Bioactive Constituents from “Triguero” Asparagus Improve the Plasma Lipid Profile and Liver Antioxidant Status in Hypercholesterolemic Rats
Abstract
:1. Introduction
2. Results
2.1. Major Bioactive Constituents in the Asparagus Fractions
2.2. Animal Food Intake and Weight Gain
2.3. Plasma Lipid Profile and Atherogenic Index (AI) Values
2.4. Liver Antioxidant Evaluation
3. Discussion
4. Experimental Section
4.1. Plant Extraction and Analysis
4.2. Animals and Diets
4.3. Plasma Lipid Profiles
4.4. Tissue Preparation and Liver Antioxidant Evaluation
4.5. Statistic Section
5. Conclusions
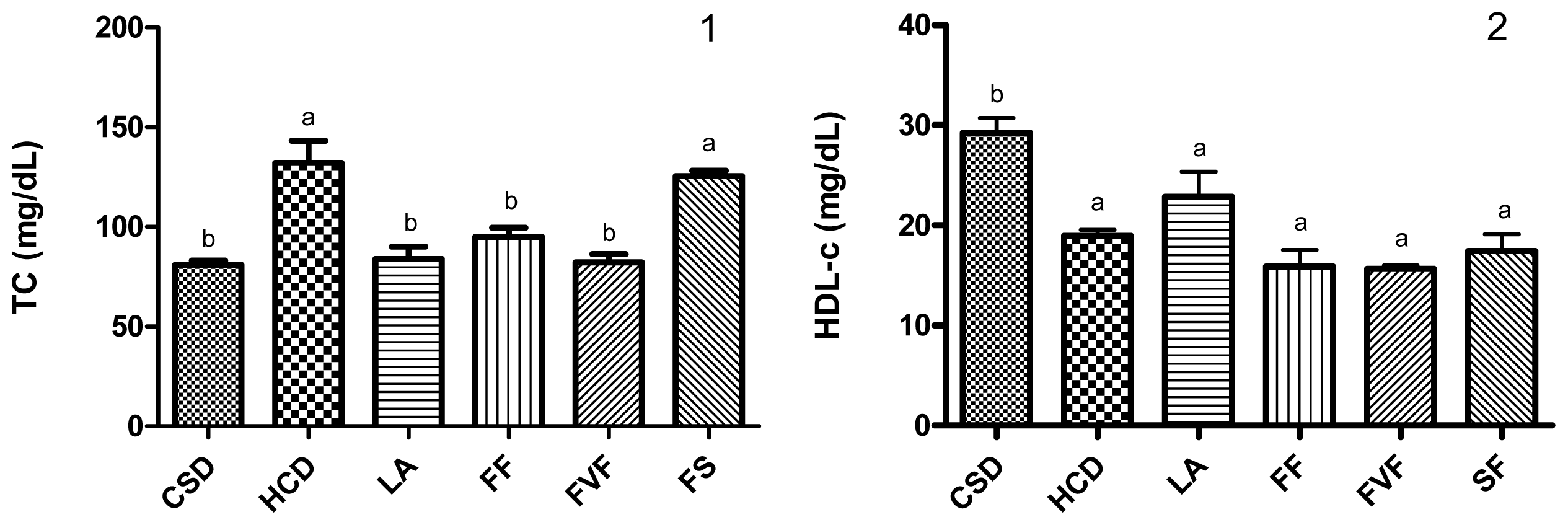
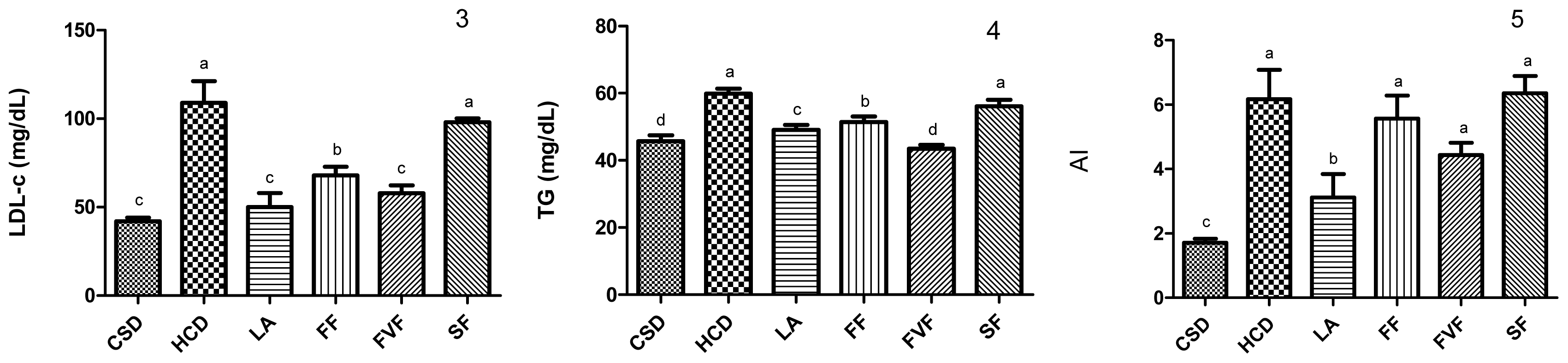
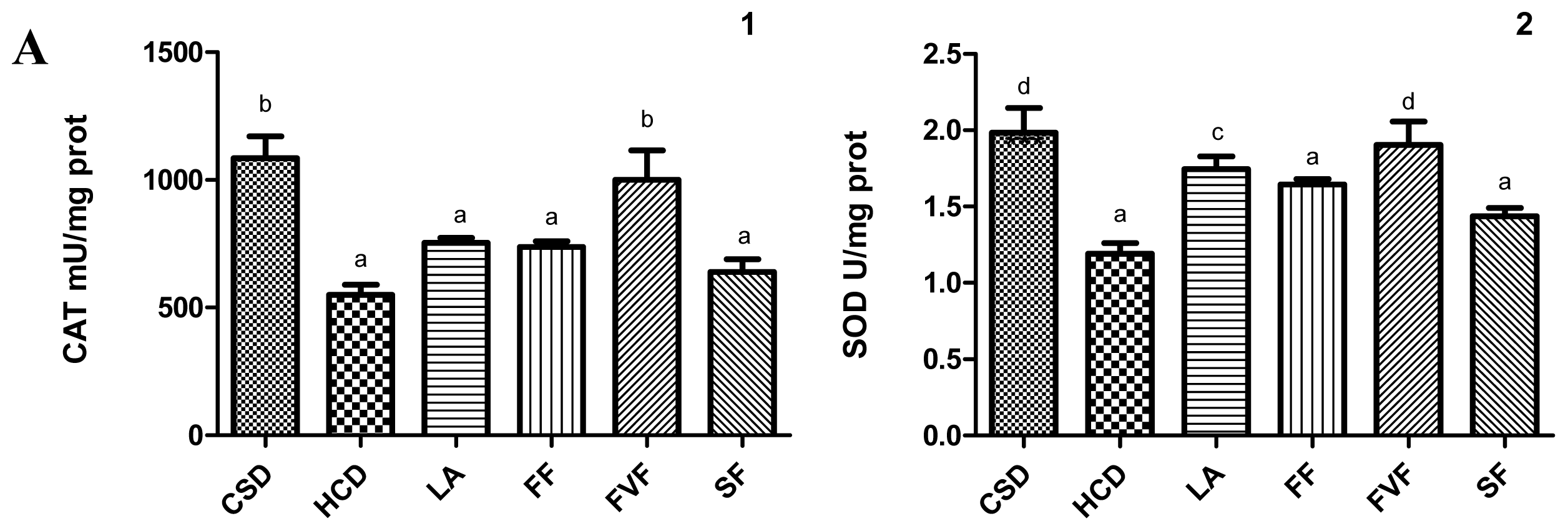
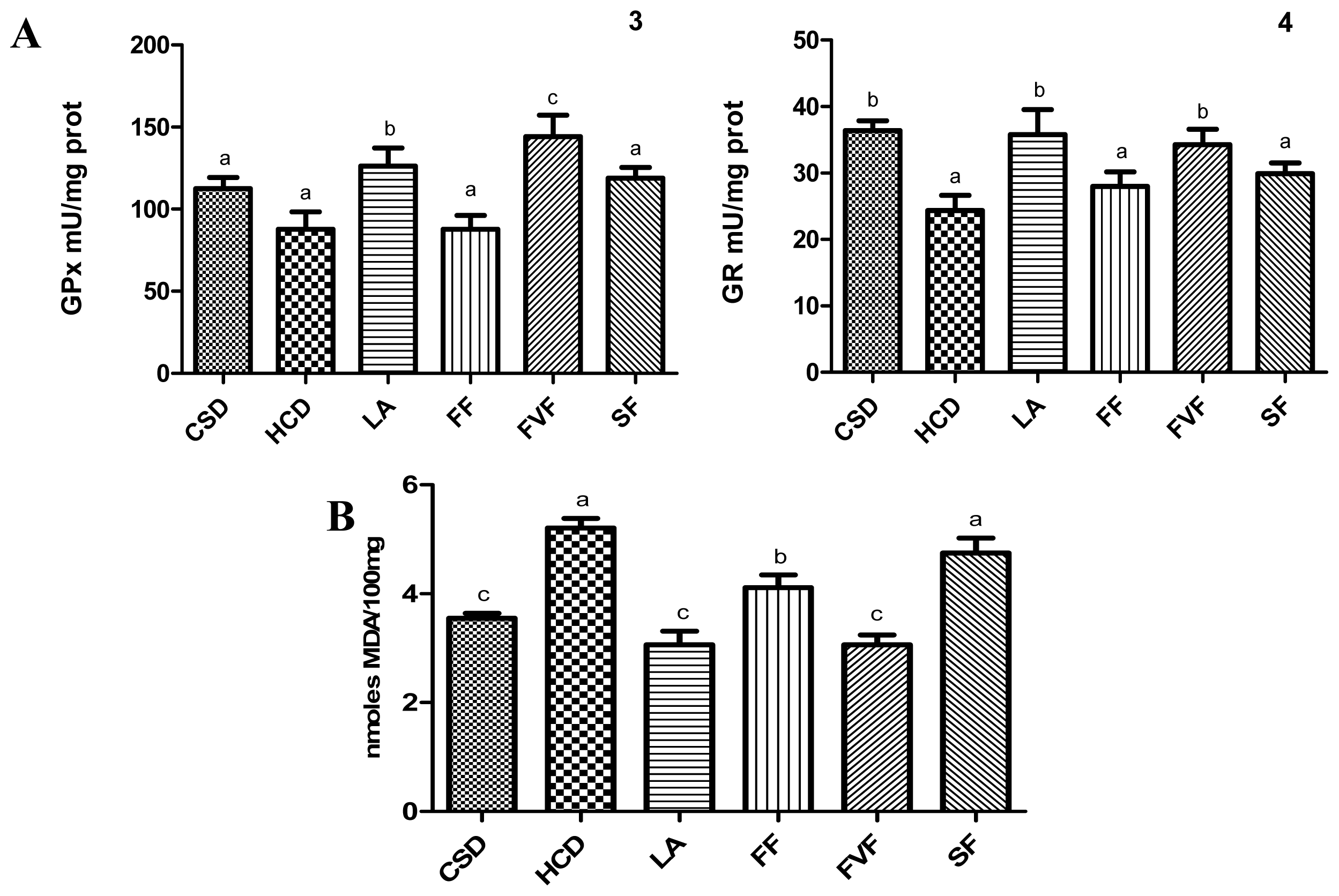
| Bioactive constituents | LA | FF | FVF | SF |
|---|---|---|---|---|
| Dietary fiber | 565 ± 14.5 | 610.0 ± 19.2 | - | - |
| Flavonoids | 10 ± 0.5 | 1.3 ± 0.1 | 110 ± 5.5 | - |
| Saponins | 5 ± 0.6 | 4.4 ± 0.4 | - | 800 ± 19.8 |
| Groups | Initial body weight | Final body weight | Weight gain (g) | Average food intake (g/day) |
|---|---|---|---|---|
| CSD | 261.5 ± 24.1 | 357.1 ± 29.5 | 95.6 ± 6.8 a | 12.9 ± 1.9 d |
| HCD | 252.8 ± 19.4 | 371.7 ± 30.1 | 118.9 ± 9.3 a | 18.7 ± 1.2 a |
| LA | 237.3 ± 17.5 | 322.2 ± 28.4 | 84.9 ± 7.4 b | 13.9 ± 0.9 c |
| FF | 257.3 ± 19.6 | 364.1 ± 34.2 | 106.8 ± 5.6 a | 13.8 ± 1.0 c |
| FVF | 245.4 ± 20.1 | 304.1 ± 27.6 | 58.7± 6.2 c | 15.1 ± 1.4 b |
| SF | 260.1 ± 22.3 | 356.1 ± 31.2 | 96.0 ± 8.2 a | 15.3 ± 1.3 b |
Acknowledgments
Conflicts of Interest
References
- Ross, R. Atherosclerosis: An inflammatory disease. N. Engl. J. Med 1999, 340, 115–126. [Google Scholar]
- Steinberg, D. Low density lipoprotein oxidation and its pathological significance. J. Biol. Chem 1997, 272, 20963–20966. [Google Scholar]
- Tiwari, A.K. Natural product antioxidants and their therapeutic potential in mitigating peroxidative modification of lipoproteins and atherosclerosis: Recent development. J. Med. Arom. Plant Sci 1999, 21, 730–741. [Google Scholar]
- Lecumberri, E.; Goya, L.; Mateos, R.; Alia, M.; Ramos, S.; Izquierdo-Pulido, M.; Bravo, L. A diet rich in dietary fiber from cocoa improves lipid profile and reduces malodialdehyde in hypercholesterolemic rats. Nutrition 2007, 23, 332–341. [Google Scholar]
- Shimizu-Ibuka, A.; Udagawa, H.; Kobayashii-Hattori, K.; Mura, K.; Tokue, C. Hypocholesterolemic effect of peanut skin and its fractions: A case record of rats fed on a High-Cholesterol diet. Biosci. Biotechnol. Biochem 2009, 73, 205–208. [Google Scholar]
- Moreno, R.; Espejo, J.A.; Cabrera, A.; Gil, J. Origin of tetraploid cultivated asparagus landraces inferred from nuclear ribosomal DNA internal transcribed spacers’ polymorphisms. Ann. Appl. Biol 2008, 153, 233–241. [Google Scholar]
- Ricardi, P.; Casali, P.; Mercati, F.; Falavigna, A.; Sunseri, F. Genetic characteization of asparagus doubled haploids collection and wild relatives. Sci. Hortic 2011, 130, 691–700. [Google Scholar]
- Fuentes-Alventosa, J.M.; Jaramillo, S.; Rodriguez-Gutierrez, G.; Cermeño, P.; Espejo, J.A.; Jiménez-Araujo, A. Flavonoid profile of green asparagus genotypes. J. Agric. Food Chem 2008, 56, 6977–6984. [Google Scholar]
- Guillén, R.; Rodríguez, R.; Jaramillo, S.; Rodríguez, G.; Espejo, J.A.; Fernández-Bolaños, J.; Heredia, A.; Jiménez, A. Antioxidants from asparagus spears: Phenolics. Acta Hortic 2008, 776, 247–254. [Google Scholar]
- Maeda, T.; Kakuta, H.; Sonoda, S.; Ueno, R.; Suzuki, T.; Oosawa, K. Antioxidants capacities of extracts from green, purple and white asparagus spears related to polyphenol concentration. Sci. Hortic 2005, 40, 1221–1224. [Google Scholar]
- Rodríguez, R.; Smith, A.; Waldron, K. Ferulic acid crooslinks in asparagus cell walls in relation to texture. J. Agric. Food Chem 2004, 52, 4740–4750. [Google Scholar]
- Vázquez-Castilla, S.; Jaramillo-Carmona, S.; Fuentes-Alventosa, J.M.; Jiménez-Araujo, A.; Rodríguez-Arcos, R.; Cermeño-Sacristán, P.; Espejo-Calvo, J.A.; Guillén-Bejarano, R. Optimization of a method for the profiling and quantification of saponins in different genotypes. J. Agric. Food Chem 2013. [Google Scholar] [CrossRef]
- Visavadiya, N.P.; Narasimhacharya, A.V. Asparagus root regulates cholesterol metabolism and improves antioxidant status in hypercholesterolemic rats. Evid. Based Compl. Alt. Med 2007, 6, 219–226. [Google Scholar]
- Zhu, X.; Zhang, W.; Zhao, J.; Wang, J.; Qu, W. Hypolipidaemic and hepatoprotective effects of ethanolic and aqueous extracts from Asparagus officinalis L. by-products in mice fed a high-fat diet. J. Sci. Food Agric 2010, 90, 1129–1135. [Google Scholar]
- García Giménez, M.D.; De la Puerta, R.; Sáenz, M.T.; Marquez-Martín, A.; Fernández-Arche, M.A. Hypocholesterolemic and hepatoprotective effects of “triguero” asparagus from Andalusian in rats fed a high cholesterol diet. Evid. Based. Compl. Alt. Med 2012. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M.; Denke, M.A. Dietary influences on serum lipids and lipoproteins. J. Lipid Res 1990, 36, 1277–1282. [Google Scholar]
- Lindstrom, J.; Peltonen, M.; Eriksson, J.G.; Louheranta, A.; Fogelholm, M.; Uusitupa, M.; Tuomilehto, J. High-fibre, low fat diet pedicts long term weight loss and decreased type 2 diabetes risk: The Finnish Diabetes Prevention Study. Diabetologia 2006, 49, 912–920. [Google Scholar]
- Shali, P.K.; Kaul, S.; Nilsson, J.; Cercek, B. Exploiting the vascular protective effects of high-density lipoprotein and its apolipoproteins: An idea whose time for testing is coming. Circulation 2001, 104, 2376–2383. [Google Scholar]
- Assmann, G.; Nofer, J. Atheroprotective effects of high-density lipoproteins. Annu. Rev. Med 2003, 54, 321–341. [Google Scholar]
- Zhu, X.; Zhang, W.; Pang, X.; Wang, J.; Zhao, J.; Qu, W. Hypolipidemic effect of N-butanol extract from Asparagus officinalis L. in mice fed a high-fat diet. Phytother. Res 2011, 25, 119–1124. [Google Scholar]
- Romero, A.L.; West, K.L.; Zern, T.; Fernandez, M.L. The seeds from Plantago ovata lower plasma lipids by altering hepatic and bile acid metabolism in guinea pigs. J. Nutr 2002, 132, 1194–1198. [Google Scholar]
- Bolkent, S.; Yanardag, R.; Karabulut-Bulan, O.; Yesilyaprak, B. Protective role of Melissa officinalis L. extract on liver of hyperlipidemic rats: A morphological and biochemical study. J. Ethnopharmacol 2005, 99, 391–398. [Google Scholar]
- Qin, Y.; Wu, X.; Huang, W.; Gong, G.; Li, D.; He, Y.; Zhao, Y. Acute toxicity and sub-chronic toxicity of steroidal saponins from Dioscorea zingiberensis C.H.Wright in rodents. J. Ethnopharmacol 2009, 126, 543–550. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol 1990, 186, 1–85. [Google Scholar]
- Ali, M.S.; Mudagal, M.P.; Goli, D. Cardioprotective effect of tetrahydrocurcumin and rutin on lipid peroxides and antioxidants in experimentally induced myocardial infarction in rats. Pharmazie 2009, 64, 132–136. [Google Scholar]
- Toyokuni, S.; Tanaka, T.; Kawaguchi, W.; Fang, N.R.; Ozeki, M.; Akatsuka, S. Effects of the phenolic contents of Mauritian endemic plant extract on promoter activities of antioxidant enzymes. Free Radic. Res 2003, 37, 1215–1224. [Google Scholar]
- Ranaivo, H.R.; Rakotoarison, O.; Tesse, A.; Schott, C.; Randriantsoa, A.; Lobstein, A. Cedrelopsis grevei induced hypotension and improved endothelial vasodilatation through an increase of Cu/Zn SOD protein expression. Am. J. Physiol. Heart Circ. Physiol 2004, 286, 775–781. [Google Scholar]
- Guillén-Bejarano, R.; Rodríguez-Gutiérrez, G.; Fuentes Alventosa, J.M.; Jaramillo-Carmona, S.; Rodríguez-Arcos, R.; Jiménez-Araujo, A.; Fernández-Bolaños, J. Procedimiento de Obtención de Compuestos Funcionales de Origen Vegetal Es. Patent P2001030951, 2012.
- Lee, S.C.; Prosky, L.; De Vries, J.W. Determination of the total, soluble and insoluble dietary fiber in food enzymatic, gravimetric method, MES-TRIS buffer: Collaborative study. J. Assoc. Off. Anal. Chem 1992, 75, 395–416. [Google Scholar]
- Fuentes-Alventosa, J.M.; Jaramillo, S.; Rodríguez, G.; Rodríguez, R.; Fernández, J.; Guillén, R.; Espejo, J.A.; Jiménez, A. Effect of the extraction method on phytochemical composition and antioxidant activity of high dietary fibre powders obtained from asparagus by-products. Food Chem 2009, 116, 484–490. [Google Scholar]
- Friedewald, W.T.; Levy, R.I.; Fradrickson, D.S. Estimation of concentration of low-density lipoprotein cholesterol in plasma without the use of preparative ultracentrifuge. Clinical Chem 1972, 18, 494–502. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantization of micrograms quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 1976, 7, 248–274. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- McCord, J.M.; Fridovich, I. Superoxide dismutase, an enzymatic function for erythrocuprein (hemocuprein). J. Biol. Chem 1969, 244, 6049–6055. [Google Scholar]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun 1976, 71, 952–958. [Google Scholar]
- Calberg, I.; Mannervik, B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem 1975, 250, 5475–80. [Google Scholar]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malondialdehyde and 4-Hydroxynonenal. Methods Enzymol 1990, 186, 407–421. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vázquez-Castilla, S.; De la Puerta, R.; Garcia-Gimenez, M.D.; Fernández-Arche, M.A.; Guillén-Bejarano, R. Bioactive Constituents from “Triguero” Asparagus Improve the Plasma Lipid Profile and Liver Antioxidant Status in Hypercholesterolemic Rats. Int. J. Mol. Sci. 2013, 14, 21227-21239. https://doi.org/10.3390/ijms141121227
Vázquez-Castilla S, De la Puerta R, Garcia-Gimenez MD, Fernández-Arche MA, Guillén-Bejarano R. Bioactive Constituents from “Triguero” Asparagus Improve the Plasma Lipid Profile and Liver Antioxidant Status in Hypercholesterolemic Rats. International Journal of Molecular Sciences. 2013; 14(11):21227-21239. https://doi.org/10.3390/ijms141121227
Chicago/Turabian StyleVázquez-Castilla, Sara, Rocío De la Puerta, María Dolores Garcia-Gimenez, María Angeles Fernández-Arche, and Rafael Guillén-Bejarano. 2013. "Bioactive Constituents from “Triguero” Asparagus Improve the Plasma Lipid Profile and Liver Antioxidant Status in Hypercholesterolemic Rats" International Journal of Molecular Sciences 14, no. 11: 21227-21239. https://doi.org/10.3390/ijms141121227
APA StyleVázquez-Castilla, S., De la Puerta, R., Garcia-Gimenez, M. D., Fernández-Arche, M. A., & Guillén-Bejarano, R. (2013). Bioactive Constituents from “Triguero” Asparagus Improve the Plasma Lipid Profile and Liver Antioxidant Status in Hypercholesterolemic Rats. International Journal of Molecular Sciences, 14(11), 21227-21239. https://doi.org/10.3390/ijms141121227





