biswas, S.; McCormack, M.; Eberlin, K.R.; Nguyen, J.T.; Tatlidede, H.S.; et al. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc. Natl. Acad. Sci. USA 2008, 105, 3551–3556. [Google Scholar]
- Bates, J.M.; Akerlund, J.; Mittge, E.; Guillemin, K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe 2007, 2, 371–382. [Google Scholar]
- Geddes, K.; Philpott, D.J. A new role for intestinal alkaline phosphatase in gut barrier maintenance. Gastroenterology 2008, 135, 8–12. [Google Scholar]
- Bol-Schoenmakers, M.; Fiechter, D.; Raaben, W.; Hassing, I.; Bleumink, R.; Kruijswijk, D.; Maijoor, K.; Tersteeg-Zijderveld, M.; Brands, R.; Pieters, R. Intestinal alkaline phosphatase contributes to the reduction of severe intestinal epithelial damage. Eur. J. Pharmacol 2010, 633, 71–77. [Google Scholar]
- Sun, C.; Zhu, Z. Agglutinin; Science Press: Beijing, China, 1986; p. 113. [Google Scholar]
- Palmer, R.M.; Pusztai, A.; Bain, P.; Grant, G. Changes in rates of tissue protein synthesis in rats induced in vivo by consumption of kidney bean (Phaseolus vulgaris) lectins. Comp. Biochem. Physiol 1987, 88, 179–183. [Google Scholar]
- Grant, A.L.; Holland, R.E.; Thomas, J.W.; King, K.J.; Liesman, J.S. The effects of dietary amines on the small intestine in calves fed soybean protein. J. Nutr 1989, 119, 1034–1036. [Google Scholar]
- Zang, J.J.; Ma, Y.X.; Piao, X.S.; Wang, J.J. Research advance of antinutritional effects of soybean agglutinin. China Feed 2008, 6, 8–11. [Google Scholar]
- Liu, F.F. Effects of soybean agglutinin on intestinal epithelia cell of Rabbit Cultured in vitro and labeling on its target site. Master’s Thesis, Jilin Agricultural University, Changchun, June 2011. [Google Scholar]
- Makino, K.; Shinagawa, H.; Amemura, M.; Kawamoto, T.; Yamada, M.; Nakata, A. Signal transduction in the phosphate regular of escherichia coli involves phosphotransfer between PhoR and PhoB Proteins. J. Mol. Biol 1989, 210, 551–559. [Google Scholar]
- Chang, D.C.; Meng, C. A localized elevation of cytosolic free calcium is associated with cytokinesis in the zebrafish embryo. J. Cell Biol 1995, 131, 1539–1545. [Google Scholar]
- Xu, N.; Luo, K.Q.; Chang, D.C. Ca2+ signal blockers can inhibit M/A transition in mammalian cells by interfering with the spindle checkpoint. Biochem. Biophys. Res. Commun 2003, 306, 737–745. [Google Scholar]
- Salgado, P.; Freire, J.P.B.; Mourato, M.; Cabral, F.; Toullec, R.; Lalles, J.P. Comparative effects of different legume protein sources in weaned piglets: Nutrient digestibility, intestinal morphology and digestive enzymes. Livest. Prod. Sci 2002, 74, 191–202. [Google Scholar]
- Madara, J.L. Regulation of the movement of solutes across tight junctions. Annu. Rev. Physiol 1998, 60, 143–159. [Google Scholar]
- Balda, M.S.; Whitney, J.A.; Flores, C.; Gonzalez, S.; Cereijido, M.; Matter, K. Functional dissociation of paracellular permeability and trans-epithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol 1996, 134, 1031–1049. [Google Scholar]
- Drago, S.; El Asmar, R.; Di Pierro, M.; Grazia Clemente, M.; Tripathi, A.; Sapone, A.; Thakar, M.; Iacono, G.; Carroccio, A.; D’Agate, C.; et al. Gliadin, zonulin and gut permeability: effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand. J. Gastroenterol 2006, 41, 408–419. [Google Scholar]
- McCarthy, K.M.; Skare, I.B.; Stankewich, M.C.; Furuse, M.; Tsukita, S.; Rogers, R.A.; Lynch, R.D.; Schneeberger, E.E. Occludin is a functional component of the tight junction. J. Cell Sci 1996, 109, 2287–2298. [Google Scholar]
- Bamforth, S.D.; Kniesel, U.; Wolburg, H.; Engelhardt, B.; Risau, W. A dominant mutant of occludin disrupts tight junction structure and function. J. Cell Sci 1999, 112, 1879–1888. [Google Scholar]
- Furuse, M.; Fujita, K.; Hirase, T.; Fujimoto, K.; Tsukita, S. Claudin-1 and -2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occluding. J. Cell Biol 1998, 141, 1539–1550. [Google Scholar]
- Furuse, M.; Sasaki, H.; Fujimoto, K.; Tsukita, S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J. Cell Biol 1998, 143, 391–401. [Google Scholar]
- Perez-Moreno, M.; Fuchs, E. Catenins: Keeping cells from getting their signals crossed. Dev. Cell 2006, 11, 601–612. [Google Scholar]
- Madara, J.L. Intestinal absorptive cell tight junctions are linked to cytoskeleton. Am. J. Physiol 1987, 253, C171–C175. [Google Scholar]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of Tight Junction Permeability by Intestinal Bacteria and Dietary Components. J. Nutr 2011, 141, 769–776. [Google Scholar]
- Zhao, Y.; Qin, G.X.; Sun, Z.W.; Che, D.S.; Bao, N.; Zhang, X.D. Effects of Soybean Agglutinin on Intestinal Barrier Permeability and Tight Junction Protein Expression in Weaned Piglets. Int. J. Mol. Sci 2011, 12. [Google Scholar] [CrossRef]
- Gordon, S.R. The effects of soybean agglutinin binding on the corneal endothelium and the re-establishment of an intact monolayer following injury. J. Tissue Viability 2011, 20, 20–29. [Google Scholar]
- Mu, C.L. Labeling of soybean agglutinin receptors and effects of soybean agglutinin on intestinal epithelial cells. Master’s Thesis, Jilin Agricultural University, Changchun, June 2011. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pan, L.; Qin, G.; Zhao, Y.; Wang, J.; Liu, F.; Che, D. Effects of Soybean Agglutinin on Mechanical Barrier Function and Tight Junction Protein Expression in Intestinal Epithelial Cells from Piglets. Int. J. Mol. Sci. 2013, 14, 21689-21704. https://doi.org/10.3390/ijms141121689
Pan L, Qin G, Zhao Y, Wang J, Liu F, Che D. Effects of Soybean Agglutinin on Mechanical Barrier Function and Tight Junction Protein Expression in Intestinal Epithelial Cells from Piglets. International Journal of Molecular Sciences. 2013; 14(11):21689-21704. https://doi.org/10.3390/ijms141121689
Chicago/Turabian StylePan, Li, Guixin Qin, Yuan Zhao, Jun Wang, Feifei Liu, and Dongsheng Che. 2013. "Effects of Soybean Agglutinin on Mechanical Barrier Function and Tight Junction Protein Expression in Intestinal Epithelial Cells from Piglets" International Journal of Molecular Sciences 14, no. 11: 21689-21704. https://doi.org/10.3390/ijms141121689




