Inhibition of Melanogenesis by Gallic Acid: Possible Involvement of the PI3K/Akt, MEK/ERK and Wnt/β-Catenin Signaling Pathways in B16F10 Cells
Abstract
:1. Introduction
2. Results
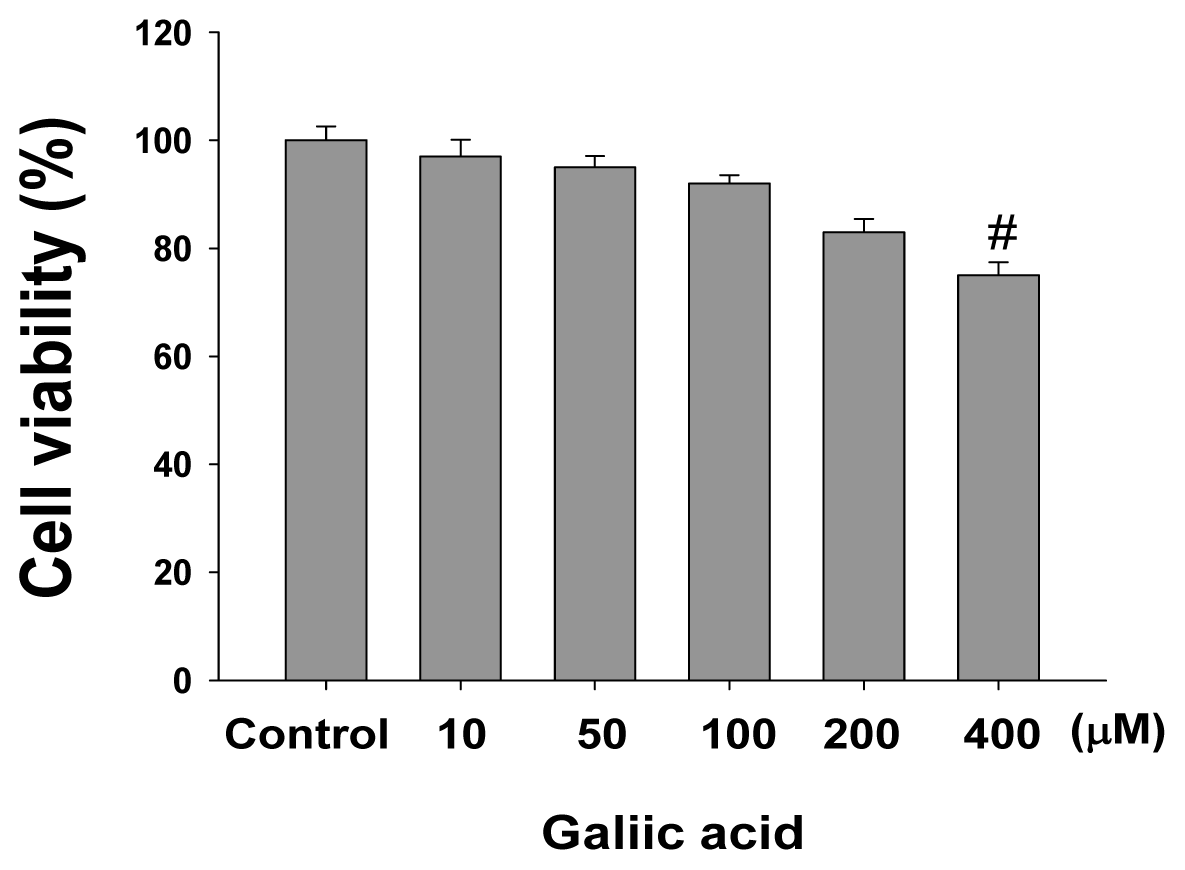
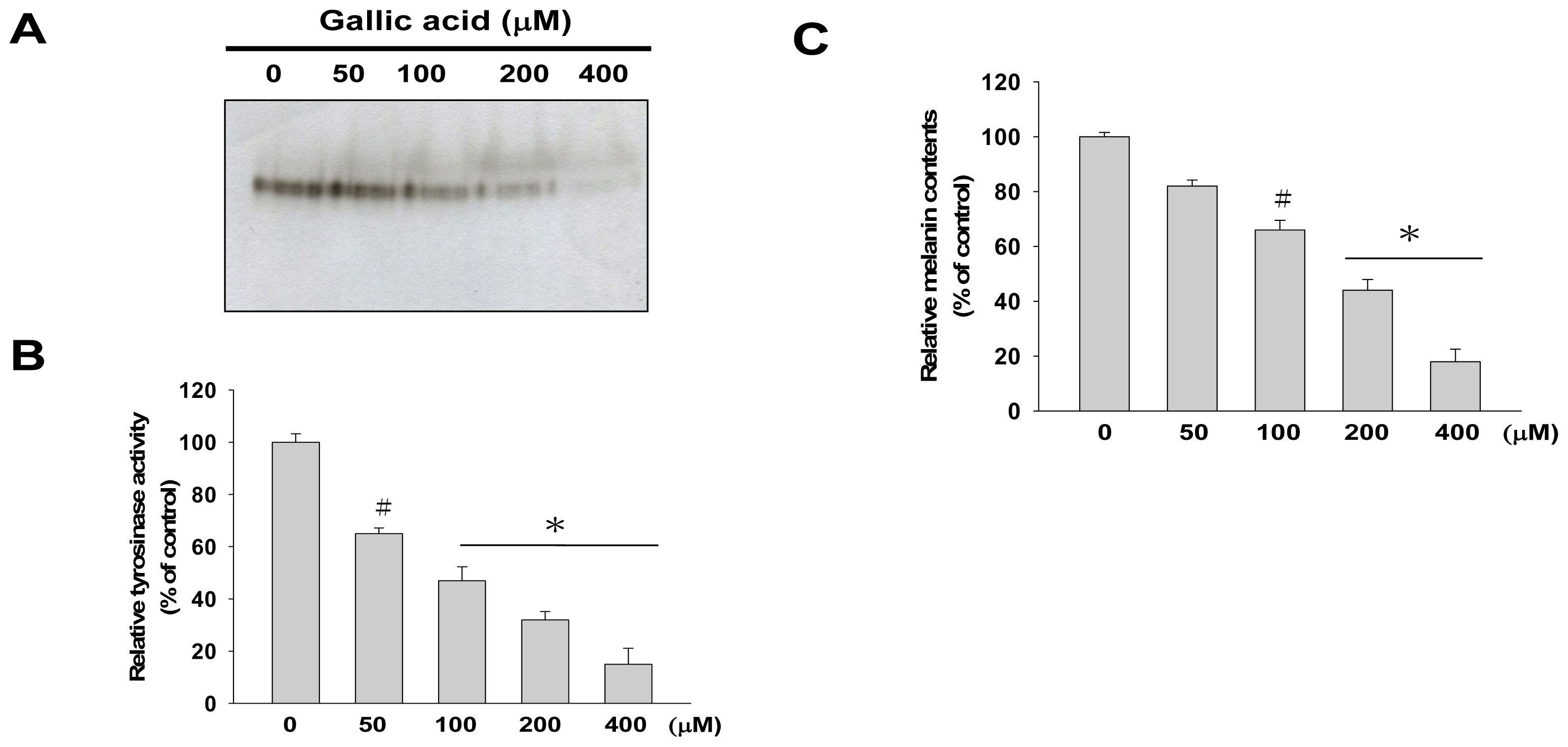
2.1. Effects of Gallic Acid on Melanin Synthesis and Tyrosinase Activity in B16F10 Cells
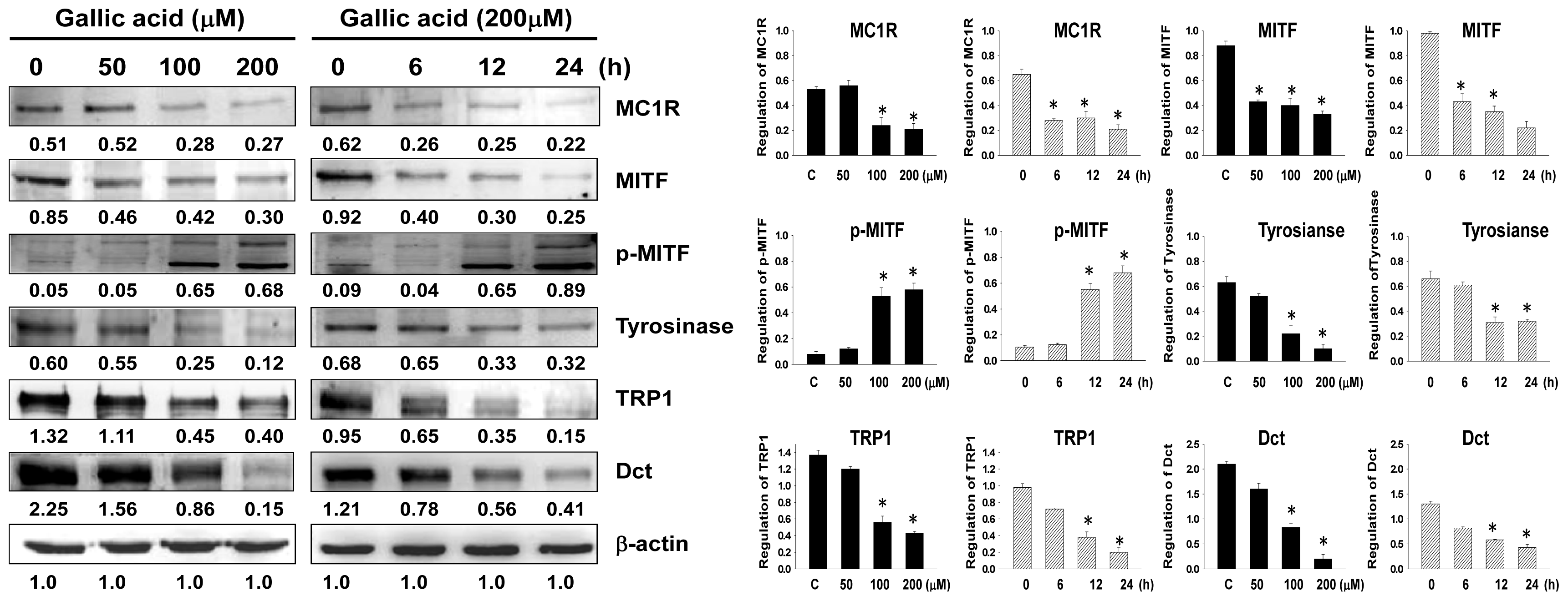
2.2. Effects of Gallic Acid on Expressions of Melanogenesis-Related Proteins
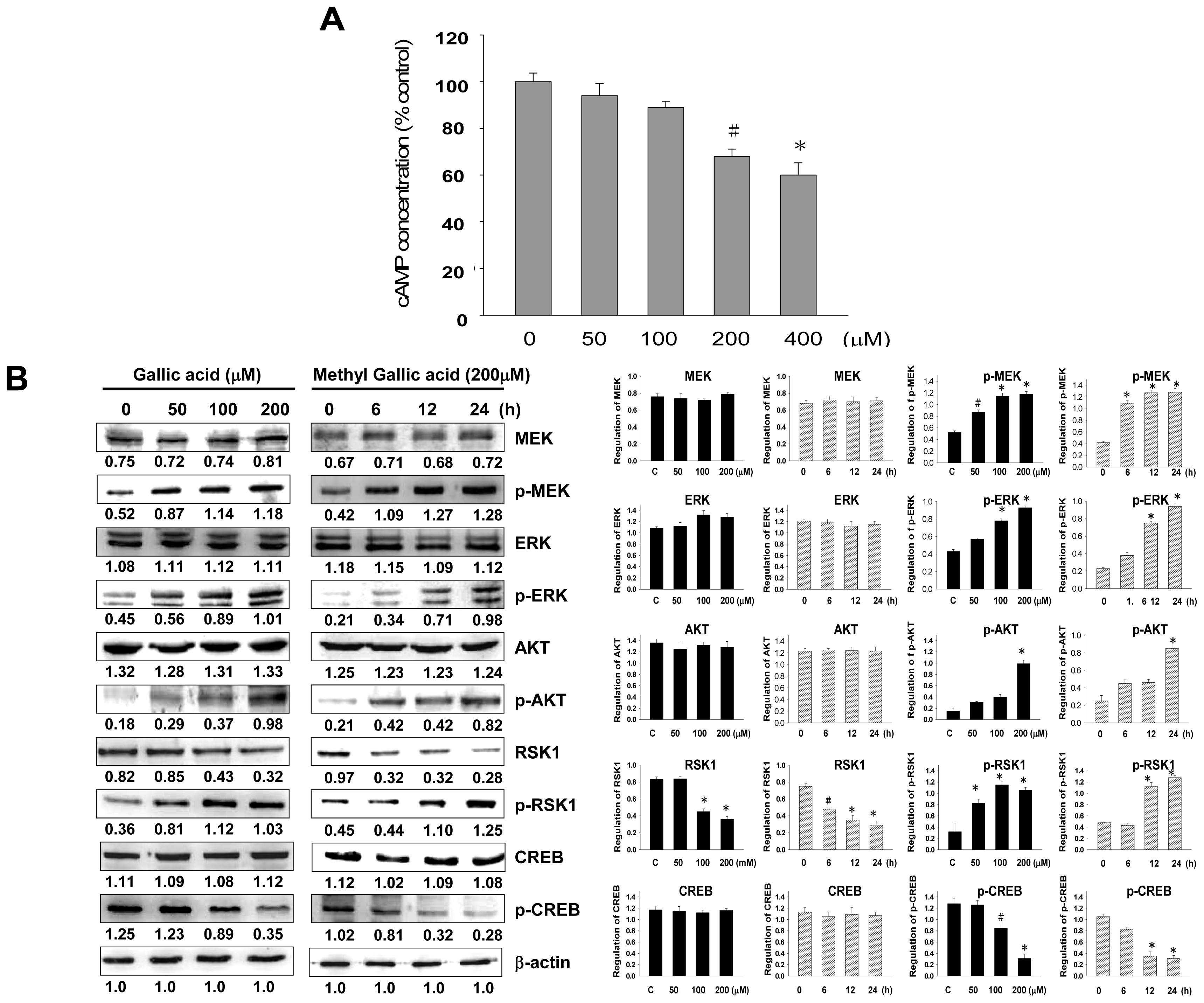
2.3. Effect of Gallic Acid on the Melanogenesis-Related Signaling Pathway
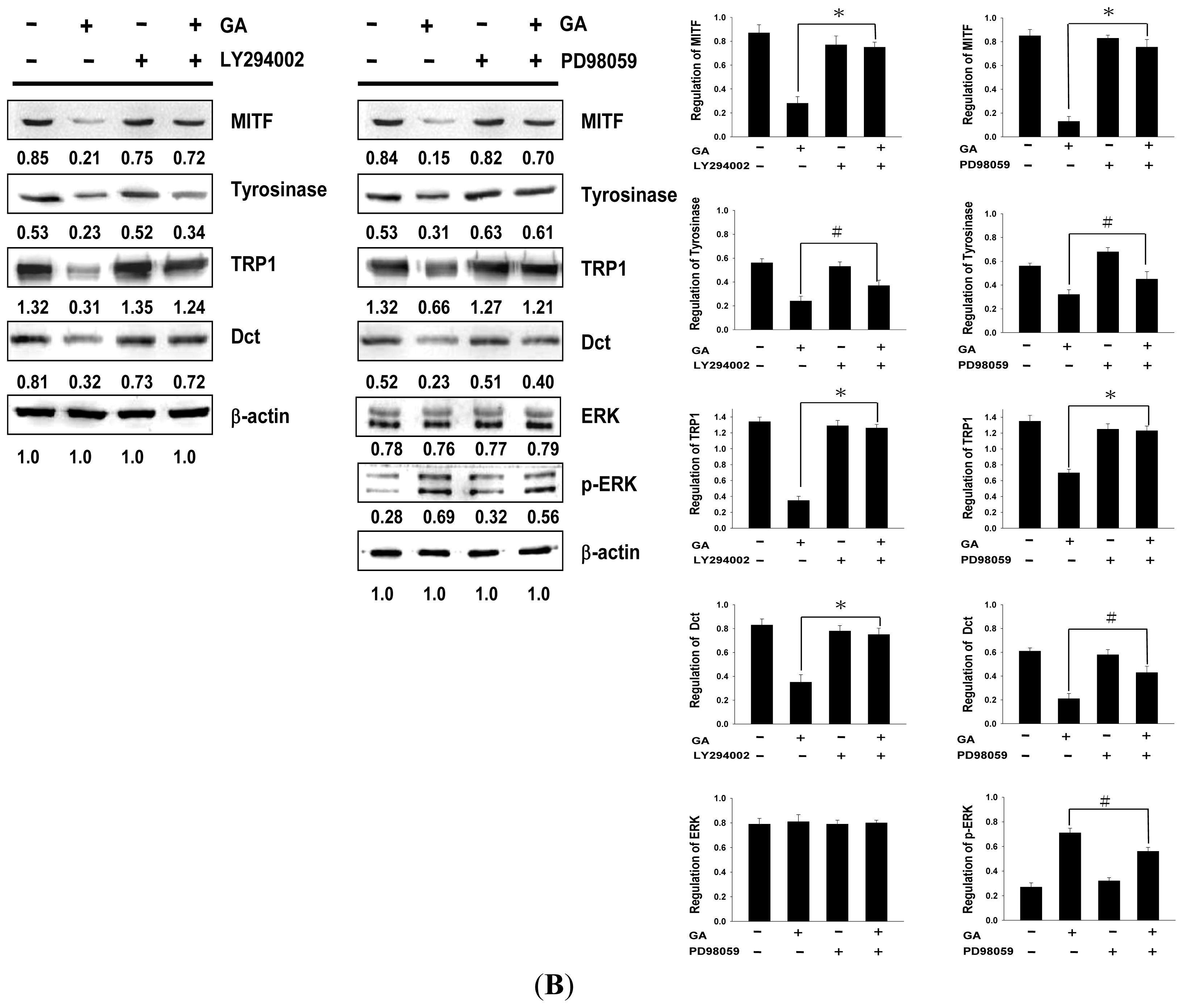
2.4. Effects of PI3K/Akt and MEK/ERK Inhibitors on Melanin Synthesis in Gallic Acid-Treated B16F10 Melanoma Cells
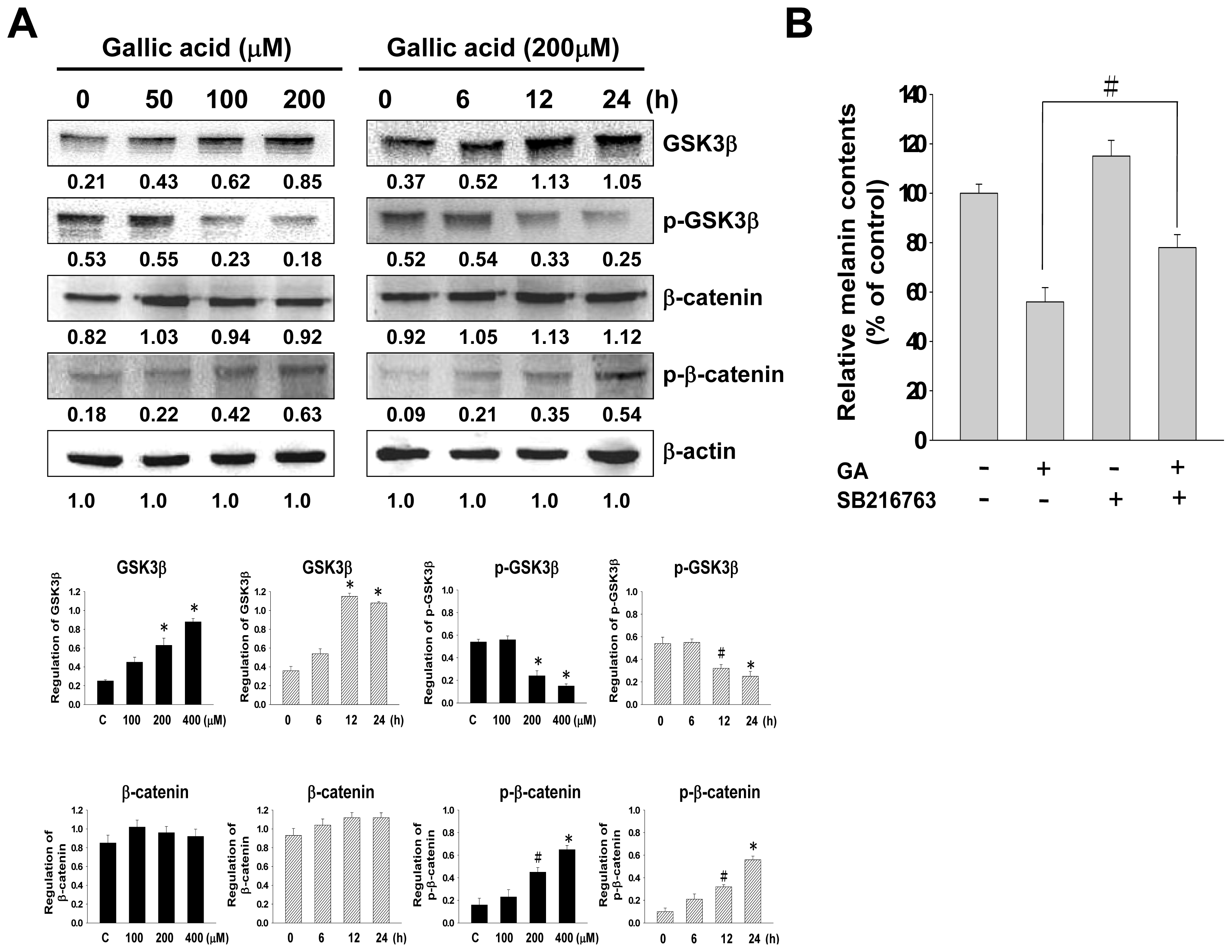
2.5. Effect of Gallic Acid on Wnt/β-Catenin Signaling Pathway
3. Discussion
4. Experimental
4.1. Chemicals and Reagents
4.2. Cell Culture and Treatment with Gallic Acid
4.3. Cell Viability Assay
4.4. Cellular Melanin Content Determination
4.5. Tyrosinase Activity Assay
4.6. Tyrosinase Activity Staining
4.7. Western Blot Analysis
4.8. cAMP Assay
5. Conclusions

Acknowledgments
Conflicts of interest
References
- Tsatmali, M.; Ancans, J.; Thody, A.J. Melanocyte function and its control by melanocortin peptides. J. Histochem. Cytochem 2002, 50, 125–133. [Google Scholar]
- Briganti, S.; Camera, E.; Picardo, M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Melanoma Res 2003, 16, 101–110. [Google Scholar]
- Kobayashi, T.; Urabe, K.; Winder, A.; Jimenez-Cervantes, C.; Imokawa, G.; Brewington, T.; Solano, F.; Garcia-Borron, J.C.; Hearing, V.J. Tyrosinase related protein 1 (TRP1) functions as a DHICA oxidase in melanin biosynthesis. EMBO J 1994, 13, 5818–5825. [Google Scholar]
- Busca, R.; Ballotti, R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res 2000, 13, 60–69. [Google Scholar]
- Bertolotto, C.; Abbe, P.; Hemesath, T.J.; Bille, K.; Fisher, D.E.; Ortonne, J.P.; Ballotti, R. Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J. Cell Biol 1998, 142, 827–835. [Google Scholar]
- Bertolotto, C.; Bille, K.; Ortonne, J.P.; Ballotti, R. Regulation of tyrosinase gene expression by cAMP in B16 melanoma cells involves two CATGTG motifs surrounding the TATA box: Implication of the microphthalmia gene product. J. Cell Biol 1996, 134, 747–755. [Google Scholar]
- Park, H.Y.; Wu, C.; Yonemoto, L.; Murphy-Smith, M.; Wu, H.; Stachur, C.M.; Gilchrest, B.A. MITF mediates cAMP-induced protein kinase C-beta expression in human melanocytes. Biochem. J 2006, 395, 571–578. [Google Scholar]
- Wu, M.; Hemesath, T.J.; Takemoto, C.M.; Horstmann, M.A.; Wells, A.G.; Price, E.R.; Fisher, D.Z.; Fisher, D.E. c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev 2000, 14, 301–312. [Google Scholar]
- Jang, J.Y.; Lee, J.H.; Kang, B.W.; Chung, K.T.; Choi, Y.H.; Choi, B.T. Dichloromethane fraction of Cimicifuga heracleifolia decreases the level of melanin synthesis by activating the ERK or AKT signaling pathway in B16F10 cells. Exp. Dermatol 2009, 18, 232–237. [Google Scholar]
- Jang, J.Y.; Kim, H.N.; Kim, Y.R.; Choi, W.Y.; Choi, Y.H.; Shin, H.K.; Choi, B.T. Partially purified components of Nardostachys chinensis suppress melanin synthesis through ERK and Akt signaling pathway with cAMP down-regulation in B16F10 cells. J. Ethnopharmacol 2011, 137, 1207–1214. [Google Scholar]
- Kim, D.S.; Jeong, Y.M.; Park, I.K.; Hahn, H.G.; Lee, H.K.; Kwon, S.B.; Jeong, J.H.; Yang, S.J.; Sohn, U.D.; Park, K.C. A new 2-imino-1,3-thiazoline derivative, KHG22394, inhibits melanin synthesis in mouse B16 melanoma cells. Biol. Pharm. Bull 2007, 30, 180–183. [Google Scholar]
- Larue, L.; Delmas, V. The WNT/beta-catenin pathway in melanoma. Front. Biosci 2006, 1, 733–742. [Google Scholar]
- Takeda, K.; Takemoto, C.; Kobayashi, I.; Watanabe, A.; Nobukuni, Y.; Fisher, D.E.; Tachibana, M. Ser298 of MITF, a mutation site in Waardenburg syndrome type 2, is a phosphorylation site with functional significance. Hum. Mol. Genet 2000, 9, 125–132. [Google Scholar]
- Yasumoto, K.; Takeda, K.; Saito, H; Watanabe, K.; Takahashi, K.; Shibahara, S. Microphthalmia-associated transcription factor interacts with LEF-1, a mediator of Wnt signaling. EMBO J 2002, 21, 2703–2714. [Google Scholar]
- Wu, J.; Saint-Jeannet, J.P.; Klein, P.S. Wnt-frizzled signaling in neural crest formation. Trends Neurosci 2003, 26, 40–45. [Google Scholar]
- Bellei, B.; Flori, E.; Izzo, E.; Maresca, V.; Picardo, M. GSK3beta inhibition promotes melanogenesis in mouse B16 melanoma cells and normal human melanocytes. Cell Signal 2008, 20, 1750–1761. [Google Scholar]
- Shahrzad, S.; Aoyagi, K.; Winter, A.; Koyama, A.; Bitsch, I. Pharmacokinetics of gallic acid and its relative bioavailability from tea in healthy humans. J. Nutr 2001, 131, 1207–1210. [Google Scholar]
- Hsu, J.D.; Kao, S.H.; Ou, T.T.; Chen, Y.J.; Li, Y.J.; Wang, C.J. Gallic acid induces G2/M phase arrest of breast cancer cell MCF-7 through stabilization of p27(Kip1) attributed to disruption of p27(Kip1)/Skp2 complex. J. Agric. Food Chem 2011, 59, 1996–2003. [Google Scholar]
- Yoon, C.H.; Chung, S.J.; Lee, S.W.; Park, Y.B.; Lee, S.K.; Park, M.C. Gallic acid, a natural polyphenolic acid, induces apoptosis and inhibits proinflammatory gene expressions in rheumatoid arthritis fibroblast-like synoviocytes. Jt. Bone Spine 2013, 80, 274–279. [Google Scholar]
- Wang, H.; Provan, G.J.; Helliwell, K. Determination of hamamelitannin, catechins and gallic acid in witch hazel bark, twig and leaf by HPLC. J. Pharm. Biomed. Anal 2003, 33, 539–544. [Google Scholar]
- Martín, S.; González-Burgos, E.; Carretero, M. E.; Gómez-Serranillos, M.P. Protective effects of Merlot red wine extract and its major polyphenols in PC12 cells under oxidative stress conditions. J. Food Sci 2013, 78, H112–H118. [Google Scholar]
- Chuang, C.Y.; Liu, H.C.; Wu, L.C.; Chen, C.Y.; Chang, J.T.; Hsu, S.L. Gallic acid induces apoptosis of lung fibroblasts via a reactive oxygen species-dependent ataxia telangiectasia mutated-p53 activation pathway. J. Agric. Food Chem 2010, 58, 2943–2951. [Google Scholar]
- Kroes, B.H.; van den Berg, A.J.; van Ufford, H.C.Q.; van Dijk, H.; Labadie, R.P. Anti-inflammatory activity of gallic acid. Planta Medica 1992, 58, 499–504. [Google Scholar]
- Kim, Y.J. Antimelanogenic and antioxidant properties of gallic acid. Biol. Pharm. Bull 2007, 30, 1052–1055. [Google Scholar]
- Khaled, M.; Larribere, L.; Bille, K.; Ortonne, J.P.; Ballotti, R.; Bertolotto, C. Microphthalmia associated transcription factor is a target of the phosphatidylinositol-3-kinase pathway. J. Investig. Dermatol 2003, 121, 831–836. [Google Scholar]
- Khaled, M.; Larribere, L.; Bille, K.; Aberdam, E.; Ortonne, J.P.; Ballotti, R.; Bertolotto, C. Glycogen synthase kinase 3beta is activated by cAMP and plays an active role in the regulation of melanogenesis. J. Biol. Chem 2002, 277, 33690–33697. [Google Scholar]
- Huh, S.; Jung, E.; Lee, J.; Roh, K.; Kim, J.D.; Park, D. Mechanisms of melanogenesis inhibition by propafenone. Arch. Dermatol. Res 2010, 302, 561–565. [Google Scholar]
- Du, J.; Miller, A.J.; Widlund, H.R.; Horstmann, M.A.; Ramaswamy, S.; Fisher, D.E. MLANA/MART1 and SILV/PMEL17/GP100 are transcriptionally regulated by MITF in melanocytes and melanoma. Am. J. Pathol 2003, 163, 333–343. [Google Scholar]
- Goding, C.R. Mitf from neural crest to melanoma: Signal transduction and transcription in the melanocyte lineage. Genes Dev 2000, 14, 1712–1728. [Google Scholar]
- Aoki, H.; Moro, O. Involvement of microphthalmia-associated transcription factor (MITF) in expression of human melanocortin-1 receptor (MC1R). Life Sci 2002, 71, 2171–2179. [Google Scholar]
- Hemesath, T.J.; Price, E.R.; Takemoto, C.; Badalian, T.; Fisher, D.E. MAP kinase links the transcription factor Microphthalmia to c-Kit signalling in melanocytes. Nature 1998, 391, 298–301. [Google Scholar]
- Lee, J.; Jung, K.; Kim, Y.S.; Park, D. Diosgenin inhibits melanogenesis through the activation of phosphatidylinositol-3-kinase pathway (PI3K) signaling. Life Sci 2007, 81, 249–254. [Google Scholar]
- Yoon, H.S.; Lee, S.R.; Ko, H.C.; Choi, S.Y.; Park, J.G.; Kim, J.K.; Kim, S.J. Involvement of extracellular signal-regulated kinase in nobiletin-induced melanogenesis in murine B16/F10 melanoma cells. Biosci. Biotechnol. Biochem 2007, 71, 1781–1784. [Google Scholar]
- Kumar, K.J.; Vani, M.G.; Wang, S.Y.; Liao, J.W.; Hsu, L.S.; Yang, H.L.; Hseu, Y.C. In vitro and in vivo studies disclosed the depigmenting effects of gallic acid: A novel skin lightening agent for hyperpigmentary skin diseases. Biofactors 2013, 39, 259–270. [Google Scholar]
- Takedam, K.; Yasumoto, K.; Takada, R.; Takada, S.; Watanabe, K.; Udono, T.; Saito, H.; Takahashi, K.; Shibahara, S. Induction of melanocyte-specific microphthalmia-associated transcription factor by Wnt-3a. J. Biol. Chem 2000, 275, 14013–14016. [Google Scholar]
- Liu, C.I.; Wang, R.Y.; Lin, J.J.; Su, J.H.; Chiu, C.C.; Chen, J.C.; Chen, J.Y.; Wu, Y.J. Proteomic profiling of the 11-dehydrosinulariolide-treated oral carcinoma cells Ca9–22: Effects on the cell apoptosis through mitochondrial-related and ER stress pathway. J. Proteomics 2012, 75, 5578–5589. [Google Scholar]
- Bellei, B.; Maresca, V.; Flori, E.; Pitisci, A.; Larue, L.; Picardo, M. p38 regulates pigmentation via proteasomal degradation of tyrosinase. J. Biol. Chem 2010, 285, 7288–7299. [Google Scholar]
- Tomita, Y.; Maeda, K.; Tagami, H. Melanocyte-stimulating properties of arachidonic acid metabolites: Possible role in postinflammatory pigmentation. Pigment Cell Melanoma Res 1992, 5, 357–361. [Google Scholar]
- Jimenez-Cervantes, C.; Valverde, P.; Garcia-Borron, J.C.; Solano, F.; Lozano, J.A. Improved tyrosinase activity stains in polyacrylamide electrophoresis gels. Pigment Cell Melanoma Res 1993, 6, 394–399. [Google Scholar]
- Neoh, C.A.; Wang, R.Y.; Din, Z.H.; Su, J.H.; Chen, Y.K.; Tsai, F.J.; Weng, S.H.; Wu, Y.J. Induction of apoptosis by sinulariolide from soft coral through mitochondrial-related and p38MAPK pathways on human bladder carcinoma cells. Mar. Drugs 2012, 10, 2893–2911. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Su, T.-R.; Lin, J.-J.; Tsai, C.-C.; Huang, T.-K.; Yang, Z.-Y.; Wu, M.-O.; Zheng, Y.-Q.; Su, C.-C.; Wu, Y.-J. Inhibition of Melanogenesis by Gallic Acid: Possible Involvement of the PI3K/Akt, MEK/ERK and Wnt/β-Catenin Signaling Pathways in B16F10 Cells. Int. J. Mol. Sci. 2013, 14, 20443-20458. https://doi.org/10.3390/ijms141020443
Su T-R, Lin J-J, Tsai C-C, Huang T-K, Yang Z-Y, Wu M-O, Zheng Y-Q, Su C-C, Wu Y-J. Inhibition of Melanogenesis by Gallic Acid: Possible Involvement of the PI3K/Akt, MEK/ERK and Wnt/β-Catenin Signaling Pathways in B16F10 Cells. International Journal of Molecular Sciences. 2013; 14(10):20443-20458. https://doi.org/10.3390/ijms141020443
Chicago/Turabian StyleSu, Tzu-Rong, Jen-Jie Lin, Chi-Chu Tsai, Tsu-Kei Huang, Zih-Yan Yang, Ming-O Wu, Yu-Qing Zheng, Ching-Chyuan Su, and Yu-Jen Wu. 2013. "Inhibition of Melanogenesis by Gallic Acid: Possible Involvement of the PI3K/Akt, MEK/ERK and Wnt/β-Catenin Signaling Pathways in B16F10 Cells" International Journal of Molecular Sciences 14, no. 10: 20443-20458. https://doi.org/10.3390/ijms141020443
APA StyleSu, T.-R., Lin, J.-J., Tsai, C.-C., Huang, T.-K., Yang, Z.-Y., Wu, M.-O., Zheng, Y.-Q., Su, C.-C., & Wu, Y.-J. (2013). Inhibition of Melanogenesis by Gallic Acid: Possible Involvement of the PI3K/Akt, MEK/ERK and Wnt/β-Catenin Signaling Pathways in B16F10 Cells. International Journal of Molecular Sciences, 14(10), 20443-20458. https://doi.org/10.3390/ijms141020443



