CD8+ T Cell-Induced Expression of Tissue Inhibitor of Metalloproteinses-1 Exacerbated Osteoarthritis
Abstract
:1. Introduction
2. Results
2.1. OA-Induced Pathological Changes in CD8−/− Mice
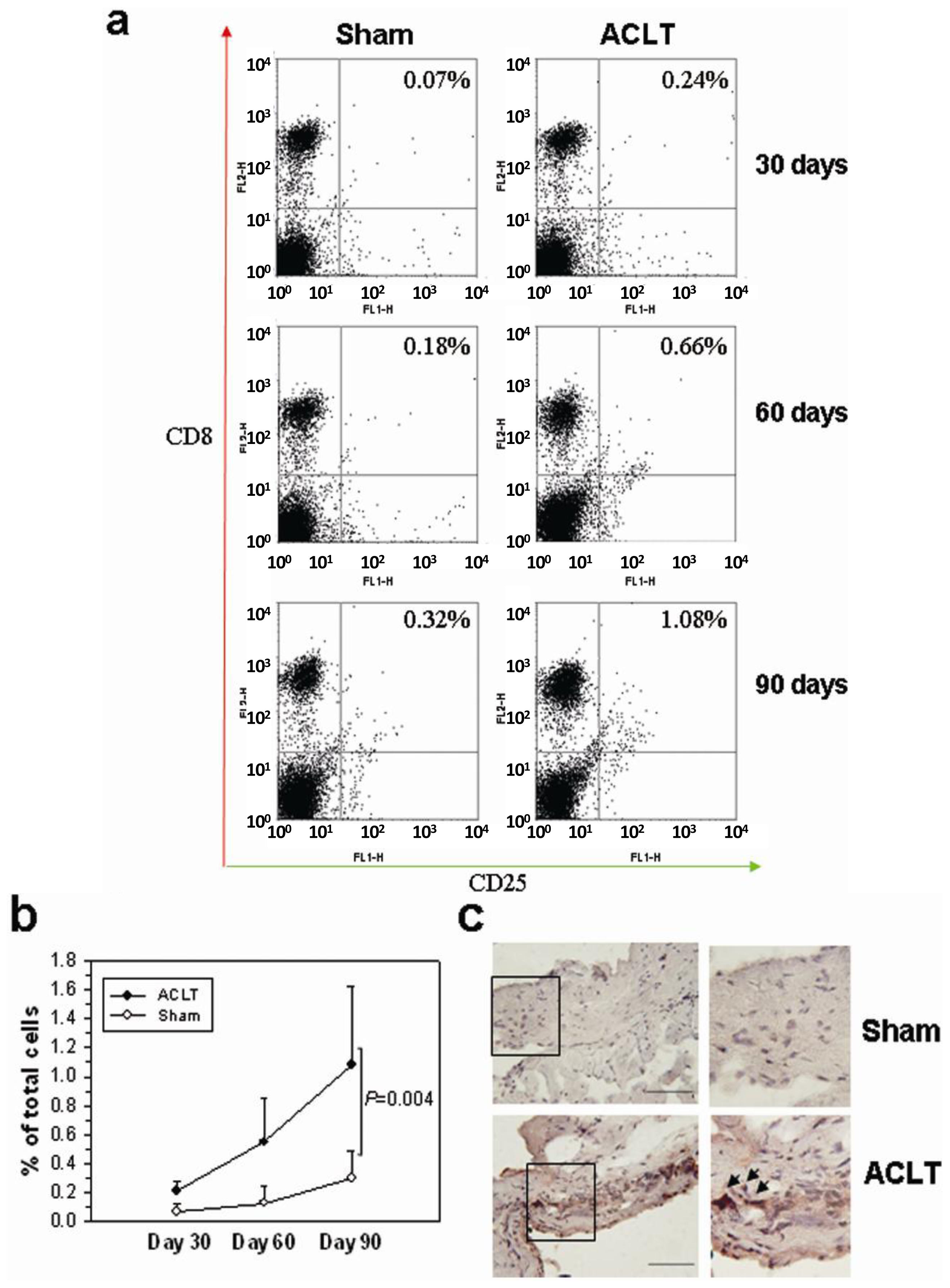
2.2. CD8+ T Cell Activation during the Progression of OA
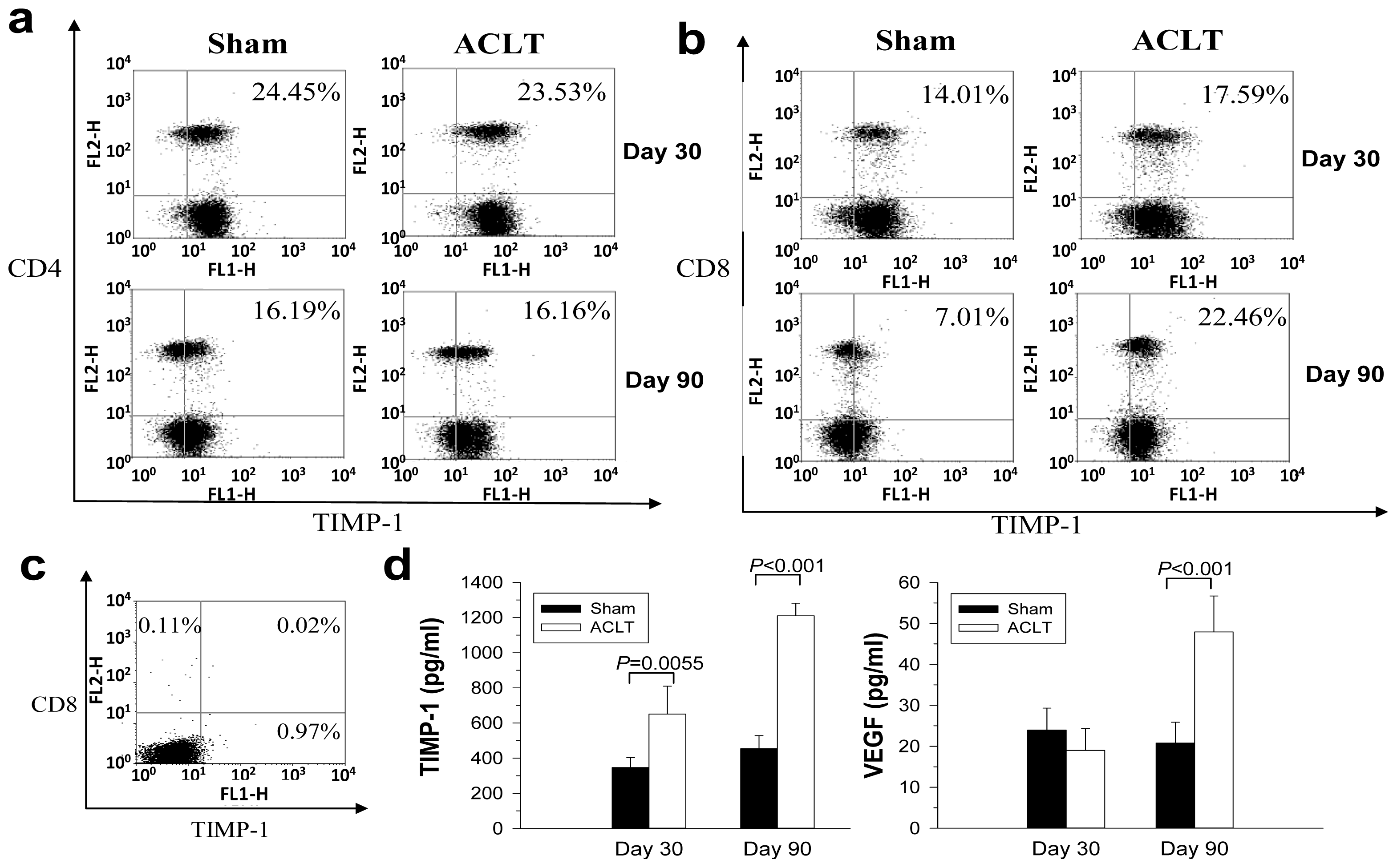
2.3. Decreased TIMP-1 Expression in CD8−/− Mice
2.4. Increased TIMP-1 Expression in the CD8+ T Cells after OA Induction
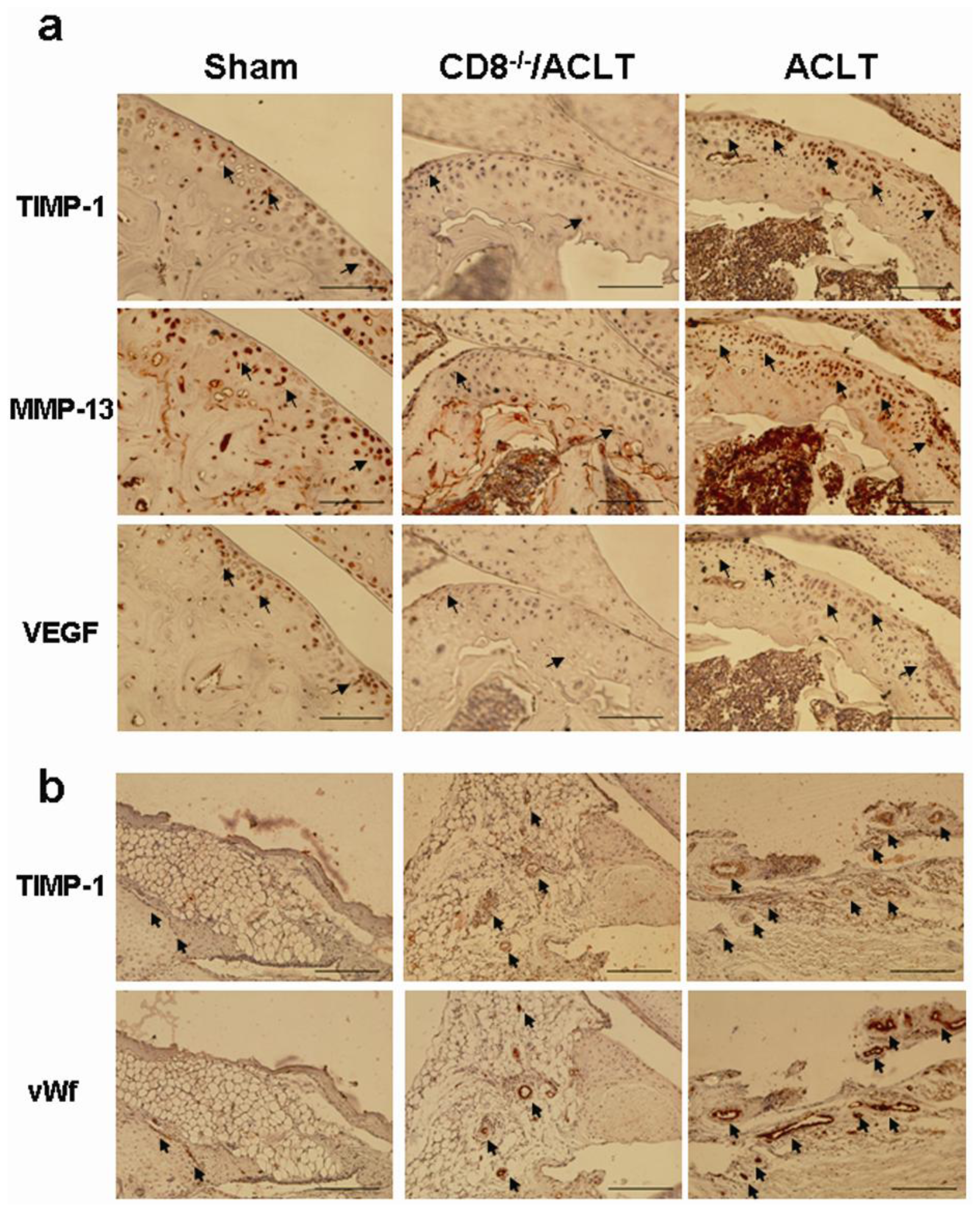
2.5. Evaluation of TIMP-1, VEGF, and MMP-13 Expression in Joints with ACLT-Induced OA
2.6. In Vivo Histopathologic Evaluation in Mice with OA after Treatment with Neutralization TIMP-1 Antibody
3. Discussion
4. Experimental Section
4.1. Animal Model
4.2. Histology Assessment
4.3. Flow Cytometry
4.4. Cytokine Array
4.5. Enzyme-Linked Immunosorbant Assay
4.6. Immunohistochemical Staining (IHC)
4.7. Statistical Analysis
5. Conclusions
Supplementary Information
ijms-14-19951-s001.pdf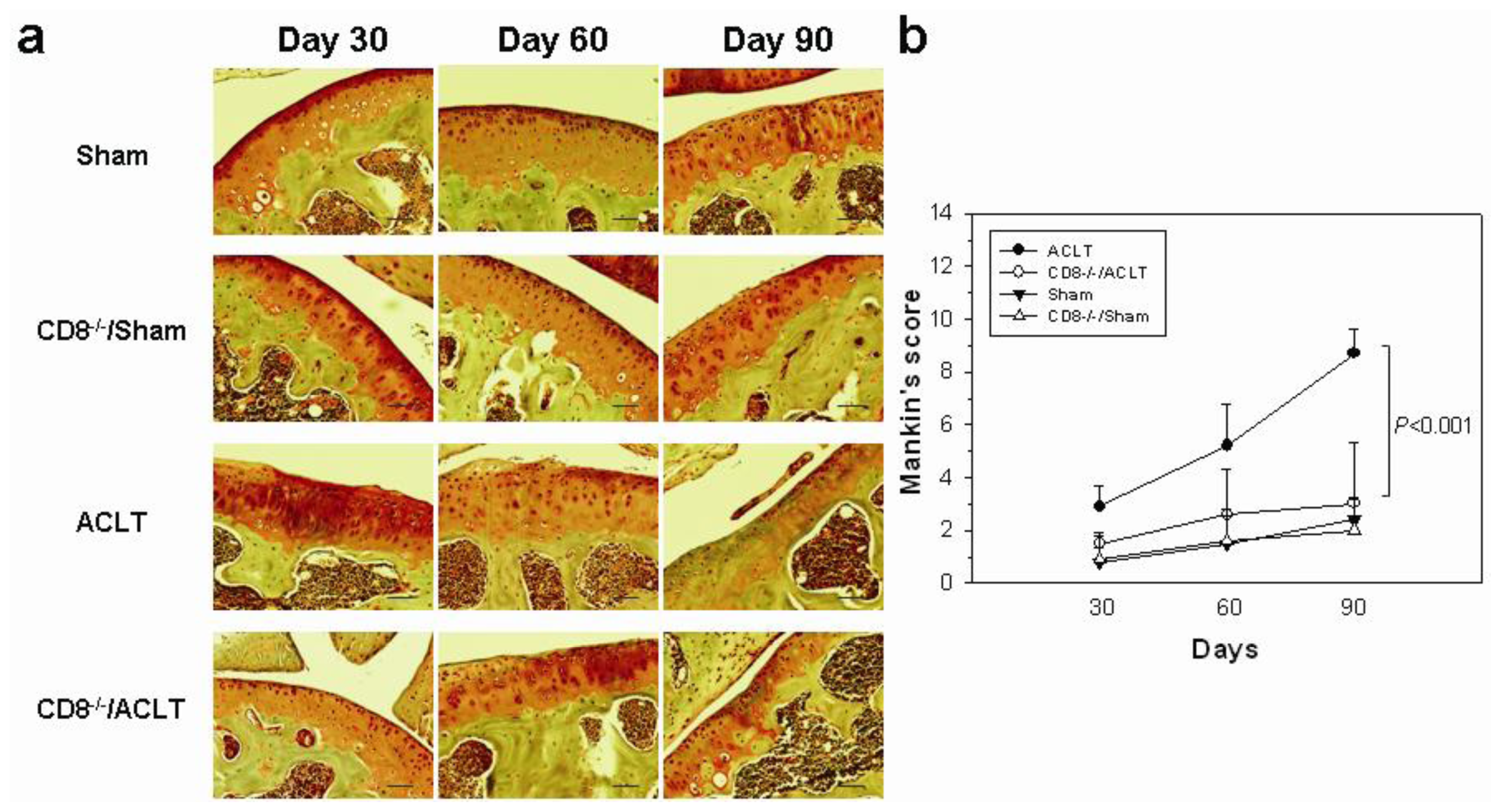




| Gene | Fold-change * | |
|---|---|---|
| ACLT | CD8−/−/ACLT | |
| TIMP-1 | 1.90 | 0.75 |
| sTNFRII | 0.59 | 0.86 |
| IL-4 | 0.76 | 1.06 |
Acknowledgments
Conflicts of Interest
References
- Sowers, M. Epidemiology of risk factors for osteoarthritis: Systemic factors. Curr. Opin. Rheumatol 2001, 13, 447–451. [Google Scholar]
- Suri, S.; Gill, S.E.; Massena de Camin, S.; Wilson, D.; McWilliams, D.F.; Walsh, D.A. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann. Rheum. Dis 2007, 66, 1423–1428. [Google Scholar]
- Walsh, D.A.; Bonnet, C.S.; Turner, E.L.; Wilson, D.; Situ, M.; McWilliams, D.F. Angiogenesis in the synovium and at the osteochondral junction in osteoarthritis. Osteoarthr. Cartil 2007, 15, 743–751. [Google Scholar]
- Fernandes, J.C.; Martel-Pelletier, J.; Pelletier, J.P. The role of cytokines in osteoarthritis pathophysiology. Biorheology 2002, 39, 237–246. [Google Scholar]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil 2013, 21, 16–21. [Google Scholar]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol 2011, 23, 471–478. [Google Scholar]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol 2011, 7, 33–42. [Google Scholar]
- Sakkas, L.I.; Scanzello, C.; Johanson, N.; Burkholder, J.; Mitra, A.; Salgame, P.; Katsetos, C.D.; Platsoucas, C.D. T cells and T-cell cytokine transcripts in the synovial membrane in patients with osteoarthritis. Clin. Diagn. Lab. Immunol 1998, 5, 430–437. [Google Scholar]
- Muir, P.; Kelly, J.L.; Marvel, S.J.; Heinrich, D.A.; Schaefer, S.L.; Manley, P.A.; Tewari, K.; Singh, A.; Suresh, M.; Hao, Z.; et al. Lymphocyte populations in joint tissues from dogs with inflammatory stifle arthritis and associated degenerative cranial cruciate ligament rupture. Vet. Surg 2011, 40, 753–761. [Google Scholar]
- Kennedy, T.D.; Plater-Zyberk, C.; Partridge, T.A.; Woodrow, D.F.; Maini, R.N. Morphometric comparison of synovium from patients with osteoarthritis and rheumatoid arthritis. J. Clin. Pathol 1988, 41, 847–852. [Google Scholar]
- Lance, E.M.; Kimura, L.H.; Manibog, C.N. The expression of major histocompatibility antigens on human articular chondrocytes. Clin. Orthop 1993, 291, 266–282. [Google Scholar]
- Sakata, M.; Masuko-Hongo, K.; Nakamura, H.; Onuma, H.; Tsuruha, J.I.; Aoki, H.; Nishioka, K.; Kato, T. Osteoarthritic articular chondrocytes stimulate autologous T cell responses in vitro. Clin. Exp. Rheumatol. 2003, 21, 704–710. [Google Scholar]
- Scanzello, C.R.; Umoh, E.; Pessler, F.; Diaz-Torne, C.; Miles, T.; Dicarlo, E.; Potter, H.G.; Mandl, L.; Marx, R.; Rodeo, S.; et al. Local cytokine profiles in knee osteoarthritis: Elevated synovial fluid interleukin-15 differentiates early from end-stage disease. Osteoarthr. Cartil 2009, 17, 1040–1048. [Google Scholar]
- Benito, M.J.; Veale, D.J.; FitzGerald, O.; van den Berg, W.B.; Bresnihan, B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis 2005, 64, 1263–1267. [Google Scholar]
- Shen, P.C.; Wu, C.L.; Jou, I.M.; Lee, C.H.; Juan, H.Y.; Lee, P.J.; Chen, S.H.; Hsieh, J.L. T helper cells promote disease progression of osteoarthritis by inducing macrophage inflammatory protein-1γ. Osteoarthr. Cartil 2011, 19, 728–736. [Google Scholar]
- Relic, B.; Guicheux, J.; Mezin, F.; Lubberts, E.; Togninalli, D.; Garcia, I.; van den Berg, W.B.; Guerne, P.A. IL-4 and IL-13, but not IL-10, protect human synoviocytes from apoptosis. J. Immunol 2001, 166, 2775–2782. [Google Scholar]
- Comabella, M.; Julià, E.; Tintoré, M.; Brieva, L.; Téllez, N.; Río, J.; López, C.; Rovira, A.; Montalban, X. Induction of serum soluble tumor necrosis factor receptor II (sTNF-RII) and interleukin-1 receptor antagonist (IL-1ra) by interferon β-1b in patients with progressive multiple sclerosis. J. Neurol 2008, 255, 1136–1141. [Google Scholar]
- Rai, M.F.; Graeve, T.; Twardziok, S.; Schmidt, M.F. Evidence for regulated interleukin-4 expression in chondrocyte-scaffolds under in vitro inflammatory conditions. PLoS One 2011, 6, e25749. [Google Scholar]
- Gomez, D.E.; Alonso, D.F.; Yoshiji, H.; Thorgeirsson, U.P. Tissue inhibitors of metalloproteinases: Structure, regulation and biological functions. Eur. J. Cell Biol 1997, 74, 111–122. [Google Scholar]
- Kevorkian, L.; Young, D.A.; Darrah, C.; Donell, S.T.; Shepstone, L.; Porter, S.; Brockbank, S.M.; Edwards, D.R.; Parker, A.E.; Clark, I.M. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum 2004, 50, 131–141. [Google Scholar]
- Leonardi, A.; Cortivo, R.; Fregona, I.; Plebani, M.; Secchi, A.G.; Abatangelo, G. Effects of Th2 cytokines on expression of collagen, MMP-1, and TIMP-1 in conjunctival fibroblasts. Invest. Ophthalmol. Vis. Sci 2003, 44, 183–189. [Google Scholar]
- Allen, S.J.; Mott, K.R.; Ljubimov, A.V.; Ghiasi, H. Exacerbation of corneal scarring in HSV-1 gK-immunized mice correlates with elevation of CD8+CD25+ T cells in corneas of ocularly infected mice. Virology 2010, 399, 11–22. [Google Scholar]
- Clark, R.T.; Nance, J.P.; Noor, S.; Wilson, E.H. T-cell production of matrix metalloproteinases and inhibition of parasite clearance by TIMP-1 during chronic Toxoplasma infection in the brain. Am. Soc. Neurochem 2011, 3, e00049. [Google Scholar]
- Suidan, G.L.; Dickerson, J.W.; Chen, Y.; McDole, J.R.; Tripathi, P.; Pirko, I.; Seroogy, K.B.; Johnson, A.J. CD8 T cell-initiated vascular endothelial growth factor expression promotes central nervous system vascular permeability under neuroinflammatory conditions. J. Immunol 2010, 184, 1031–1040. [Google Scholar]
- Nakamura, H.; Tanaka, M.; Masuko-Hongo, K.; Yudoh, K.; Kato, T.; Beppu, M.; Nishioka, K. Enhanced production of MMP-1, MMP-3, MMP-13, and RANTES by interaction of chondrocytes with autologous T cells. Rheumatol. Int 2006, 26, 984–990. [Google Scholar]
- Fransès, R.E.; McWilliams, D.F.; Mapp, P.I.; Walsh, D.A. Osteochondral angiogenesis and increased protease inhibitor expression in OA. Osteoarthr. Cartil 2010, 18, 563–571. [Google Scholar]
- Pufe, T.; Harde, V.; Petersen, W.; Goldring, M.B.; Tillmann, B.; Mentlein, R. Vascular endothelial growth factor (VEGF) induces matrix metalloproteinase expression in immortalized chondrocytes. J. Pathol 2004, 202, 367–374. [Google Scholar]
- Kim, K.S.; Choi, H.M.; Lee, Y.A.; Choi, I.A.; Lee, S.H.; Hong, S.J.; Yang, H.I.; Yoo, M.C. Expression levels and association of gelatinases MMP-2 and MMP-9 and collagenases MMP-1 and MMP-13 with VEGF in synovial fluid of patients with arthritis. Rheumatol. Int 2011, 31, 543–547. [Google Scholar]
- Jung, K.K.; Liu, X.W.; Chirco, R.; Fridman, R.; Kim, H.R. Identification of CD63 as a tissue inhibitor of metalloproteinase-1 interacting cell surface protein. EMBO 2006, 25, 3934–3942. [Google Scholar]
- Stetler-Stevenson, W.G. Tissue inhibitors of metalloproteinases in cell signaling: Metalloproteinase-independent biological activities. Sci. Signal 2008, 1. [Google Scholar] [CrossRef]
- Hill, C.L.; Seo, G.S.; Gale, D.; Totterman, S.; Gale, M.E.; Felson, D.T. Cruciate ligament integrity in osteoarthritis of the knee. Arthritis Rheum 2005, 52, 794–799. [Google Scholar]
- Catterall, J.B.; Stabler, T.V.; Flannery, C.R.; Kraus, V.B. Changes in serum and synovial fluid biomarkers after acute injury (NCT00332254). Arthritis Res. Ther 2010, 12. [Google Scholar] [CrossRef]
- Glasson, S.S.; Blanchet, T.J.; Morris, E.A. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthr. Cartil 2007, 15, 1061–1069. [Google Scholar]
- Kamekura, S.; Hoshi, K.; Shimoaka, T.; Chung, U.; Chikuda, H.; Yamada, T.; Uchida, M.; Ogata, N.; Seichi, A.; Nakamura, K.; et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthr. Cartil 2005, 13, 632–641. [Google Scholar]
- Hsieh, J.L.; Shen, P.C.; Shiau, A.L.; Jou, I.M.; Lee, C.H.; Teo, M.L.; Wang, C.R.; Chao, J.; Chao, L.; Wu, C.L. Adenovirus-mediated kallistatin gene transfer ameliorates disease progression in a rat model of osteoarthritis induced by anterior cruciate ligament transection. Hum. Gene Ther 2009, 20, 147–158. [Google Scholar]
- Mankin, H.J.; Dorfman, H.; Lippiello, L.; Zarins, A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J. Bone Joint Surg. Am 1971, 53, 523–537. [Google Scholar]
- Yoshimi, T.; Kikuchi, T.; Obara, T.; Yamaguchi, T.; Sakakibara, Y.; Itoh, H.; Iwata, H.; Miura, T. Effects of high-molecular-weight sodium hyaluronate on experimental osteoarthrosis induced by the resection of rabbit anterior cruciate ligament. Clin. Orthop. Relat. Res 1994, 298, 296–304. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hsieh, J.-L.; Shiau, A.-L.; Lee, C.-H.; Yang, S.-J.; Lee, B.-O.; Jou, I.-M.; Wu, C.-L.; Chen, S.-H.; Shen, P.-C. CD8+ T Cell-Induced Expression of Tissue Inhibitor of Metalloproteinses-1 Exacerbated Osteoarthritis. Int. J. Mol. Sci. 2013, 14, 19951-19970. https://doi.org/10.3390/ijms141019951
Hsieh J-L, Shiau A-L, Lee C-H, Yang S-J, Lee B-O, Jou I-M, Wu C-L, Chen S-H, Shen P-C. CD8+ T Cell-Induced Expression of Tissue Inhibitor of Metalloproteinses-1 Exacerbated Osteoarthritis. International Journal of Molecular Sciences. 2013; 14(10):19951-19970. https://doi.org/10.3390/ijms141019951
Chicago/Turabian StyleHsieh, Jeng-Long, Ai-Li Shiau, Che-Hsin Lee, Shiu-Ju Yang, Bih-O Lee, I-Ming Jou, Chao-Liang Wu, Shun-Hua Chen, and Po-Chuan Shen. 2013. "CD8+ T Cell-Induced Expression of Tissue Inhibitor of Metalloproteinses-1 Exacerbated Osteoarthritis" International Journal of Molecular Sciences 14, no. 10: 19951-19970. https://doi.org/10.3390/ijms141019951
APA StyleHsieh, J.-L., Shiau, A.-L., Lee, C.-H., Yang, S.-J., Lee, B.-O., Jou, I.-M., Wu, C.-L., Chen, S.-H., & Shen, P.-C. (2013). CD8+ T Cell-Induced Expression of Tissue Inhibitor of Metalloproteinses-1 Exacerbated Osteoarthritis. International Journal of Molecular Sciences, 14(10), 19951-19970. https://doi.org/10.3390/ijms141019951





