The Role of the VEGF-C/VEGFRs Axis in Tumor Progression and Therapy
Abstract
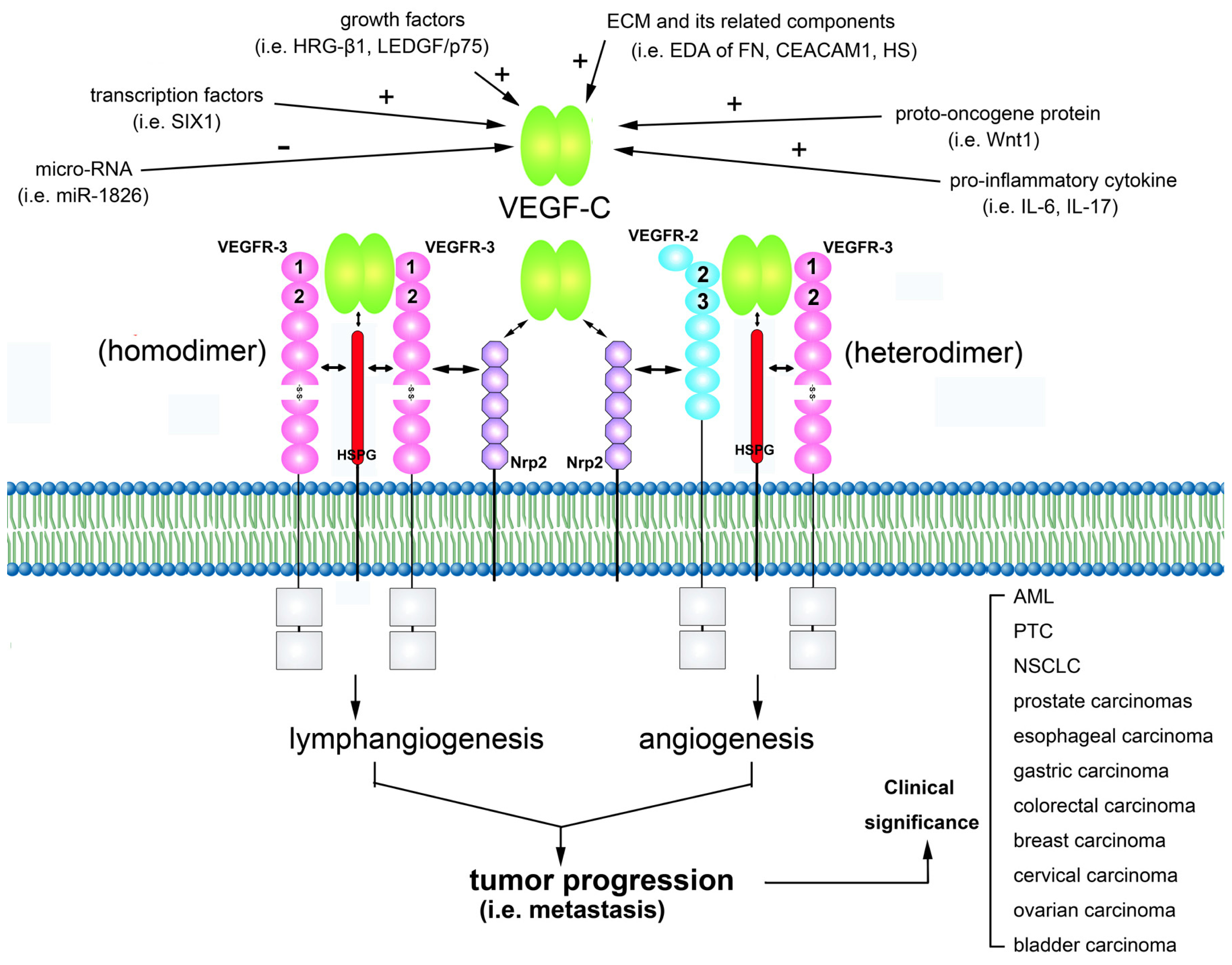
:1. Characteristics of VEGF-C
2. VEGF-C Signals via its Receptors
3. VEGF-C Is Involved in Regulating Tumor Lymphangiogenesis and Angiogenesis
4. Regulation of VEGF-C Gene Expression
5. Modulation of VEGF-C/VEGFRs Signaling Axis
6. Clinical Significance of VEGF-C Expression in Tumors
7. Mechanisms of Action of VEGF-C Targeted Therapy
7.1. Monoclonal Antibodies
7.2. IgG Fusion Proteins or Soluble Receptor Protein
7.3. Multikinase Inhibitors
7.4. RNA Interference
8. Conclusions
Acknowledgements
References
- Senger, D.R.; Galli, S.J.; Dvorak, A.M.; Perruzzi, C.A.; Harvey, V.S.; Dvorak, H.F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983, 219, 983–985. [Google Scholar]
- Ferrara, N.; Henzel, W.J. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophys. Res. Commun 1989, 161, 851–858. [Google Scholar]
- Veikkola, T.; Karkkainen, M.; Claesson-Welsh, L.; Alitalo, K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res 2000, 60, 203–212. [Google Scholar]
- Lohela, M.; Bry, M.; Tammela, T.; Alitalo, K. Vegfs and receptors involved in angiogenesis versus lymphangiogenesis. Curr. Opin. Cell Biol 2009, 21, 154–165. [Google Scholar]
- Roy, H.; Bhardwaj, S.; Yla-Herttuala, S. Biology of vascular endothelial growth factors. FEBS Lett 2006, 580, 2879–2887. [Google Scholar]
- Roskoski, R., Jr. Vascular endothelial growth factor (vegf) signaling in tumor progression. Crit. Rev. Oncol. Hematol. 2007, 62, 179–213. [Google Scholar]
- Ogawa, S.; Oku, A.; Sawano, A.; Yamaguchi, S.; Yazaki, Y.; Shibuya, M. A novel type of vascular endothelial growth factor, vegf-e (nz-7 vegf), preferentially utilizes kdr/flk-1 receptor and carries a potent mitotic activity without heparin-binding domain. J. Biol. Chem 1998, 273, 31273–31282. [Google Scholar]
- Yamazaki, Y.; Matsunaga, Y.; Tokunaga, Y.; Obayashi, S.; Saito, M.; Morita, T. Snake venom vascular endothelial growth factors (vegf-fs) exclusively vary their structures and functions among species. J. Biol. Chem 2009, 284, 9885–9891. [Google Scholar]
- Kukk, E.; Lymboussaki, A.; Taira, S.; Kaipainen, A.; Jeltsch, M.; Joukov, V.; Alitalo, K. Vegf-c receptor binding and pattern of expression with vegfr-3 suggests a role in lymphatic vascular development. Development 1996, 122, 3829–3837. [Google Scholar]
- Karkkainen, M.J.; Haiko, P.; Sainio, K.; Partanen, J.; Taipale, J.; Petrova, T.V.; Jeltsch, M.; Jackson, D.G.; Talikka, M.; Rauvala, H.; et al. Vascular endothelial growth factor c is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol 2004, 5, 74–80. [Google Scholar]
- Gore, A.V.; Swift, M.R.; Cha, Y.R.; Lo, B.; McKinney, M.C.; Li, W.; Castranova, D.; Davis, A.; Mukouyama, Y.S.; Weinstein, B.M. Rspo1/wnt signaling promotes angiogenesis via vegfc/vegfr3. Development 2011, 138, 4875–4886. [Google Scholar]
- Hirakawa, S.; Brown, L.F.; Kodama, S.; Paavonen, K.; Alitalo, K.; Detmar, M. Vegf-c-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood 2007, 109, 1010–1017. [Google Scholar]
- Cao, Y.; Linden, P.; Farnebo, J.; Cao, R.; Eriksson, A.; Kumar, V.; Qi, J.H.; Claesson-Welsh, L.; Alitalo, K. Vascular endothelial growth factor c induces angiogenesis in vivo. Proc. Natl. Acad. Sci. USA 1998, 95, 14389–14394. [Google Scholar]
- Joukov, V.; Pajusola, K.; Kaipainen, A.; Chilov, D.; Lahtinen, I.; Kukk, E.; Saksela, O.; Kalkkinen, N.; Alitalo, K. A novel vascular endothelial growth factor, vegf-c, is a ligand for the flt4 (vegfr-3) and kdr (vegfr-2) receptor tyrosine kinases. EMBO J 1996, 15, 290–298. [Google Scholar]
- Paavonen, K.; Horelli-Kuitunen, N.; Chilov, D.; Kukk, E.; Pennanen, S.; Kallioniemi, O.P.; Pajusola, K.; Olofsson, B.; Eriksson, U.; Joukov, V.; et al. Novel human vascular endothelial growth factor genes vegf-b and vegf-c localize to chromosomes 11q13 and 4q34, respectively. Circulation 1996, 93, 1079–1082. [Google Scholar]
- Fitz, L.J.; Morris, J.C.; Towler, P.; Long, A.; Burgess, P.; Greco, R.; Wang, J.; Gassaway, R.; Nickbarg, E.; Kovacic, S.; et al. Characterization of murine flt4 ligand/vegf-c. Oncogene 1997, 15, 613–618. [Google Scholar]
- Chilov, D.; Kukk, E.; Taira, S.; Jeltsch, M.; Kaukonen, J.; Palotie, A.; Joukov, V.; Alitalo, K. Genomic organization of human and mouse genes for vascular endothelial growth factor c. J. Biol. Chem 1997, 272, 25176–25183. [Google Scholar]
- Li, X.; Eriksson, U. Novel vegf family members: Vegf-b, vegf-c and vegf-d. Int. J. Biochem. Cell Biol 2001, 33, 421–426. [Google Scholar]
- Joukov, V.; Sorsa, T.; Kumar, V.; Jeltsch, M.; Claesson-Welsh, L.; Cao, Y.; Saksela, O.; Kalkkinen, N.; Alitalo, K. Proteolytic processing regulates receptor specificity and activity of vegf-c. EMBO J 1997, 16, 3898–3911. [Google Scholar]
- Leppanen, V.M.; Prota, A.E.; Jeltsch, M.; Anisimov, A.; Kalkkinen, N.; Strandin, T.; Lankinen, H.; Goldman, A.; Ballmer-Hofer, K.; Alitalo, K. Structural determinants of growth factor binding and specificity by vegf receptor 2. Proc. Natl. Acad. Sci. USA 2010, 107, 2425–2430. [Google Scholar]
- Joukov, V.; Kumar, V.; Sorsa, T.; Arighi, E.; Weich, H.; Saksela, O.; Alitalo, K. A recombinant mutant vascular endothelial growth factor-c that has lost vascular endothelial growth factor receptor-2 binding, activation, and vascular permeability activities. J. Biol. Chem 1998, 273, 6599–6602. [Google Scholar]
- Jeltsch, M.; Karpanen, T.; Strandin, T.; Aho, K.; Lankinen, H.; Alitalo, K. Vascular endothelial growth factor (vegf)/vegf-c mosaic molecules reveal specificity determinants and feature novel receptor binding patterns. J. Biol. Chem 2006, 281, 12187–12195. [Google Scholar]
- Kluger, M.S.; Colegio, O.R. Lymphangiogenesis linked to vegf-c from tumor-associated macrophages: Accomplices to metastasis by cutaneous squamous cell carcinoma? J. Invest. Dermatol 2011, 131, 17–19. [Google Scholar]
- Ji, R.C. Macrophages are important mediators of either tumor- or inflammation-induced lymphangiogenesis. Cell. Mol. Life Sci 2012, 69, 897–914. [Google Scholar]
- Skobe, M.; Hawighorst, T.; Jackson, D.G.; Prevo, R.; Janes, L.; Velasco, P.; Riccardi, L.; Alitalo, K.; Claffey, K.; Detmar, M. Induction of tumor lymphangiogenesis by vegf-c promotes breast cancer metastasis. Nat. Med 2001, 7, 192–198. [Google Scholar]
- Mattila, M.M.; Ruohola, J.K.; Karpanen, T.; Jackson, D.G.; Alitalo, K.; Harkonen, P.L. Vegf-c induced lymphangiogenesis is associated with lymph node metastasis in orthotopic mcf-7 tumors. Int. J. Cancer 2002, 98, 946–951. [Google Scholar]
- Mandriota, S.J.; Jussila, L.; Jeltsch, M.; Compagni, A.; Baetens, D.; Prevo, R.; Banerji, S.; Huarte, J.; Montesano, R.; Jackson, D.G.; et al. Vascular endothelial growth factor-c-mediated lymphangiogenesis promotes tumour metastasis. EMBO J 2001, 20, 672–682. [Google Scholar]
- Laakkonen, P.; Waltari, M.; Holopainen, T.; Takahashi, T.; Pytowski, B.; Steiner, P.; Hicklin, D.; Persaud, K.; Tonra, J.R.; Witte, L.; et al. Vascular endothelial growth factor receptor 3 is involved in tumor angiogenesis and growth. Cancer Res 2007, 67, 593–599. [Google Scholar]
- Chien, M.H.; Ku, C.C.; Johansson, G.; Chen, M.W.; Hsiao, M.; Su, J.L.; Inoue, H.; Hua, K.T.; Wei, L.H.; Kuo, M.L. Vascular endothelial growth factor-c (vegf-c) promotes angiogenesis by induction of cox-2 in leukemic cells via the vegf-r3/jnk/ap-1 pathway. Carcinogenesis 2009, 30, 2005–2013. [Google Scholar]
- Miettinen, M.; Rikala, M.S.; Rys, J.; Lasota, J.; Wang, Z.F. Vascular endothelial growth factor receptor 2 as a marker for malignant vascular tumors and mesothelioma: An immunohistochemical study of 262 vascular endothelial and 1640 nonvascular tumors. Am. J. Surg. Pathol 2012, 36, 629–639. [Google Scholar]
- Su, J.L.; Yang, P.C.; Shih, J.Y.; Yang, C.Y.; Wei, L.H.; Hsieh, C.Y.; Chou, C.H.; Jeng, Y.M.; Wang, M.Y.; Chang, K.J.; et al. The vegf-c/flt-4 axis promotes invasion and metastasis of cancer cells. Cancer Cell 2006, 9, 209–223. [Google Scholar]
- Su, J.L.; Chen, P.S.; Chien, M.H.; Chen, P.B.; Chen, Y.H.; Lai, C.C.; Hung, M.C.; Kuo, M.L. Further evidence for expression and function of the vegf-c/vegfr-3 axis in cancer cells. Cancer Cell 2008, 13, 557–560. [Google Scholar]
- Koch, S.; Tugues, S.; Li, X.; Gualandi, L.; Claesson-Welsh, L. Signal transduction by vascular endothelial growth factor receptors. Biochem. J 2011, 437, 169–183. [Google Scholar]
- Dixelius, J.; Makinen, T.; Wirzenius, M.; Karkkainen, M.J.; Wernstedt, C.; Alitalo, K.; Claesson-Welsh, L. Ligand-induced vascular endothelial growth factor receptor-3 (vegfr-3) heterodimerization with vegfr-2 in primary lymphatic endothelial cells regulates tyrosine phosphorylation sites. J. Biol. Chem 2003, 278, 40973–40979. [Google Scholar]
- Nilsson, I.; Bahram, F.; Li, X.; Gualandi, L.; Koch, S.; Jarvius, M.; Soderberg, O.; Anisimov, A.; Kholova, I.; Pytowski, B.; et al. Vegf receptor 2/-3 heterodimers detected in situ by proximity ligation on angiogenic sprouts. EMBO J 2010, 29, 1377–1388. [Google Scholar]
- Dosch, D.D.; Ballmer-Hofer, K. Transmembrane domain-mediated orientation of receptor monomers in active vegfr-2 dimers. FASEB J 2010, 24, 32–38. [Google Scholar]
- Goldman, J.; Rutkowski, J.M.; Shields, J.D.; Pasquier, M.C.; Cui, Y.; Schmokel, H.G.; Willey, S.; Hicklin, D.J.; Pytowski, B.; Swartz, M.A. Cooperative and redundant roles of vegfr-2 and vegfr-3 signaling in adult lymphangiogenesis. FASEB J 2007, 21, 1003–1012. [Google Scholar]
- Tvorogov, D.; Anisimov, A.; Zheng, W.; Leppanen, V.M.; Tammela, T.; Laurinavicius, S.; Holnthoner, W.; Helotera, H.; Holopainen, T.; Jeltsch, M.; et al. Effective suppression of vascular network formation by combination of antibodies blocking vegfr ligand binding and receptor dimerization. Cancer Cell 2010, 18, 630–640. [Google Scholar]
- Feng, Y.; Hu, J.; Ma, J.; Feng, K.; Zhang, X.; Yang, S.; Wang, W.; Zhang, J.; Zhang, Y. Rnai-mediated silencing of vegf-c inhibits non-small cell lung cancer progression by simultaneously down-regulating the cxcr4, ccr7, vegfr-2 and vegfr-3-dependent axes-induced erk, p38 and akt signalling pathways. Eur. J. Cancer 2011, 47, 2353–2363. [Google Scholar]
- Jeltsch, M.; Kaipainen, A.; Joukov, V.; Meng, X.; Lakso, M.; Rauvala, H.; Swartz, M.; Fukumura, D.; Jain, R.K.; Alitalo, K. Hyperplasia of lymphatic vessels in vegf-c transgenic mice. Science 1997, 276, 1423–1425. [Google Scholar]
- Makinen, T.; Jussila, L.; Veikkola, T.; Karpanen, T.; Kettunen, M.I.; Pulkkanen, K.J.; Kauppinen, R.; Jackson, D.G.; Kubo, H.; Nishikawa, S.; et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble vegf receptor-3. Nat. Med 2001, 7, 199–205. [Google Scholar]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 2010, 140, 460–476. [Google Scholar]
- Khromova, N.; Kopnin, P.; Rybko, V.; Kopnin, B.P. Downregulation of vegf-c expression in lung and colon cancer cells decelerates tumor growth and inhibits metastasis via multiple mechanisms. Oncogene 2012, 31, 1389–1397. [Google Scholar]
- Hoshida, T.; Isaka, N.; Hagendoorn, J.; di Tomaso, E.; Chen, Y.L.; Pytowski, B.; Fukumura, D.; Padera, T.P.; Jain, R.K. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-c increases metastasis by increasing delivery of cancer cells to lymph nodes: Therapeutic implications. Cancer Res 2006, 66, 8065–8075. [Google Scholar]
- Witzenbichler, B.; Asahara, T.; Murohara, T.; Silver, M.; Spyridopoulos, I.; Magner, M.; Principe, N.; Kearney, M.; Hu, J.S.; Isner, J.M. Vascular endothelial growth factor-c (vegf-c/vegf-2) promotes angiogenesis in the setting of tissue ischemia. Am. J. Pathol 1998, 153, 381–394. [Google Scholar]
- Dumont, D.J.; Jussila, L.; Taipale, J.; Lymboussaki, A.; Mustonen, T.; Pajusola, K.; Breitman, M.; Alitalo, K. Cardiovascular failure in mouse embryos deficient in vegf receptor-3. Science 1998, 282, 946–949. [Google Scholar]
- Ober, E.A.; Olofsson, B.; Makinen, T.; Jin, S.W.; Shoji, W.; Koh, G.Y.; Alitalo, K.; Stainier, D.Y. Vegfc is required for vascular development and endoderm morphogenesis in zebrafish. EMBO Rep 2004, 5, 78–84. [Google Scholar]
- Valtola, R.; Salven, P.; Heikkila, P.; Taipale, J.; Joensuu, H.; Rehn, M.; Pihlajaniemi, T.; Weich, H.; deWaal, R.; Alitalo, K. Vegfr-3 and its ligand vegf-c are associated with angiogenesis in breast cancer. Am. J. Pathol 1999, 154, 1381–1390. [Google Scholar]
- Tammela, T.; Zarkada, G.; Wallgard, E.; Murtomaki, A.; Suchting, S.; Wirzenius, M.; Waltari, M.; Hellstrom, M.; Schomber, T.; Peltonen, R.; et al. Blocking vegfr-3 suppresses angiogenic sprouting and vascular network formation. Nature 2008, 454, 656–660. [Google Scholar]
- Kumar, B.; Chile, S.A.; Ray, K.B.; Reddy, G.E.; Addepalli, M.K.; Kumar, A.S.; Ramana, V.; Rajagopal, V. Vegf-c differentially regulates vegf-a expression in ocular and cancer cells; promotes angiogenesis via rhoa mediated pathway. Angiogenesis 2011, 14, 371–380. [Google Scholar]
- Shinriki, S.; Jono, H.; Ueda, M.; Ota, K.; Ota, T.; Sueyoshi, T.; Oike, Y.; Ibusuki, M.; Hiraki, A.; Nakayama, H.; et al. Interleukin-6 signalling regulates vascular endothelial growth factor-c synthesis and lymphangiogenesis in human oral squamous cell carcinoma. J. Pathol 2011, 225, 142–150. [Google Scholar]
- Chen, X.; Xie, Q.; Cheng, X.; Diao, X.; Cheng, Y.; Liu, J.; Xie, W.; Chen, Z.; Zhu, B. Role of interleukin-17 in lymphangiogenesis in non-small-cell lung cancer: Enhanced production of vascular endothelial growth factor c in non-small-cell lung carcinoma cells. Cancer Sci 2010, 101, 2384–2390. [Google Scholar]
- Huang, C.L.; Liu, D.; Ishikawa, S.; Nakashima, T.; Nakashima, N.; Yokomise, H.; Kadota, K.; Ueno, M. Wnt1 overexpression promotes tumour progression in non-small cell lung cancer. Eur. J. Cancer 2008, 44, 2680–2688. [Google Scholar]
- Gherghe, C.M.; Duan, J.; Gong, J.; Rojas, M.; Klauber-Demore, N.; Majesky, M.; Deb, A. Wnt1 is a proangiogenic molecule, enhances human endothelial progenitor function, and increases blood flow to ischemic limbs in a hgf-dependent manner. FASEB J 2011, 25, 1836–1843. [Google Scholar]
- Niederleithner, H.; Heinz, M.; Tauber, S.; Bilban, M.; Pehamberger, H.; Sonderegger, S.; Knofler, M.; Bracher, A.; Berger, W.; Loewe, R.; et al. Wnt1 is anti-lymphangiogenic in a melanoma mouse model. J. Invest. Dermatol 2012, 132, 2235–2244. [Google Scholar]
- Xiang, L.; Xie, G.; Ou, J.; Wei, X.; Pan, F.; Liang, H. The extra domain a of fibronectin increases vegf-c expression in colorectal carcinoma involving the pi3k/akt signaling pathway. PLoS One 2012, 7, e35378. [Google Scholar]
- Ilan, N.; Elkin, M.; Vlodavsky, I. Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int. J. Biochem. Cell Biol 2006, 38, 2018–2039. [Google Scholar]
- Cohen-Kaplan, V.; Naroditsky, I.; Zetser, A.; Ilan, N.; Vlodavsky, I.; Doweck, I. Heparanase induces vegf c and facilitates tumor lymphangiogenesis. Int. J. Cancer J 2008, 123, 2566–2573. [Google Scholar]
- Oliveira-Ferrer, L.; Tilki, D.; Ziegeler, G.; Hauschild, J.; Loges, S.; Irmak, S.; Kilic, E.; Huland, H.; Friedrich, M.; Ergun, S. Dual role of carcinoembryonic antigen-related cell adhesion molecule 1 in angiogenesis and invasion of human urinary bladder cancer. Cancer Res 2004, 64, 8932–8938. [Google Scholar]
- Tsai, P.W.; Shiah, S.G.; Lin, M.T.; Wu, C.W.; Kuo, M.L. Up-regulation of vascular endothelial growth factor c in breast cancer cells by heregulin-beta 1. A critical role of p38/nuclear factor-kappa b signaling pathway. J. Biol. Chem 2003, 278, 5750–5759. [Google Scholar]
- Sapoznik, S.; Cohen, B.; Tzuman, Y.; Meir, G.; Ben-Dor, S.; Harmelin, A.; Neeman, M. Gonadotropin-regulated lymphangiogenesis in ovarian cancer is mediated by ledgf-induced expression of vegf-c. Cancer Res 2009, 69, 9306–9314. [Google Scholar]
- Wang, C.A.; Jedlicka, P.; Patrick, A.N.; Micalizzi, D.S.; Lemmer, K.C.; Deitsch, E.; Casas-Selves, M.; Harrell, J.C.; Ford, H.L. Six1 induces lymphangiogenesis and metastasis via upregulation of vegf-c in mouse models of breast cancer. J. Clin. Invest 2012, 122, 1895–1906. [Google Scholar]
- Hirata, H.; Hinoda, Y.; Ueno, K.; Shahryari, V.; Tabatabai, Z.L.; Dahiya, R. Microrna-1826 targets vegfc, beta-catenin (ctnnb1) and mek1 (map2k1) in human bladder cancer. Carcinogenesis 2012, 33, 41–48. [Google Scholar]
- Kawamura, H.; Li, X.; Goishi, K.; van Meeteren, L.A.; Jakobsson, L.; Cebe-Suarez, S.; Shimizu, A.; Edholm, D.; Ballmer-Hofer, K.; Kjellen, L.; et al. Neuropilin-1 in regulation of vegf-induced activation of p38mapk and endothelial cell organization. Blood 2008, 112, 3638–3649. [Google Scholar]
- Herzog, B.; Pellet-Many, C.; Britton, G.; Hartzoulakis, B.; Zachary, I.C. Vegf binding to nrp1 is essential for vegf stimulation of endothelial cell migration, complex formation between nrp1 and vegfr2, and signaling via fak tyr407 phosphorylation. Mol. Biol. Cell 2011, 22, 2766–2776. [Google Scholar]
- Herzog, Y.; Kalcheim, C.; Kahane, N.; Reshef, R.; Neufeld, G. Differential expression of neuropilin-1 and neuropilin-2 in arteries and veins. Mech. Dev 2001, 109, 115–119. [Google Scholar]
- Staton, C.A.; Kumar, I.; Reed, M.W.; Brown, N.J. Neuropilins in physiological and pathological angiogenesis. J. Pathol 2007, 212, 237–248. [Google Scholar]
- Bernatchez, P.N.; Rollin, S.; Soker, S.; Sirois, M.G. Relative effects of vegf-a and vegf-c on endothelial cell proliferation, migration and paf synthesis: Role of neuropilin-1. J. Cell. Biochem 2002, 85, 629–639. [Google Scholar]
- Karpanen, T.; Heckman, C.A.; Keskitalo, S.; Jeltsch, M.; Ollila, H.; Neufeld, G.; Tamagnone, L.; Alitalo, K. Functional interaction of vegf-c and vegf-d with neuropilin receptors. FASEB J 2006, 20, 1462–1472. [Google Scholar]
- Caunt, M.; Mak, J.; Liang, W.C.; Stawicki, S.; Pan, Q.; Tong, R.K.; Kowalski, J.; Ho, C.; Reslan, H.B.; Ross, J.; et al. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell 2008, 13, 331–342. [Google Scholar]
- Xu, Y.; Yuan, L.; Mak, J.; Pardanaud, L.; Caunt, M.; Kasman, I.; Larrivee, B.; Del Toro, R.; Suchting, S.; Medvinsky, A.; et al. Neuropilin-2 mediates vegf-c-induced lymphatic sprouting together with vegfr3. J. Cell Biol 2010, 188, 115–130. [Google Scholar]
- Favier, B.; Alam, A.; Barron, P.; Bonnin, J.; Laboudie, P.; Fons, P.; Mandron, M.; Herault, J.P.; Neufeld, G.; Savi, P.; et al. Neuropilin-2 interacts with vegfr-2 and vegfr-3 and promotes human endothelial cell survival and migration. Blood 2006, 108, 1243–1250. [Google Scholar]
- Fuster, M.M.; Wang, L.; Castagnola, J.; Sikora, L.; Reddi, K.; Lee, P.H.; Radek, K.A.; Schuksz, M.; Bishop, J.R.; Gallo, R.L.; et al. Genetic alteration of endothelial heparan sulfate selectively inhibits tumor angiogenesis. J. Cell Biol 2007, 177, 539–549. [Google Scholar]
- Yin, X.; Truty, J.; Lawrence, R.; Johns, S.C.; Srinivasan, R.S.; Handel, T.M.; Fuster, M.M. A critical role for lymphatic endothelial heparan sulfate in lymph node metastasis. Mol. Cancer 2010, 9, 316. [Google Scholar]
- Yin, X.; Johns, S.C.; Lawrence, R.; Xu, D.; Reddi, K.; Bishop, J.R.; Varner, J.A.; Fuster, M.M. Lymphatic endothelial heparan sulfate deficiency results in altered growth responses to vascular endothelial growth factor-c (vegf-c). J. Biol. Chem 2011, 286, 14952–14962. [Google Scholar]
- Ebos, J.M.; Bocci, G.; Man, S.; Thorpe, P.E.; Hicklin, D.J.; Zhou, D.; Jia, X.; Kerbel, R.S. A naturally occurring soluble form of vascular endothelial growth factor receptor 2 detected in mouse and human plasma. Mol. Cancer Res 2004, 2, 315–326. [Google Scholar]
- Albuquerque, R.J.; Hayashi, T.; Cho, W.G.; Kleinman, M.E.; Dridi, S.; Takeda, A.; Baffi, J.Z.; Yamada, K.; Kaneko, H.; Green, M.G.; et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat. Med 2009, 15, 1023–1030. [Google Scholar]
- Pavlakovic, H.; Becker, J.; Albuquerque, R.; Wilting, J.; Ambati, J. Soluble vegfr-2: An antilymphangiogenic variant of vegf receptors. Ann. N. Y. Acad. Sci 2010, 1207, E7–E15. [Google Scholar]
- Ebos, J.M.; Lee, C.R.; Bogdanovic, E.; Alami, J.; van Slyke, P.; Francia, G.; Xu, P.; Mutsaers, A.J.; Dumont, D.J.; Kerbel, R.S. Vascular endothelial growth factor-mediated decrease in plasma soluble vascular endothelial growth factor receptor-2 levels as a surrogate biomarker for tumor growth. Cancer Res 2008, 68, 521–529. [Google Scholar]
- Becker, J.; Pavlakovic, H.; Ludewig, F.; Wilting, F.; Weich, H.A.; Albuquerque, R.; Ambati, J.; Wilting, J. Neuroblastoma progression correlates with downregulation of the lymphangiogenesis inhibitor svegfr-2. Clin. Cancer Res 2010, 16, 1431–1441. [Google Scholar]
- Kikuchi, S.; Obata, Y.; Yagyu, K.; Lin, Y.; Nakajima, T.; Kobayashi, O.; Kikuichi, M.; Ushijima, R.; Kurosawa, M.; Ueda, J. Reduced serum vascular endothelial growth factor receptor-2 (svegfr-2) and svegfr-1 levels in gastric cancer patients. Cancer Sci 2011, 102, 866–869. [Google Scholar]
- Fielder, W.; Graeven, U.; Ergun, S.; Verago, S.; Kilic, N.; Stockschlader, M.; Hossfeld, D.K. Expression of flt4 and its ligand vegf-c in acute myeloid leukemia. Leukemia 1997, 11, 1234–1237. [Google Scholar]
- De Jonge, H.J.; Weidenaar, A.C.; ter Elst, A.; Boezen, H.M.; Scherpen, F.J.; Bouma-Ter Steege, J.C.; Kaspers, G.J.; Goemans, B.F.; Creutzig, U.; Zimmermann, M.; et al. Endogenous vascular endothelial growth factor-c expression is associated with decreased drug responsiveness in childhood acute myeloid leukemia. Clin. Cancer Res 2008, 14, 924–930. [Google Scholar]
- De Jonge, H.J.; Valk, P.J.; Veeger, N.J.; ter Elst, A.; den Boer, M.L.; Cloos, J.; de Haas, V.; van den Heuvel-Eibrink, M.M.; Kaspers, G.J.; Zwaan, C.M.; et al. High vegfc expression is associated with unique gene expression profiles and predicts adverse prognosis in pediatric and adult acute myeloid leukemia. Blood 2010, 116, 1747–1754. [Google Scholar]
- Bunone, G.; Vigneri, P.; Mariani, L.; Buto, S.; Collini, P.; Pilotti, S.; Pierotti, M.A.; Bongarzone, I. Expression of angiogenesis stimulators and inhibitors in human thyroid tumors and correlation with clinical pathological features. Am. J. Pathol 1999, 155, 1967–1976. [Google Scholar]
- Yu, X.M.; Lo, C.Y.; Chan, W.F.; Lam, K.Y.; Leung, P.; Luk, J.M. Increased expression of vascular endothelial growth factor c in papillary thyroid carcinoma correlates with cervical lymph node metastases. Clin. Cancer Res 2005, 11, 8063–8069. [Google Scholar]
- Yu, X.M.; Lo, C.Y.; Lam, A.K.; Leung, P.; Luk, J.M. Serum vascular endothelial growth factor c correlates with lymph node metastases and high-risk tumor profiles in papillary thyroid carcinoma. Ann. Surg 2008, 247, 483–489. [Google Scholar]
- Kajita, T.; Ohta, Y.; Kimura, K.; Tamura, M.; Tanaka, Y.; Tsunezuka, Y.; Oda, M.; Sasaki, T.; Watanabe, G. The expression of vascular endothelial growth factor c and its receptors in non-small cell lung cancer. Bri. J. Cancer 2001, 85, 255–260. [Google Scholar]
- Takizawa, H.; Kondo, K.; Fujino, H.; Kenzaki, K.; Miyoshi, T.; Sakiyama, S.; Tangoku, A. The balance of vegf-c and vegfr-3 mrna is a predictor of lymph node metastasis in non-small cell lung cancer. Bri. J. Cancer 2006, 95, 75–79. [Google Scholar]
- Kadota, K.; Huang, C.L.; Liu, D.; Ueno, M.; Kushida, Y.; Haba, R.; Yokomise, H. The clinical significance of lymphangiogenesis and angiogenesis in non-small cell lung cancer patients. Eur. J. Cancer 2008, 44, 1057–1067. [Google Scholar]
- Datta, K.; Muders, M.; Zhang, H.; Tindall, D.J. Mechanism of lymph node metastasis in prostate cancer. Future Oncol 2010, 6, 823–836. [Google Scholar]
- Tsurusaki, T.; Kanda, S.; Sakai, H.; Kanetake, H.; Saito, Y.; Alitalo, K.; Koji, T. Vascular endothelial growth factor-c expression in human prostatic carcinoma and its relationship to lymph node metastasis. Bri. J. Cancer 1999, 80, 309–313. [Google Scholar]
- Li, R.; Younes, M.; Wheeler, T.M.; Scardino, P.; Ohori, M.; Frolov, A.; Ayala, G. Expression of vascular endothelial growth factor receptor-3 (vegfr-3) in human prostate. Prostate 2004, 58, 193–199. [Google Scholar]
- Zeng, Y.; Opeskin, K.; Baldwin, M.E.; Horvath, L.G.; Achen, M.G.; Stacker, S.A.; Sutherland, R.L.; Williams, E.D. Expression of vascular endothelial growth factor receptor-3 by lymphatic endothelial cells is associated with lymph node metastasis in prostate cancer. Clin. Cancer Res 2004, 10, 5137–5144. [Google Scholar]
- Duff, S.E.; Li, C.; Jeziorska, M.; Kumar, S.; Saunders, M.P.; Sherlock, D.; O’Dwyer, S.T.; Jayson, G.C. Vascular endothelial growth factors c and d and lymphangiogenesis in gastrointestinal tract malignancy. Bri. J. Cancer 2003, 89, 426–430. [Google Scholar]
- Kitadai, Y.; Amioka, T.; Haruma, K.; Tanaka, S.; Yoshihara, M.; Sumii, K.; Matsutani, N.; Yasui, W.; Chayama, K. Clinicopathological significance of vascular endothelial growth factor (vegf)-c in human esophageal squamous cell carcinomas. Int. J. Cancer 2001, 93, 662–666. [Google Scholar]
- Mobius, C.; Freire, J.; Becker, I.; Feith, M.; Brucher, B.L.; Hennig, M.; Siewert, J.R.; Stein, H.J. Vegf-c expression in squamous cell carcinoma and adenocarcinoma of the esophagus. World J. Surg. 2007, 31, 1768–1772, discussion 1773–1764. [Google Scholar]
- Tanaka, T.; Ishiguro, H.; Kuwabara, Y.; Kimura, M.; Mitsui, A.; Katada, T.; Shiozaki, M.; Naganawa, Y.; Fujii, Y.; Takeyama, H. Vascular endothelial growth factor c (vegf-c) in esophageal cancer correlates with lymph node metastasis and poor patient prognosis. J. Exp. Clin. Cancer Res 2010, 29, 83. [Google Scholar]
- Yonemura, Y.; Endo, Y.; Fujita, H.; Fushida, S.; Ninomiya, I.; Bandou, E.; Taniguchi, K.; Miwa, K.; Ohoyama, S.; Sugiyama, K.; et al. Role of vascular endothelial growth factor c expression in the development of lymph node metastasis in gastric cancer. Clin. Cancer Res 1999, 5, 1823–1829. [Google Scholar]
- Amioka, T.; Kitadai, Y.; Tanaka, S.; Haruma, K.; Yoshihara, M.; Yasui, W.; Chayama, K. Vascular endothelial growth factor-c expression predicts lymph node metastasis of human gastric carcinomas invading the submucosa. Eur. J. Cancer 2002, 38, 1413–1419. [Google Scholar]
- Gao, P.; Zhou, G.Y.; Zhang, Q.H.; Su, Z.X.; Zhang, T.G.; Xiang, L.; Wang, Y.; Zhang, S.L.; Mu, K. Lymphangiogenesis in gastric carcinoma correlates with prognosis. J. Pathol 2009, 218, 192–200. [Google Scholar]
- Han, F.H.; Li, H.M.; Zheng, D.H.; He, Y.L.; Zhan, W.H. The effect of the expression of vascular endothelial growth factor (vegf)-c and vegf receptor-3 on the clinical outcome in patients with gastric carcinoma. Eur. J. Surg. Oncol 2010, 36, 1172–1179. [Google Scholar]
- Kodama, M.; Kitadai, Y.; Tanaka, M.; Kuwai, T.; Tanaka, S.; Oue, N.; Yasui, W.; Chayama, K. Vascular endothelial growth factor c stimulates progression of human gastric cancer via both autocrine and paracrine mechanisms. Clin. Cancer Res 2008, 14, 7205–7214. [Google Scholar]
- Akagi, K.; Ikeda, Y.; Miyazaki, M.; Abe, T.; Kinoshita, J.; Maehara, Y.; Sugimachi, K. Vascular endothelial growth factor-c (vegf-c) expression in human colorectal cancer tissues. Br. J. Cancer 2000, 83, 887–891. [Google Scholar]
- George, M.L.; Tutton, M.G.; Janssen, F.; Arnaout, A.; Abulafi, A.M.; Eccles, S.A.; Swift, R.I. Vegf-a, vegf-c, and vegf-d in colorectal cancer progression. Neoplasia 2001, 3, 420–427. [Google Scholar]
- Hanrahan, V.; Currie, M.J.; Gunningham, S.P.; Morrin, H.R.; Scott, P.A.; Robinson, B.A.; Fox, S.B. The angiogenic switch for vascular endothelial growth factor (vegf)-a, vegf-b, vegf-c, and vegf-d in the adenoma-carcinoma sequence during colorectal cancer progression. J. Pathol 2003, 200, 183–194. [Google Scholar]
- Kinoshita, J.; Kitamura, K.; Kabashima, A.; Saeki, H.; Tanaka, S.; Sugimachi, K. Clinical significance of vascular endothelial growth factor-c (vegf-c) in breast cancer. Breast Cancer Res. Treat 2001, 66, 159–164. [Google Scholar]
- Mohammed, R.A.; Green, A.; El-Shikh, S.; Paish, E.C.; Ellis, I.O.; Martin, S.G. Prognostic significance of vascular endothelial cell growth factors-a, -c and -d in breast cancer and their relationship with angio- and lymphangiogenesis. Bri. J. Cancer 2007, 96, 1092–1100. [Google Scholar]
- Wu, Q.W.; She, H.Q.; Liang, J.; Huang, Y.F.; Yang, Q.M.; Yang, Q.L.; Zhang, Z.M. Expression and clinical significance of extracellular matrix protein 1 and vascular endothelial growth factor-c in lymphatic metastasis of human breast cancer. BMC Cancer 2012, 12, 47. [Google Scholar]
- Nakamura, Y.; Yasuoka, H.; Tsujimoto, M.; Yoshidome, K.; Nakahara, M.; Nakao, K.; Nakamura, M.; Kakudo, K. Nitric oxide in breast cancer: Induction of vascular endothelial growth factor-c and correlation with metastasis and poor prognosis. Clin. Cancer Res 2006, 12, 1201–1207. [Google Scholar]
- Ueda, M.; Terai, Y.; Yamashita, Y.; Kumagai, K.; Ueki, K.; Yamaguchi, H.; Akise, D.; Hung, Y.C.; Ueki, M. Correlation between vascular endothelial growth factor-c expression and invasion phenotype in cervical carcinomas. Int. J. Cancer 2002, 98, 335–343. [Google Scholar]
- Gombos, Z.; Xu, X.; Chu, C.S.; Zhang, P.J.; Acs, G. Peritumoral lymphatic vessel density and vascular endothelial growth factor c expression in early-stage squamous cell carcinoma of the uterine cervix. Clin. Cancer Res 2005, 11, 8364–8371. [Google Scholar]
- Yokoyama, Y.; Charnock-Jones, D.S.; Licence, D.; Yanaihara, A.; Hastings, J.M.; Holland, C.M.; Emoto, M.; Umemoto, M.; Sakamoto, T.; Sato, S.; et al. Vascular endothelial growth factor-d is an independent prognostic factor in epithelial ovarian carcinoma. Br. J. Cancer 2003, 88, 237–244. [Google Scholar]
- Nishida, N.; Yano, H.; Komai, K.; Nishida, T.; Kamura, T.; Kojiro, M. Vascular endothelial growth factor c and vascular endothelial growth factor receptor 2 are related closely to the prognosis of patients with ovarian carcinoma. Cancer 2004, 101, 1364–1374. [Google Scholar]
- Ueda, M.; Hung, Y.C.; Terai, Y.; Kanda, K.; Kanemura, M.; Futakuchi, H.; Yamaguchi, H.; Akise, D.; Yasuda, M.; Ueki, M. Vascular endothelial growth factor-c expression and invasive phenotype in ovarian carcinomas. Clin. Cancer Res 2005, 11, 3225–3232. [Google Scholar]
- Miyata, Y.; Kanda, S.; Ohba, K.; Nomata, K.; Hayashida, Y.; Eguchi, J.; Hayashi, T.; Kanetake, H. Lymphangiogenesis and angiogenesis in bladder cancer: Prognostic implications and regulation by vascular endothelial growth factors-a, -c, and -d. Clin. Cancer Res 2006, 12, 800–806. [Google Scholar]
- Rinderknecht, M.; Villa, A.; Ballmer-Hofer, K.; Neri, D.; Detmar, M. Phage-derived fully human monoclonal antibody fragments to human vascular endothelial growth factor-c block its interaction with vegf receptor-2 and 3. PLoS One 2010, 5, e11941. [Google Scholar]
- Roberts, N.; Kloos, B.; Cassella, M.; Podgrabinska, S.; Persaud, K.; Wu, Y.; Pytowski, B.; Skobe, M. Inhibition of vegfr-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of vegfr-2. Cancer Res 2006, 66, 2650–2657. [Google Scholar]
- Burton, J.B.; Priceman, S.J.; Sung, J.L.; Brakenhielm, E.; An, D.S.; Pytowski, B.; Alitalo, K.; Wu, L. Suppression of prostate cancer nodal and systemic metastasis by blockade of the lymphangiogenic axis. Cancer Res 2008, 68, 7828–7837. [Google Scholar]
- Lin, J.; Lalani, A.S.; Harding, T.C.; Gonzalez, M.; Wu, W.W.; Luan, B.; Tu, G.H.; Koprivnikar, K.; VanRoey, M.J.; He, Y.; et al. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble vegfr-3 decoy receptor. Cancer Res 2005, 65, 6901–6909. [Google Scholar]
- Zhang, D.; Li, B.; Shi, J.; Zhao, L.; Zhang, X.; Wang, C.; Hou, S.; Qian, W.; Kou, G.; Wang, H.; et al. Suppression of tumor growth and metastasis by simultaneously blocking vascular endothelial growth factor (vegf)-a and vegf-c with a receptor-immunoglobulin fusion protein. Cancer Res 2010, 70, 2495–2503. [Google Scholar]
- Heckman, C.A.; Holopainen, T.; Wirzenius, M.; Keskitalo, S.; Jeltsch, M.; Yla-Herttuala, S.; Wedge, S.R.; Jurgensmeier, J.M.; Alitalo, K. The tyrosine kinase inhibitor cediranib blocks ligand-induced vascular endothelial growth factor receptor-3 activity and lymphangiogenesis. Cancer Res 2008, 68, 4754–4762. [Google Scholar]
- Matsui, J.; Funahashi, Y.; Uenaka, T.; Watanabe, T.; Tsuruoka, A.; Asada, M. Multi-kinase inhibitor e7080 suppresses lymph node and lung metastases of human mammary breast tumor mda-mb-231 via inhibition of vascular endothelial growth factor-receptor (vegf-r) 2 and vegf-r3 kinase. Clin. Cancer Res 2008, 14, 5459–5465. [Google Scholar]
- Kodera, Y.; Katanasaka, Y.; Kitamura, Y.; Tsuda, H.; Nishio, K.; Tamura, T.; Koizumi, F. Sunitinib inhibits lymphatic endothelial cell functions and lymph node metastasis in a breast cancer model through inhibition of vascular endothelial growth factor receptor 3. Breast Cancer Res 2011, 13, R66. [Google Scholar]
- Pai, S.I.; Lin, Y.Y.; Macaes, B.; Meneshian, A.; Hung, C.F.; Wu, T.C. Prospects of rna interference therapy for cancer. Gene Ther 2006, 13, 464–477. [Google Scholar]
- Zhang, H.; Yin, Y.; Zhang, L.; Zheng, X.; Gao, D.; Chen, K.; Zhang, Y. The effects of vascular endothelial growth factor c knockdown in esophageal squamous cell carcinoma. J. Cancer Res. Clin. Oncol 2012, 138, 133–139. [Google Scholar]
- Shibata, M.A.; Morimoto, J.; Shibata, E.; Otsuki, Y. Combination therapy with short interfering rna vectors against vegf-c and vegf-a suppresses lymph node and lung metastasis in a mouse immunocompetent mammary cancer model. Cancer Gene Ther 2008, 15, 776–786. [Google Scholar]
- Wang, F.Q.; Barfield, E.; Dutta, S.; Pua, T.; Fishman, D.A. Vegfr-2 silencing by small interference rna (sirna) suppresses lpa-induced epithelial ovarian cancer (eoc) invasion. Gynecol. Oncol 2009, 115, 414–423. [Google Scholar]
- Kurenova, E.V.; Hunt, D.L.; He, D.; Fu, A.D.; Massoll, N.A.; Golubovskaya, V.M.; Garces, C.A.; Cance, W.G. Vascular endothelial growth factor receptor-3 promotes breast cancer cell proliferation, motility and survival in vitro and tumor formation in vivo. Cell Cycle 2009, 8, 2266–2280. [Google Scholar]
- Saharinen, P.; Eklund, L.; Pulkki, K.; Bono, P.; Alitalo, K. Vegf and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol. Med 2011, 17, 347–362. [Google Scholar]
- ClinicalTrials. Available online: http://www.clinicaltrials.gov/ accessed on 17 December 2012.
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, J.-C.; Chang, Y.-W.; Hong, C.-C.; Yu, Y.-H.; Su, J.-L. The Role of the VEGF-C/VEGFRs Axis in Tumor Progression and Therapy. Int. J. Mol. Sci. 2013, 14, 88-107. https://doi.org/10.3390/ijms14010088
Chen J-C, Chang Y-W, Hong C-C, Yu Y-H, Su J-L. The Role of the VEGF-C/VEGFRs Axis in Tumor Progression and Therapy. International Journal of Molecular Sciences. 2013; 14(1):88-107. https://doi.org/10.3390/ijms14010088
Chicago/Turabian StyleChen, Jui-Chieh, Yi-Wen Chang, Chih-Chen Hong, Yang-Hao Yu, and Jen-Liang Su. 2013. "The Role of the VEGF-C/VEGFRs Axis in Tumor Progression and Therapy" International Journal of Molecular Sciences 14, no. 1: 88-107. https://doi.org/10.3390/ijms14010088



