Dietary Polyunsaturated Fatty Acids and Inflammation: The Role of Phospholipid Biosynthesis
Abstract
:1. Introduction
2. Trends in Dietary Fatty Acid Composition
3. Dietary Polyunsaturated Fatty Acids and Inflammation
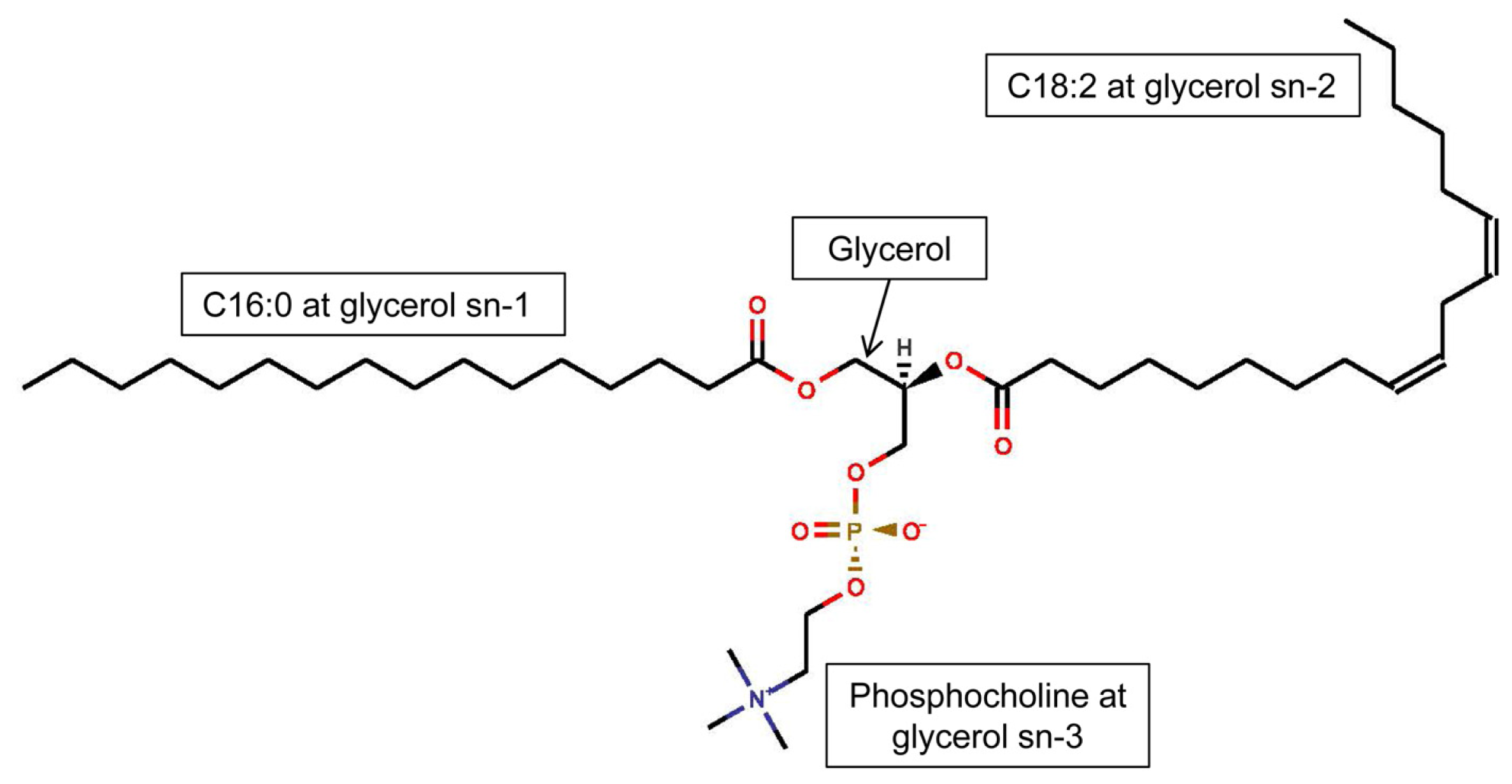
3.1. Phospholipids of Inflammatory Cells
3.2. Inflammatory Pathways Influenced by Fatty Acid Components of Phospholipids
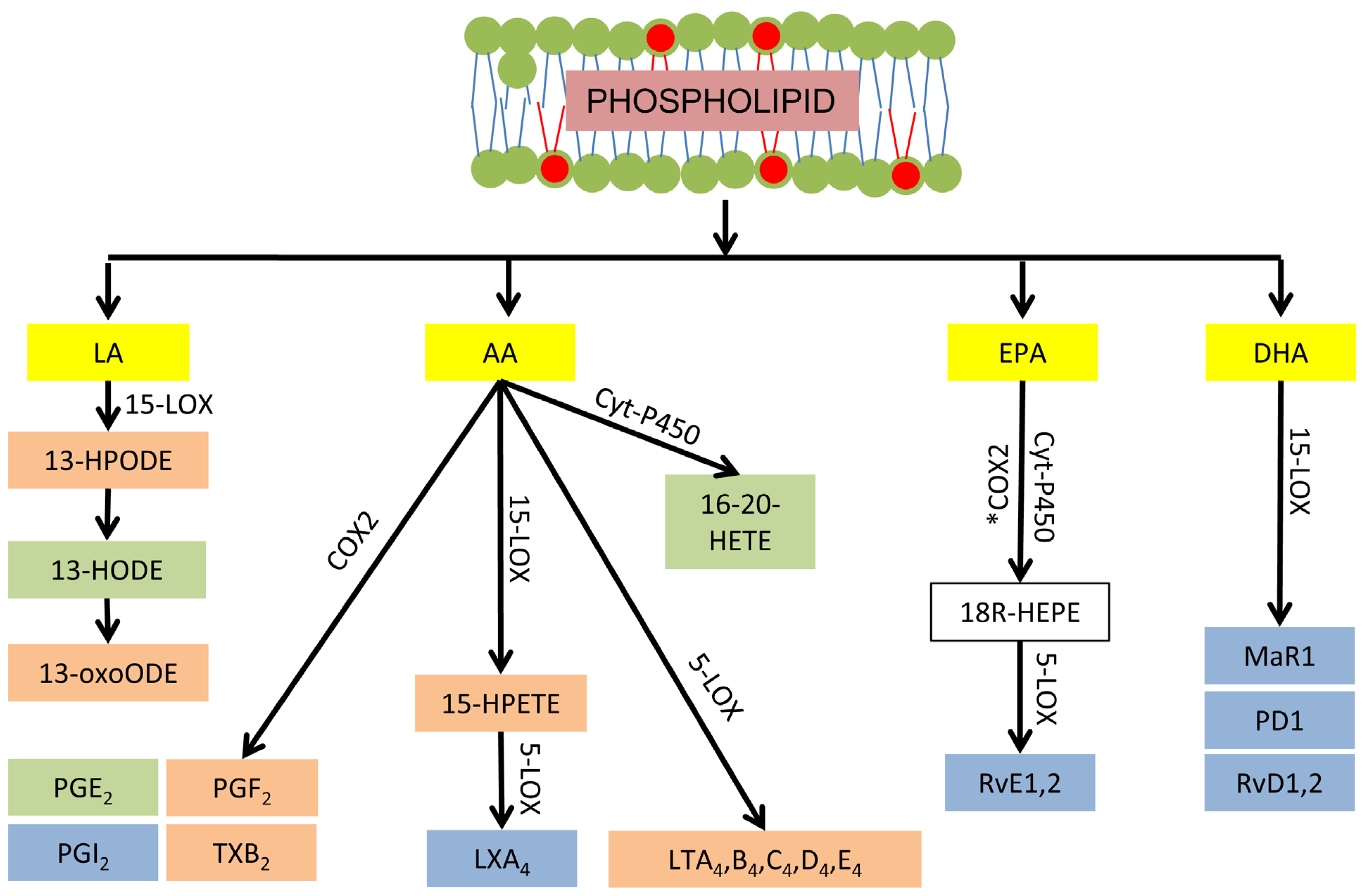
3.3. Phospholipid-Dependent Biosynthesis of Lipid Mediators
3.4. Dietary PUFA Influence Inflammation through Lipid Mediators
3.5. Further Lipid Mediator Research
4. Delivery and Utilization of Dietary PUFA for Phospholipid Biosynthesis
4.1. Digestion and Absorption of Dietary PUFA
4.2. Phospholipid Substrate from Non-Dietary PUFA
4.3. Effects of Disease, Physiological State, and Other Nutrients on Phospholipid Biosynthesis
4.4. Regulation of the Acyl Composition of Phospholipid
5. Conclusions


Acknowledgments
Conflicts of Interest
References
- Calder, P.C. Fatty acids and inflammation: The cutting edge between food and pharma. Eur. J. Pharmacol 2011, 668, S50–S58. [Google Scholar]
- Calder, P.C.; Albers, R.; Antoine, J.M.; Blum, S.; Bourdet-Sicard, R.; Ferns, G.; Folkerts, G.; Friedmann, P.; Frost, G.; Guarner, F. Inflammatory disease processes and interactions with nutrition. Br. J. Nutr 2009, 101, 1–45. [Google Scholar]
- Simopoulos, A.P. Evolutionary aspects of diet, the ω-6/ω-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother 2006, 60, 502–507. [Google Scholar]
- Contreras, G.A.; Sordillo, L.M. Lipid mobilization and inflammatory responses during the transition period of dairy cows. Comp. Immunol. Microbiol. Infect. Dis 2011, 34, 281–289. [Google Scholar]
- Sordillo, L.M.; Contreras, G.A.; Aitken, S.L. Metabolic factors affecting the inflammatory response of periparturient dairy cows. Anim. Health Res. Rev 2009, 10, 53–63. [Google Scholar]
- Harris, W.S.; Poston, W.C.; Haddock, C.K. Tissue n-3 and n-6 fatty acids and risk for coronary heart disease events. Atherosclerosis 2007, 193, 1–10. [Google Scholar]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol 2000, 1, 31–39. [Google Scholar]
- Stillwell, W.; Wassall, S.R. Docosahexaenoic acid: Membrane properties of a unique fatty acid. Chem. Phys. Lipids 2003, 126, 1–27. [Google Scholar]
- Poulsen, R.C.; Gotlinger, K.H.; Serhan, C.N.; Kruger, M.C. Identification of inflammatory and proresolving lipid mediators in bone marrow and their lipidomic profiles with ovariectomy and ω-3 intake. Am. J. Hematol 2008, 83, 437–445. [Google Scholar]
- Bruss, M.L. Lipids and Ketones. In Clinical Biochemistry of Domestic Animals, 6th ed.; Kaneko, J.J., Harvey, J.W., Bruss,, M.L., Eds.; Elsevier: St. Louis, MO, USA, 2008; pp. 81–115. [Google Scholar]
- Bauman, D.E.; Harvatine, K.J.; Lock, A.L. Nutrigenomics, rumen-derived bioactive fatty acids, and the regulation of milk fat synthesis. Annu. Rev. Nutr 2011, 31, 299–319. [Google Scholar]
- Vance, D.E.; Vance, J.E. Phospholipid Biosynthesis in Eukaryotes. In Biochemistry of Lipids, Lipoproteins and Membranes, 5th ed.; Vance, D.E., Vance, J.E., Eds.; Elsevier: St. Louis, MO, USA, 2008; pp. 213–244. [Google Scholar]
- Loewen, C.J.R. Lipids as conductors in the orchestra of life. F1000 Biol. Rep 2012. [Google Scholar] [CrossRef]
- Young, B.P.; Shin, J.J.H.; Orij, R.; Chao, J.T.; Li, S.C.; Guan, X.L.; Khong, A.; Jan, E.; Wenk, M.R.; Prinz, W.A. Phosphatidic acid is a pH biosensor that links membrane biogenesis to metabolism. Sci. Signal 2010, 329, 1085–1088. [Google Scholar]
- Blasbalg, T.L.; Hibbeln, J.R.; Ramsden, C.E.; Majchrzak, S.F.; Rawlings, R.R. Changes in consumption of ω-3 and ω-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr 2011, 93, 950–962. [Google Scholar]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr 2005, 81, 341–354. [Google Scholar]
- Bang, H.O.; Dyerberg, J.; Hjørne, N. The composition of food consumed by greenland eskimos. Acta Med. Scand 1976, 200, 69–73. [Google Scholar]
- Elias, P.M.; Brown, B.E.; Ziboh, V.A. The permeability barrier in essential fatty acid deficiency: Evidence for a direct role for linoleic acid in barrier function. J. Invest. Dermatol 1980, 74, 230–233. [Google Scholar]
- Delion, S.; Chalon, S.; Hérault, J.; Guilloteau, D.; Besnard, J.C.; Durand, G. Chronic dietary α-linolenic acid deficiency alters dopaminergic and serotoninergic neurotransmission in rats. J. Nutr 1994, 124, 2466–2476. [Google Scholar]
- Guillou, H.; Zadravec, D.; Martin, P.G.P.; Jacobsson, A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog. Lipid Res 2010, 49, 186–199. [Google Scholar]
- Rule, D.C.; Broughton, K.S.; Shellito, S.M.; Maiorano, G. Comparison of muscle fatty acid profiles and cholesterol concentrations of bison, beef cattle, elk, and chicken. J. Anim. Sci 2002, 80, 1202–1211. [Google Scholar]
- White, S.L.; Bertrand, J.A.; Wade, M.R.; Washburn, S.P.; Green, J.T., Jr.; Jenkins, T.C. Comparison of fatty acid content of milk from Jersey and Holstein cows consuming pasture or a total mixed ration. J. Dairy Sci 2001, 84, 2295–2301. [Google Scholar]
- Douglas, G.; Rehage, J.; Beaulieu, A.; Bahaa, A.; Drackley, J. Prepartum nutrition alters fatty acid composition in plasma, adipose tissue, and liver lipids of periparturient dairy cows. J. Dairy Sci 2007, 90, 2941–2959. [Google Scholar]
- Contreras, G.; Mattmiller, S.; Raphael, W.; Gandy, J.; Sordillo, L. Enhanced n-3 phospholipid content reduces inflammatory responses in bovine endothelial cells. J. Dairy Sci 2012, 95, 7137–7150. [Google Scholar]
- Lawrence, T.; Gilroy, D.W. Chronic inflammation: A failure of resolution? Int. J. Exp. Pathol 2007, 88, 85–94. [Google Scholar]
- Virtanen, J.K.; Mozaffarian, D.; Chiuve, S.E.; Rimm, E.B. Fish consumption and risk of major chronic disease in men. Am. J. Clin. Nutr 2008, 88, 1618–1625. [Google Scholar]
- Galli, C.; Risé, P. Fish consumption, ω-3 fatty acids and cardiovascular disease. The science and the clinical trials. Nutr. Health 2009, 20, 11–20. [Google Scholar]
- Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Lancet 1999, 354, 447–455.
- Rose, D.P.; Connolly, J.M. Ω-3 fatty acids as cancer chemopreventive agents. Pharmacol. Ther 1999, 83, 217–244. [Google Scholar]
- Trebble, T.; Arden, N.K.; Stroud, M.A.; Wootton, S.A.; Burdge, G.C.; Miles, E.A.; Ballinger, A.B.; Thompson, R.L.; Calder, P.C. Inhibition of tumour necrosis factor-α and interleukin 6 production by mononuclear cells following dietary fish-oil supplementation in healthy men and response to antioxidant co-supplementation. Br. J. Nutr 2003, 90, 405–412. [Google Scholar]
- Bosch, J.; Gerstein, H.C.; Dagenais, G.R.; Diaz, R.; Dyal, L.; Jung, H.; Maggiono, A.P.; Probstfield, J.; Ramachandran, A.; Riddle, M.C.; et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N. Engl. J. Med 2012, 367, 309–318. [Google Scholar]
- Rizos, E.C.; Ntzani, E.E.; Bika, E.; Kostapanos, M.S.; Elisaf, M.S. Association between ω-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA 2012, 308, 1024–1033. [Google Scholar]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr 2006, 83, 1467S–1476S. [Google Scholar]
- De Mello, V.D.F.; Kolehmanien, M.; Schwab, U.; Pulkkinen, L.; Uusitupa, M. Gene expression of peripheral blood mononuclear cells as a tool in dietary intervention studies: What do we know so far? Mol. Nutr. Food Res 2012, 56, 1160–1172. [Google Scholar]
- Andreyev, A.Y.; Fahy, E.; Guan, Z.; Kelly, S.; Li, X.; McDonald, J.G.; Milne, S.; Myers, D.; Park, H.; Ryan, A.; et al. Subcellular organelle lipidomics in TLR-4-activated macrophages. J. Lipid Res 2010, 51, 2785–2797. [Google Scholar]
- Quehenberger, O.; Armando, A.M.; Brown, A.H.; Milne, S.B.; Myers, D.S.; Merrill, A.H.; Bandyopadhyay, S.; Jones, K.N.; Kelly, S.; Shaner, R.L. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res 2010, 51, 3299–3305. [Google Scholar]
- Dowhan, W.; Bogdanov, M.; Mileykovskaya, E. Functional Roles of Lipids in Membranes. In Biochemistry of Lipids, Lipoproteins and Membranes, 5th ed.; Vance, D.E., Vance, J.E., Eds.; Elsevier: St. Louis, MO, USA, 2008; pp. 1–38. [Google Scholar]
- Paradies, G.; Petrosillo, G.; Paradies, V.; Reiter, R.J.; Ruggiero, F.M. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J. Pineal Res 2010, 48, 297–310. [Google Scholar]
- Musatov, A.; Robinson, N.C. Susceptibility of mitochondrial electron-transport complexes to oxidative damage. Focus on cytochrome c oxidase. Free Radic. Res 2012, 46, 1313–1326. [Google Scholar]
- Yin, H.; Zhu, M. Free radical oxidation of cardiolipin: chemical mechanisms, detection and implication in apoptosis, mitochondrial dysfunction and human diseases. Free Radic. Res 2012, 46, 959–974. [Google Scholar]
- Corl, C.; Gandy, J.; Sordillo, L. Platelet activating factor production and proinflammatory gene expression in endotoxin-challenged bovine mammary endothelial cells. J. Dairy Sci 2008, 91, 3067–3078. [Google Scholar]
- Lee, J.Y.; Sohn, K.H.; Rhee, S.H.; Hwang, D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J. Biol. Chem 2001, 276, 16683–16689. [Google Scholar]
- Suganami, T.; Tanimoto-Koyama, K.; Nishida, J.; Itoh, M.; Yuan, X.; Mizuarai, S.; Kotani, H.; Yamaoka, S.; Miyake, K.; Aoe, S.; et al. Role of the Toll-like receptor 4/NF-κB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler. Thromb. Vasc. Biol 2007, 27, 84–91. [Google Scholar]
- Kim, F.; Pham, M.; Luttrell, I.; Bannerman, D.D.; Tupper, J.; Thaler, J.; Hawn, T.R.; Raines, E.W.; Schwartz, M.W. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ. Res 2007, 100, 1589–1596. [Google Scholar]
- Wong, S.W.; Kwon, M.J.; Choi, A.M.; Kim, H.P.; Nakahira, K.; Hwang, D.H. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J. Biol. Chem 2009, 284, 27384–27392. [Google Scholar]
- Li, X.; Yu, Y.; Funk, C.D. Cyclooxygenase-2 induction in macrophages is modulated by docosahexaenoic acid via interactions with free fatty acid receptor 4 (FFA4). FASEB J 2013. [Google Scholar] [CrossRef]
- Edwards, I.J.; Flaherty, J.T. Ω-3 fatty acids and PPARg in cancer. PPAR Res 2008, 2008, 358052:1–358052:14. [Google Scholar]
- Dumlao, D.S.; Buczynski, M.W.; Norris, P.C.; Harkewicz, R.; Dennis, E.A. High-throughput lipidomic analysis of fatty acid derived eicosanoids and N-acylethanolamines. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2011, 1811, 724–736. [Google Scholar]
- Dubois, R.N.; Abramson, S.B.; Crofford, L.; Gupta, R.A.; Simon, L.S.A.; van de Putte, L.B.; Lipsky, P.E. Cyclooxygenase in biology and disease. FASEB J 1998, 12, 1063–1073. [Google Scholar]
- Kuhn, H.; Thiele, B.J. The diversity of the lipoxygenase family: Many sequence data but little information on biological significance. FEBS Lett 1999, 449, 7–11. [Google Scholar]
- Serhan, C.N.; Petasis, N.A. Resolvins and protectins in inflammation resolution. Chem. Rev 2011, 111, 5922–5943. [Google Scholar]
- Calder, P.C. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr 2006, 83, S1505–S1519. [Google Scholar]
- Altmann, R.; Hausmann, M.; Spöttl, T.; Gruber, M.; Bull, A.W.; Menzel, K.; Vogl, D.; Herfarth, H.; Schölmerich, J.; Falk, W.; et al. 13-Oxo-ODE is an endogenous ligand for PPARγ in human colonic epithelial cells. Biochem. Pharmacol 2007, 74, 612–622. [Google Scholar]
- Natarajan, R.; Reddy, M.A.; Malik, K.U.; Fatima, S.; Khan, B.V. Signaling mechanisms of nuclear factor-κB-mediated activation of inflammatory genes by 13-hydroperoxyoctadecadienoic acid in cultured vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol 2001, 21, 1408–1413. [Google Scholar]
- Ogawa, E.; Owada, Y.; Ikawa, S.; Adachi, Y.; Egawa, T.; Nemoto, K.; Suzuki, K.; Hishinuma, T.; Kawashima, H.; Kondo, H.; et al. Epidermal FABP (FABP5) regulates keratinocyte differentiation by 13(S)-HODE-mediated activation of the NF-κB signaling pathway. J. Invest. Dermatol 2011, 131, 604–612. [Google Scholar]
- Ylä-Herttuala, S.; Rosenfeld, M.E.; Parthasarathy, S.; Glass, C.K.; Sigal, E.; Witztum, J.L.; Steinberg, D. Colocalization of 15-lipoxygenase mRNA and protein with epitopes of oxidized low density lipoprotein in macrophage-rich areas of atherosclerotic lesions. Proc. Natl. Acad. Sci. USA 1990, 87, 6959–6963. [Google Scholar]
- Xu, L.; Davis, T.A.; Porter, N.A. Rate constants for peroxidation of polyunsaturated fatty acids and sterols in solution and in liposomes. J. Am. Chem. Soc 2009, 131, 13037–13044. [Google Scholar]
- Yoshida, Y.; Niki, E. Hydroxyoctadecadienoic Acid (HODE) as a Marker of Linoleic Acid Oxidation. In Biomarkers for Antioxidant Defense and Oxidative Damage: Principles and Practical Applications; Aldini, G., Yeum, K.-J., Niki, E., Russell, R.M., Eds.; Wiley: Ames, IA, USA, 2010; pp. 85–97. [Google Scholar]
- Dussault, P.; Lee, I.Q. The total synthesis of 15(S)-HPETE (hydroperoxyeicosatetraenoic acid). J. Org. Chem 1992, 57, 1952–1954. [Google Scholar]
- Ramsden, C.E.; Ringel, A.; Feldstein, A.E.; Taha, A.Y.; MacIntosh, B.A.; Hibbeln, J.R.; Majchrzak-Hong, S.F.; Faurot, K.R.; Rapoport, S.I.; Cheon, Y.; et al. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot Essent. Fat. Acids 2012, 87, 135–141. [Google Scholar]
- Kelley, D.S.; Taylor, P.C.; Nelson, G.J.; Mackey, B.E. Arachidonic acid supplementation enhances synthesis of eicosanoids without suppressing immune functions in young healthy men. Lipids 1998, 33, 125–130. [Google Scholar]
- Hardardottir, I.; Kinsella, J.E. Tumor necrosis factor production by murine resident peritoneal macrophages is enhanced by dietary n-3 polyunsaturated fatty acids. Biochim. Biophys. Acta 1991, 1095, 187–195. [Google Scholar]
- Zuo, X.; Shureiqi, I. Eicosanoid profiling in colon cancer: Emergence of a pattern. Prostaglandins Lipid Mediat 2013, 104–105, 139–143. [Google Scholar]
- Wang, D.; DuBois, R.N. Prostaglandins and cancer. Gut 2006, 55, 115–122. [Google Scholar]
- Vangaveti, V.; Baune, B.T.; Kennedy, R.L. Review: Hydroxyoctadecadienoic acids: Novel regulators of macrophage differentiation and atherogenesis. Ther. Adv. Endocrinol. Metab 2010, 1, 51–60. [Google Scholar]
- Spite, M.; Norling, L.V.; Summers, L.; Yang, R.; Cooper, D.; Petasis, N.A.; Flower, R.J.; Perretti, M.; Serhan, C.N. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 2009, 461, 1287–1291. [Google Scholar]
- Norling, L.V.; Dalli, J.; Flower, R.J.; Serhan, C.N.; Perretti, M. Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: Receptor-dependent actions. Arterioscler. Thromb. Vasc. Biol 2012, 32, 1970–1978. [Google Scholar]
- Rong, S.; Cao, Q.; Liu, M.; Seo, J.; Jia, L.; Boudyguina, E.; Gebre, A.K.; Colvin, P.L.; Smith, T.L.; Murphy, R.C.; et al. Macrophage 12/15 lipoxygenase expression increases plasma and hepatic lipid levels and exacerbates atherosclerosis. J. Lipid Res 2012, 53, 686–695. [Google Scholar]
- Cyrus, T.; Witztum, J.L.; Rader, D.J.; Tangirala, R.; Fazio, S.; Linton, M.; Funk, C.D. Disruption of the 12/15-lipoxygenase gene diminishes atherosclerosis in apo E-deficient mice. J. Clin. Invest 1999, 103, 1597–1604. [Google Scholar]
- Johnson, G.H.; Fritsche, K. Effect of dietary linoleic acid on markers of inflammation in healthy persons: A systematic review of randomized controlled trials. J. Acad. Nutr. Diet 2012, 112, 1029–1041. [Google Scholar]
- Gertow, K.; Nobili, E.; Folkersen, L.; Newman, J.W.; Pedersen, T.L.; Ekstrand, J.; Swedenborg, J.; Kuhn, H.; Wheelock, C.E.; Hansson, G.K.; et al. 12- and 15-lipoxygenases in human carotid atherosclerotic lesions: Associations with cerebrovascular symptoms. Atherosclerosis 2011, 215, 411–416. [Google Scholar]
- Huo, Y.; Zhao, L.; Hyman, M.C.; Shashkin, P.; Harry, B.L.; Burcin, T.; Forlow, S.B.; Stark, M.A.; Smith, D.F.; Clarke, S.; et al. Critical role of macrophage 12/15-lipoxygenase for atherosclerosis in apolipoprotein E-deficient mice. Circulation 2004, 110, 2024–2031. [Google Scholar]
- Stachowska, E.; Dziedziejko, V.; Safranow, K.; Jakubowska, K.; Olszewska, M.; Machaliñski, B.; Chlubek, D. Effect of conjugated linoleic acids on the activity and mRNA expression of 5- and 15-lipoxygenases in human macrophages. J. Agric. Food Chem 2007, 55, 5335–5342. [Google Scholar]
- Contreras, G.; Raphael, W.; Mattmiller, S.; Gandy, J.; Sordillo, L. Nonesterified fatty acids modify inflammatory response and eicosanoid biosynthesis in bovine endothelial cells. J. Dairy Sci 2012, 95, 5011–5023. [Google Scholar]
- Brinckmann, R.; Schnurr, K.; Heydeck, D.; Rosenbach, T.; Kolde, G.; Kühn, H. Membrane translocation of 15-lipoxygenase in hematopoietic cells is calcium-dependent and activates the oxygenase activity of the enzyme. Blood 1998, 91, 64–74. [Google Scholar]
- Zou, H.; Yuan, C.; Dong, L.; Sidhu, R.S.; Hong, Y.H.; Kuklev, D.V.; Smith, W.L. Human cyclooxygenase-1 activity and its responses to COX inhibitors are allosterically regulated by nonsubstrate fatty acids. J. Lipid Res 2012, 53, 1336–1347. [Google Scholar]
- Kühn, H.; Belkner, J.; Suzuki, H.; Yamamoto, S. Oxidative modification of human lipoproteins by lipoxygenases of different positional specificities. J. Lipid Res 1994, 35, 1749–1759. [Google Scholar]
- Soberman, R.; Harper, T.; Betteridge, D.; Lewis, R.; Austen, K. Characterization and separation of the arachidonic acid 5-lipoxygenase and linoleic acid ω-6 lipoxygenase (arachidonic acid 15-lipoxygenase) of human polymorphonuclear leukocytes. J. Biol. Chem 1985, 260, 4508–4515. [Google Scholar]
- Kuhn, H.; Barnett, J.; Grunberger, D.; Baecker, P.; Chow, J.; Nguyen, B.; Bursztyn-Pettegrew, H.; Chan, H.; Sigal, E. Overexpression, purification and characterization of human recombinant 15-lipoxygenase. Biochim. Biophys. Acta Lipids Lipid Metab 1993, 1169, 80–89. [Google Scholar]
- Norris, P.C.; Dennis, E.A. Ω-3 fatty acids cause dramatic changes in TLR4 and purinergic eicosanoid signaling. Proc. Natl. Acad. Sci. USA 2012, 109, 8517–8522. [Google Scholar]
- Hung, N.D.; Kim, M.R.; Sok, D.E. Oral administration of 2-docosahexaenoyl lysophosphatidylcholine displayed anti-inflammatory effects on zymosan A-induced peritonitis. Inflammation 2011, 34, 147–160. [Google Scholar]
- Levy, B.D.; Bonnans, C.; Silverman, E.S.; Palmer, L.J.; Marigowda, G.; Israel, E. Diminished lipoxin biosynthesis in severe asthma. Am. J. Respir. Crit. Care Med 2005, 172, 824–830. [Google Scholar]
- Healy, D.A.; Wallace, F.A.; Miles, E.A.; Calder, P.C.; Newsholme, P. Effect of low-to-moderate amounts of dietary fish oil on neutrophil lipid composition and function. Lipids 2000, 35, 763–768. [Google Scholar]
- Rees, D.; Miles, E.A.; Banerjee, T.; Wells, S.J.; Roynette, C.E.; Wahle, K.W.; Calder, P.C. Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: A comparison of young and older men. Am. J. Clin. Nutr 2006, 83, 331–342. [Google Scholar]
- Woodworth, H.L.; McCaskey, S.J.; Duriancik, D.M.; Clinthorne, J.F.; Langohr, I.M.; Gardner, E.M.; Fenton, J.I. Dietary fish oil alters T lymphocyte cell populations and exacerbates disease in a mouse model of inflammatory colitis. Cancer Res 2010, 70, 7960–7969. [Google Scholar]
- Fitzgerald, G.A. Coxibs and cardiovascular disease. N. Engl. J. Med 2004, 351, 1709–1711. [Google Scholar]
- Cheng, Y.; Austin, S.C.; Rocca, B.; Koller, B.H.; Coffman, T.M.; Grosser, T.; Lawson, J.A.; FitzGerald, G.A. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science 2002, 296, 539–541. [Google Scholar]
- Xiong, A.; Yu, W.; Tiwary, R.; Sanders, B.G.; Kline, K. Distinct roles of different forms of vitamin E in DHA-induced apoptosis in triple-negative breast cancer cells. Mol. Nutr. Food Res 2012, 56, 923–934. [Google Scholar]
- Kitessa, S.M.; Gulati, S.K.; Ashes, J.R.; Fleck, E.; Scott, T.W.; Nichols, P.D. Utilisation of fish oil in ruminants: II. Transfer of fish oil fatty acids into goats’ milk. Anim. Feed Sci. Technol 2001, 89, 201–208. [Google Scholar]
- Okuyama, H.; Kobayashi, T.; Watanabe, S. Dietary fatty acids—The n-6/n-3 balance and chronic elderly diseases. Excess linoleic acid and relative n-3 deficiency syndrome seen in Japan. Prog. Lipid Res 1996, 35, 409–457. [Google Scholar]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, ω-3 fatty acids, and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol 2003, 23, e20–e30. [Google Scholar]
- Emken, E.A.; Adlof, R.O.; Gulley, R.M. Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochim. Biophys. Acta BBA Lipids Lipid Metab 1994, 1213, 277–288. [Google Scholar]
- Van den Top, A.M.; Geelen, M.J.H.; Wensing, T.; Wentink, G.H.; van ’t Klooster, A.T.; Beynen, A.C. Higher postpartum hepatic triacylglycerol concentrations in dairy cows with free rather than restricted access to feed during the dry period are associated with lower activities of hepatic glycerolphosphate acyltransferase. J. Nutr 1996, 126, 76–85. [Google Scholar]
- Contreras, G.A.; O’Boyle, N.J.; Herdt, T.H.; Sordillo, L.M. Lipomobilization in periparturient dairy cows influences the composition of plasma nonesterified fatty acids and leukocyte phospholipid fatty acids. J. Dairy Sci 2010, 93, 2508–2516. [Google Scholar]
- Kaplan, A.; Lee, V.F. Serum lipid levels in infants and mothers at parturition. Clin. Chim. Acta 1965, 12, 258–263. [Google Scholar]
- Shuster, D.; Lee, E.; Kehrli, M., Jr. Bacterial growth, inflammatory cytokine production, and neutrophil recruitment during coliform mastitis in cows within ten days after calving, compared with cows at midlactation. Am. J. Vet. Res 1996, 57, 1569–1575. [Google Scholar]
- Iwasaki, Y.; Yamane, T. Enzymatic synthesis of structured lipids. Adv. Biochem. Eng. Biotechnol 2004, 90, 760–760. [Google Scholar]
- Triggiani, M.; Oriente, A.; Marone, G. Differential roles for triglyceride and phospholipid pools of arachidonic acid in human lung macrophages. J. Immunol 1994, 152, 1394–1403. [Google Scholar]
- Strawford, A.; Antelo, F.; Christiansen, M.; Hellerstein, M.K. Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H2O. Am. J. Physiol. Endocrinol. Metab 2004, 286, E577–E588. [Google Scholar]
- Hodson, L.; Skeaff, C.M.; Fielding, B.A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid Res 2008, 47, 348–380. [Google Scholar]
- Kennedy, E.P.; Weiss, S.B. The function of cytidine coenzymes in the biosynthesis of phospholipides. J. Biol. Chem 1956, 222, 193–214. [Google Scholar]
- Liu, Q.; Siloto, R.M.P.; Lehner, R.; Stone, S.J.; Weselake, R.J. Acyl-CoA:diacylglycerol acyltransferase: Molecular biology, biochemistry and biotechnology. Prog. Lipid Res 2012, 51, 350–377. [Google Scholar]
- The LIPID MAPS–Nature Lipidomics Gateway. Available online: http://www.lipidmaps.org (accessed on 24 May 2012).
- Kanter, J.E.; Kramer, F.; Barnhart, S.; Averill, M.M.; Vivekanandan-Giri, A.; Vickery, T.; Li, L.O.; Becker, L.; Yuan, W.; Chait, A.; et al. Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl-CoA synthetase 1. Proc. Natl. Acad. Sci. USA 2012, 109, E715–E724. [Google Scholar]
- Takeuchi, K.; Reue, K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am. J. Physiol. Endocrinol. Metab 2009, 296, E1195–E1209. [Google Scholar]
- Bionaz, M.; Loor, J.J. ACSL1, AGPAT6, FABP3, LPIN1, and SLC27A6 are the most abundant isoforms in bovine mammary tissue and their expression is affected by stage of lactation. J. Nutr 2008, 138, 1019–1024. [Google Scholar]
- Loor, J.J.; Dann, H.M.; Everts, R.E.; Oliveira, R.; Green, C.A.; Guretzky, N.A.J.; Rodriguez-Zas, S.L.; Lewin, H.A.; Drackley, J.K. Temporal gene expression profiling of liver from periparturient dairy cows reveals complex adaptive mechanisms in hepatic function. Physiol. Genomics 2005, 23, 217–226. [Google Scholar]
- Bladergroen, B.A.; Wensing, T.; van Golde, L.M.G.; Geelen, M.J.H. Reversible translocation of CTP:phosphocholine cytidylyltransferase from cytosol to membranes in the adult bovine liver around parturition. Biochim. Biophys. Acta Lipids Lipid Metab 1998, 1391, 233–240. [Google Scholar]
- Xu, A.; Wang, Y.; Xu, J.Y.; Stejskal, D.; Tam, S.; Zhang, J.; Wat, N.M.S.; Wong, W.K.; Lam, K.S.L. Adipocyte fatty acid–binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin. Chem 2006, 52, 405–413. [Google Scholar]
- Sharma, N.K.; Langberg, K.A.; Mondal, A.K.; Das, S.K. Phospholipid biosynthesis genes and susceptibility to obesity: Analysis of expression and polymorphisms. PLoS One 2013, 8, e65303. [Google Scholar]
- Lands, W.E. Metabolism of glycerolipides: A comparison of lecithin and triglyceride synthesis. J. Biol. Chem 1958, 231, 883–888. [Google Scholar]
- Mulligan, C.M.; Sparagna, G.C.; le, C.H.; de Mooy, A.B.; Routh, M.A.; Holmes, M.G.; Hickson-Bick, D.L.; Zarini, S.; Murphy, R.C.; Xu, F.Y.; et al. Dietary linoleate preserves cardiolipin and attenuates mitochondrial dysfunction in the failing rat heart. Cardiovasc. Res 2012, 94, 460–468. [Google Scholar]
- Shindou, H.; Hishikawa, D.; Harayama, T.; Yuki, K.; Shimizu, T. Recent progress on acyl CoA: Lysophospholipid acyltransferase research. In J. Lipid Res; 2009; Volume 50, pp. S46–S51. [Google Scholar]
- Coleman, R.A.; Lewin, T.M.; van Horn, C.G.; Gonzalez-Baró, M.R. Do long-chain acyl-CoA synthetases regulate fatty acid entry into synthetic versus degradative pathways? J. Nutr 2002, 132, 2123–2126. [Google Scholar]
- Turkish, A.; Sturley, S.L. Regulation of triglyceride metabolism. I. Eukaryotic neutral lipid synthesis: “Many ways to skin ACAT or a DGAT”. Am. J. Physiol. Gastrointest. Liver Physiol 2007, 292, G953–G957. [Google Scholar]
- Yen, C.-L.E.; Stone, S.J.; Koliwad, S.; Harris, C.; Farese, R.V. Thematic review series: Glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res 2008, 49, 2283–2301. [Google Scholar]
- Zammit, V.A.; Buckett, L.K.; Turnbull, A.V.; Wure, H.; Proven, A. Diacylglycerol acyltransferases: Potential roles as pharmacological targets. Pharmacol. Ther 2008, 118, 295–302. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Raphael, W.; Sordillo, L.M. Dietary Polyunsaturated Fatty Acids and Inflammation: The Role of Phospholipid Biosynthesis. Int. J. Mol. Sci. 2013, 14, 21167-21188. https://doi.org/10.3390/ijms141021167
Raphael W, Sordillo LM. Dietary Polyunsaturated Fatty Acids and Inflammation: The Role of Phospholipid Biosynthesis. International Journal of Molecular Sciences. 2013; 14(10):21167-21188. https://doi.org/10.3390/ijms141021167
Chicago/Turabian StyleRaphael, William, and Lorraine M. Sordillo. 2013. "Dietary Polyunsaturated Fatty Acids and Inflammation: The Role of Phospholipid Biosynthesis" International Journal of Molecular Sciences 14, no. 10: 21167-21188. https://doi.org/10.3390/ijms141021167
APA StyleRaphael, W., & Sordillo, L. M. (2013). Dietary Polyunsaturated Fatty Acids and Inflammation: The Role of Phospholipid Biosynthesis. International Journal of Molecular Sciences, 14(10), 21167-21188. https://doi.org/10.3390/ijms141021167




